Abstract
In a previous study, we generated novel antithrombopoietin receptor agonist antibodies as therapeutic candidates. In this report, we investigated the in vivo effects of one of these antibodies, MA01G4344U, on primary human hematopoietic cells using xenotransplantation. NOD/Shi-scid, IL-2Rγnull (NOG) mice were pretreated by total-body irradiation and received a transplant of human cord blood–derived CD34+ cells. Weekly intraperitoneal injection of MA01G4344U (100 μg/mouse per week) or Peg-rhMGDF (5 μg/mouse per week) or phosphate-buffered saline (PBS) was performed. Human cells in peripheral blood were analyzed by flow cytometry and bone marrow cells were analyzed by flow cytometry and colony assay. MA01G4344U successfully increased the number of human CD41+ platelets and human CD45+ cells in peripheral blood. In the bone marrow, MA01G4344U increased the number of human CD45+/CD34+ cells, which resulted in more multilineage progenitor cells. The efficacy of MA01G4344U in promoting primary human hematopoietic cells in vivo suggests its therapeutic potential for thrombocytopenic and pancytopenic disorders.
Introduction
Thrombopoietin (THPO) is known as the pivotal hematopoietic growth factor for megakaryocyte development and platelet production.1,2 The expression of thrombopoietin receptor (MPL) is not restricted to the megakaryocytic lineage, but is found in hematopoietic stem/progenitor cells (HSCs/PCs).3-5 Recently we reported the generation of novel anti-MPL agonist antibodies using a human antibody–producing mouse system.6 We also used subclass switching of the hinge region to improve the agonistic activities of the antibodies. One of the antibodies, MA01G4344U, has a constant region of human IgG4 and an upper hinge region of human IgG3.6 MA01G4344U is specific for human MPL and does not cross-react with mouse Mpl. In this study, to evaluate the in vivo efficacy of MA01G4344U in human primary hematopoietic cells, we used a xenotransplanted mouse model
NOD/Shi-scid, IL-2Rγnull (NOG) is a mouse strain that exhibits severe immunodeficiency with lack of T, B, and natural killer (NK) cells. It has been reported that the human hematopoietic system can be effectively reconstructed in NOG mice by transplanting human hematopoietic stem cells (HSCs).7 Human umbilical cord blood is known to contain HSCs/PCs and is used as a stem cell source for therapeutic transplantation.7-10 CD34 is expressed by early hematopoietic cells and commonly used as a human HSC/PC marker.11 In this study, human cord blood CD34+ (CBCD34+) cells were transplanted into irradiated NOG mice. Then MA01G4344U was administered to the mice from the next day of transplantation. We used CD41 (integrin alpha 2b) as a marker of platelets, and CD45 (transmembrane protein-tyrosine phosphatase receptor type C) as a pan-white blood cell marker. The numbers of human platelets and total white blood cells in the peripheral blood were analyzed by flow cytometry. The numbers of human hematopoietic progenitor cells (HPCs) in the bone marrow were also analyzed by flow cytometry and colony-formation assay. Our results show that the treatment with MA01G4344U was effective for increasing human platelets and HPCs in vivo.
Methods
Transplantation
NOG mice were supplied by the Central Institute for Experimental Animals (Kanagawa, Japan). Six- to 8-week-old male mice were used for the experiments. Four hours before transplantation, the mice received total body irradiation (TBI) with x-ray at 2 grays. Human CBCD34+ cells were suspended at 5 × 104 cells/mL in MEM-alpha medium containing 0.1% bovine serum albumin (BSA). We injected 200 μL of the cell suspension into each mouse intravenously ( 000 cells/mouse). The cord blood was donated by healthy volunteers under their informed consent in accordance with the Declaration of Helsinki.
Administration
Antibody administration started on the day after transplantation. MA01G4344U was intraperitoneally injected (100 μg/mouse per week). Pegylated-recombinant human megakaryocyte growth and development factor (Peg-rhMGDF), a molecule consisting of the receptor-binding portion of human THPO conjugated to a polyethylene glycol moiety, was intraperitoneally injected (5 μg/mouse per week) into a second group of mice. PBS was used as a vehicle control. There were 11 mice per group.
