In this issue of Blood, Bussel and colleagues report on the safety and efficacy of long-term administration of romiplostim, a recently FDA-approved TSA, for the treatment of chronic ITP.
In adults, immune thrombocytopenic purpura (ITP) is largely a chronic disorder. Unfortunately, currently available medical therapies for ITP are not well suited to chronic use. Corticosteroids are associated with a well-known litany of cumulative toxicities. Like corticosteroids, immune globulin and anti-Rh(D) are highly effective as rescue therapy, but their use in the maintenance setting is limited, in part because of the inconvenience of regular intravenous infusion. Many thirdline medical therapies are similarly plagued by agent-specific cumulative toxicities and limited response rates. Although splenectomy, the most widely recommended “chronic” therapy, is effective in the majority of patients, some have persistent thrombocytopenia. In addition, splenectomy is associated with approximately a 1% risk of overwhelming sepsis that endures for the lifetime of the patient. Hence, in spite of the multitude of treatments available for ITP, some patients with chronic severe disease have few good options and suffer substantial treatment-related morbidity.
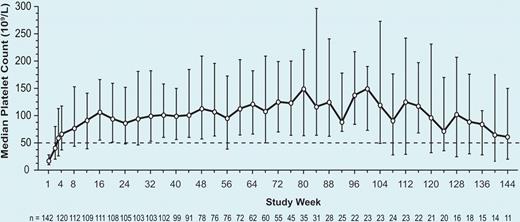
In this light, the study by Bussel et al is greeted with cautious enthusiasm. In previously reported trials, all of which were less than or equal to 24 weeks' duration, romiplostim increased platelet counts and was well tolerated in a majority of subjects.1-3 Nonetheless, a small number of patients developed reticulin deposition in the bone marrow, raising concerns about the safety of long-term romiplostim administration. In the present analysis, an ongoing, single-arm, open-label extension study of 142 subjects who participated in previous romiplostim trials, patients were treated for a mean of 69 weeks (maximum 3 years).4 Platelet response, defined as a platelet count greater than or equal to 50 × 109/L and a doubling from baseline, was achieved in 87% of subjects. As the figure illustrates, this response was sustained for the duration of the study, though it may be inflated by dropout bias, a phenomenon in which subjects with a diminished response are more likely to drop out of the study than those maintaining a good response to romiplostim. Nonetheless, consistent with the observed rise in platelet count, bleeding events decreased over time.
Median platelet count by study week. See the complete figure in the article beginning on page 2161.
Median platelet count by study week. See the complete figure in the article beginning on page 2161.
Despite these promising results, concerns persist about the safety of long-term romiplostim use, particularly with respect to the risk of bone marrow fibrosis. In a planned subgroup analysis, 9 patients underwent a bone marrow biopsy before entering the extension study and after 3 to 9 months of therapy. Of the 6 patients with evaluable baseline and follow-up biopsies, 1 patient demonstrated mildly increased reticulin staining after 3 months of therapy. Biopsies on at least 7 additional subjects were performed, presumably for clinical indications, such as new abnormalities on the peripheral blood smear or loss of response to romiplostim, and showed varying degrees of reticulin deposition. Trichrome staining for type I collagen fibrosis, performed in 5 of the 8 patients with marrow reticulin, was absent. Immunophenotyping and cytogenetics, also performed in a subset of these patients, did not demonstrate evidence of a clonal disorder. Follow-up bone marrow biopsies were performed in only 2 patients after discontinuation of romiplostim and demonstrated improvement in 1 patient and no change in the other. However, neither patient showed complete regression of marrow reticulin.
Several important questions remain regarding romiplostim-induced reticulin fibrosis. First, because routine bone marrow biopsies were not performed as part of this study, the true incidence of reticulin deposition with romiplostim could not be assessed. Moreover, the natural history of romiplostim-induced reticulin deposition is unknown. Reversibility of bone marrow reticulin associated with short-term TSA administration has been documented in mice5 and in patients with acute myeloid leukemia.6 The effects of long-term administration are less certain, although it bears remembering that transgenic mice that overexpress thrombopoietin in their bone marrow develop a phenotype similar to primary myelofibrosis.7 Prospective clinical studies that include bone marrow biopsies before and after treatment with romiplostim at prespecified time points will be required to address these critical questions.
In the search for better chronic therapies for a chronic disease, Bussel et al provide encouraging news. Nevertheless, a central tenet of ITP management is to administer therapy only when the bleeding risk outweighs the adverse effects associated with treatment.8 With this in mind, romiplostim and other TSAs should be considered for chronic therapy only with great caution until more is known about their safety in this setting.
Conflict-of-interest disclosure: C.S.A. consulted for GSK. A.C. declares no competing financial interests. ■