Peripheral blood analysis
Peripheral blood samples were collected from the retro-orbital plexus at 2, 4, and 6 weeks after transplantation under anesthesia. Total platelet counts were measured using the KX-21 Hematology Analyzer system (Sysmex, Hyogo, Japan). To examine the counts of human cells in the peripheral blood, flow cytometric analysis was performed using the FACSCalibur system (Becton Dickinson, San Jose, CA). Total platelets were gated by forward and side scatter. Then human and mouse platelets were distinguished using anti–human CD41-PE (R7058; Dako, Glostrup, Denmark) and anti–mouse CD41-FITC (553848; BD Pharmingen, San Diego, CA). Flow-Count beads (Beckman Coulter, Hialeah, FL) were used as an internal standard to measure cell counts within the samples concomitantly. For detection of CD45+ cells, anti–human CD45-APC (IM2473; Beckman Coulter) and anti–mouse CD45-PE (553081; BD Pharmingen) antibodies were used. The counts of human CD41+ and CD45+ cells per microliter of blood were calculated by multiplying the cell counts by the dilution factors.
Bone marrow analysis
Six weeks after transplantation, all mice were killed to collect the bone marrow cells from the femurs. The whole bone marrow cells were analyzed by flow cytometry. In addition, anti–human CD34-PE antibody was used to count the HSC/PC fraction. Colony assay was performed to count the HPCs contained in the bone marrow cells. The MethoCult system (StemCell Technologies, Vancouver, BC) was used with the following recombinant human cytokines: EPO (4 IU/mL), SCF (100 ng/mL), IL-3 (20 ng/mL), and GM-CSF (10 ng/mL). We plated 105 whole bone marrow cells per dish. The cells were then incubated at 37°C, 5% CO2, 5%O2 for 14 days. The colonies of erythrocytes (Es), granulocytes/macrophages (GMs), and granulocytes/erythrocytes/macrophages (GEMs) were counted under light microscopy.
Authorization
All the experiments in this report have been authorized by the institutional ethics committee and by the institutional experimental animal ethics committee of Kyowa Hakkin Kirin.
Statistical analysis
Student t test was used to determine statistical difference in the results. A P value less than .05 was considered statistically significant.
Results and discussion
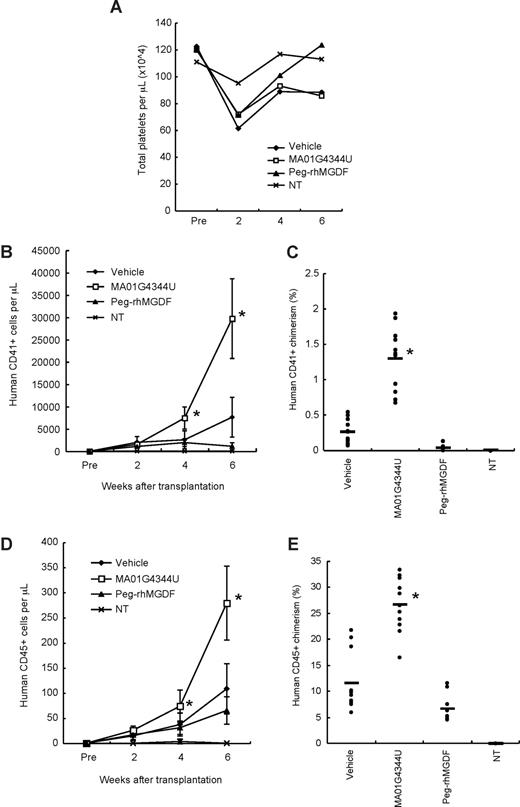
NOG mice were pretreated by TBI, and human CBCD34+ cells were transplanted intravenously. Weekly administration of MA01G4344U, or Peg-rhMGDF, or vehicle control, began the day after transplantation. The total platelet counts were transiently and mildly reduced by the pretreatment when measured at 2 weeks after transplantation, but recovered by week 4 (Figure 1A). No drug-related toxicity was observed through the experiment. The human CD41+ cells in peripheral blood significantly increased in the antibody-treated group at weeks 4 and 6, indicating that MA01G4344U successfully promoted production of human platelets (Figure 1B). Peripheral chimerisms of CD41+ cells at week 6 were 1.28% (MA01G4344U group) versus 0.26% (vehicle group) (Figure 1C). MA01G4344U also increased peripheral counts of human CD45+ cells (Figure 1D). Peripheral CD45+ chimerism of the MA01G4344U group reached 26.7%, whereas that of the vehicle group was 11.6% (Figure 1E), indicating that the antibody affected not only the megakaryocytic lineage but other hematopoietic lineages. Interestingly, treatment with Peg-rhMGDF did not increase either human CD41+ or human CD45+ cells. Instead, it resulted in a slight decrease of human cells. This may be explained by the species cross-reactivity of Peg-rhMGDF, which stimulated mouse cells to proliferate and suppressed human cells competitively. The results indicated that our xenotransplantation system is not useful for comparison of human-specific MA01G4344U and other MPL agonists cross-reactive for both human and mouse.
Peripheral blood analysis. (A) Total platelet counts in mice that received a transplant of human CBCD34+ cells. NT indicates not transplanted. (B) Counts of human CD41+ cells (platelets) in peripheral blood. The graph shows the mean plus or minus SD. (C) Percentage of human CD41+ cells in peripheral blood at week 6 after transplantation. • represent each individual. The bars represent the average values. (D) Counts of human CD45+ cells in peripheral blood (mean ± SD). (E) Percentage of human CD45+ chimeric cells in peripheral blood at week 6 after transplantation. Asterisks show statistically significant differences in comparison with the vehicle control group (P < .05).
Peripheral blood analysis. (A) Total platelet counts in mice that received a transplant of human CBCD34+ cells. NT indicates not transplanted. (B) Counts of human CD41+ cells (platelets) in peripheral blood. The graph shows the mean plus or minus SD. (C) Percentage of human CD41+ cells in peripheral blood at week 6 after transplantation. • represent each individual. The bars represent the average values. (D) Counts of human CD45+ cells in peripheral blood (mean ± SD). (E) Percentage of human CD45+ chimeric cells in peripheral blood at week 6 after transplantation. Asterisks show statistically significant differences in comparison with the vehicle control group (P < .05).
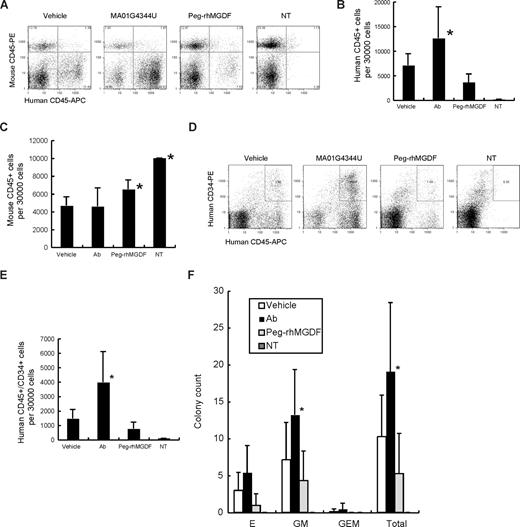
Bone marrow cells were collected from the femurs at week 6. Cellularity did not change among Peg-rhMGDF–, antibody-, and vehicle-treated groups (data not shown). Flow cytometric analysis was performed to count the human and mouse cells. Representative results of flow cytometry analyzing human CD45+ cells are shown in Figure 2A. Human CD45+ cells were significantly increased in the antibody-treated group (Figure 2A,B). As seen in peripheral blood, there was a slight decrease of human CD45+ cells in the Peg-rhMGDF–treated group. Mouse CD45+ cells were increased in the Peg-rhMGDF–treated group (Figure 2C), indicating that Peg-rhMGDF stimulated proliferation of mouse cells dominantly. Furthermore, the human CD45+/CD34+ fraction containing HSCs/PCs was also increased in the antibody-treated group (Figure 2D,E). Colony assay revealed that colony-forming unit erythrocyte (CFU-E), granulocyte/macrophage (-GM), and granulocyte/erythrocyte/macrophage (-GEM) were increased in the antibody-treated group (Figure 2F). The data indicated that treatment with MA01G4344U resulted in an increase of not only human platelets but also progenitors of other myeloid lineages such as erythrocytes and granulocytes-macrophages.
Bone marrow analysis. (A) Representative results of flow cytometry analyzing human CD45+ cells. (B) Counts of human CD45+ cells per 30 000 bone marrow cells. Ab indicates MA01G4344U; NT, not transplanted. (C) Counts of mouse CD45+ cells per 30 000 bone marrow cells (mean + SD). Asterisks show statistically significant differences in comparison with the vehicle control group (P < .05). (D) Representative results of flow cytometry analyzing human CD45+/CD34+ cells. (E) Counts of human CD45+/CD34+ cells per 30 000 bone marrow cells (mean + SD). Asterisks show statistically significant differences in comparison with the vehicle control group (P < .05). (F) Colony assay results (mean + SD). E indicates erythrocyte; GM, granulocyte/macrophage; and GEM, granulocyte/erythrocyte/macrophage. The “Total” represents E + GM + GEM. Asterisks show statistically significant differences in comparison with the vehicle control group (P < .05).
Bone marrow analysis. (A) Representative results of flow cytometry analyzing human CD45+ cells. (B) Counts of human CD45+ cells per 30 000 bone marrow cells. Ab indicates MA01G4344U; NT, not transplanted. (C) Counts of mouse CD45+ cells per 30 000 bone marrow cells (mean + SD). Asterisks show statistically significant differences in comparison with the vehicle control group (P < .05). (D) Representative results of flow cytometry analyzing human CD45+/CD34+ cells. (E) Counts of human CD45+/CD34+ cells per 30 000 bone marrow cells (mean + SD). Asterisks show statistically significant differences in comparison with the vehicle control group (P < .05). (F) Colony assay results (mean + SD). E indicates erythrocyte; GM, granulocyte/macrophage; and GEM, granulocyte/erythrocyte/macrophage. The “Total” represents E + GM + GEM. Asterisks show statistically significant differences in comparison with the vehicle control group (P < .05).
In this study, we showed enhancement of platelets in peripheral blood and HPCs in bone marrow by MA01G4344U treatment using primary human hematopoietic cells. These results suggest the therapeutic potential of this antibody for thrombocytopenic and pancytopenic disorders.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr Katsumi Yazaki for kindly providing research materials; Drs Kazuhiro Motoki and Uichi Nishiyama for discussions and suggestions; and Naoko Tago for excellent technical assistance.
Authorship
Contribution: M.K. and T.H. designed research, conducted research, performed experiments, analyzed data, and wrote the paper; Y.I., H.M., and S.K. conducted research; and C. Emuta, Y.C., K.T., and C. Endo performed experiments and analyzed data.
Conflict-of-interest disclosure: All the authors are employees of Kyowa Hakko Kirin Co, Ltd.
Correspondence: Masayuki Kai, Innovative Drug Research Laboratories, Research Division, Kyowa Hakko Kirin Co, Ltd, 3 Miyahara, Takasaki, Gunma, 370-1295, Japan; e-mail: masayuki.kai@kyowa-kirin.co.jp; or Tetsuya Hagiwara, Frontier Laboratory, Kyowa Hakko Kirin Co., Ltd., 3 Miyahara, Takasaki, Gunma, 370-1295, Japan; e-mail: tetsuya.hagiwara@kyowa-kirin.co.jp.
References
Author notes
*M.K. and T.H. contributed equally to this work.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal