Extradomain B of fibronectin (ED-B FN) is expressed in the vasculature of hematopoietic malignancies and appears to be an effective target for monoclonal antibody–based therapies.
Wherever there is cancer, there will be blood and blood vessels to support tumor growth. While the majority of anticancer drugs are designed to directly kill tumor cells, recent strategies are focusing on drugs that indirectly kill cancer cells by targeting the tumor microenvironment and tumor vasculature. This strategy gained a significant boost with the approval of the angiogenesis inhibitor bevacizumab by the Food and Drug Administration (FDA) for the treatment of a variety of solid tumors. In addition to angiogenesis inhibitors, which primarily target the vascular endothelial growth factors (VEGFs) and their receptors, novel antivascular agents are currently under development for cancer therapy.
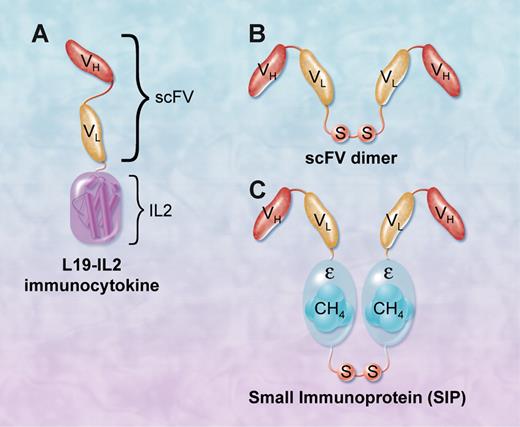
L19 monoclonal antibody derivatives that target ED-B FN. (A) L19-IL2 immunocytokine consists of a single-chain Fv fragment fused to IL-2 molecule. (B) Single-chain Fv dimer. (C) Small immunoprotein (SIP) consists of 2 scFv fragments fused by a CH4 domain of the human IgE, which mediates its homodimerization. SIP can be further conjugated with radioisotopes to deliver radiation to the site of tumors. Professional illustration by A. Y. Chen.
L19 monoclonal antibody derivatives that target ED-B FN. (A) L19-IL2 immunocytokine consists of a single-chain Fv fragment fused to IL-2 molecule. (B) Single-chain Fv dimer. (C) Small immunoprotein (SIP) consists of 2 scFv fragments fused by a CH4 domain of the human IgE, which mediates its homodimerization. SIP can be further conjugated with radioisotopes to deliver radiation to the site of tumors. Professional illustration by A. Y. Chen.
Fibronectin (FN) is a large adhesive glycoprotein found in plasma and tissue extracellular matrix.1 FN binds to a large number of cell-surface transmembrane proteins called integrins. FN was one of the first genes reported to undergo alternative splicing, resulting in 3 isoforms, IIICS, extradomain (ED)-A, and ED-B. The ED-B, which was identified more than 20 years ago, consists of 91 amino acids that are identical in several vertebrates, including mice and humans.2 This domain arises by alternative splicing exclusively at the sites of tissue remodeling and is considered a marker of angiogenesis. Extradomain B of fibronectin (ED-B FN) is usually absent in plasma and normal adult tissues, but abundantly present around newly formed blood vessels, including cancer neovasculature. Thus, the preferential localization of ED-B FN to tumor vasculature makes it a good target for cancer therapy. To test the potential clinical activity of ED-B FN–targeted therapy, a 150-kDa human IgG1 antibody to ED-B FN (termed L19) was developed. Furthermore, L19 derivatives, including a dimeric single-chain Fv (scFv) fragment (50 kDa), and a small immunoprotein (SIP), were also developed for preclinical and clinical testing. The SIP-L19 (80 kDa) consists of 2 scFv fragments fused by a CH4 domain of the human IgE, which mediates its homodimerization. Because ED-B FN was originally described to be associated with neovasculature of solid tumors, L19 and its derivatives, including radiolabeled SIP and immunocytokine conjugates, were evaluated in animal models bearing a variety of solid tumors with promising early results.3
In this issue of Blood, investigators extend the knowledge previously obtained from solid tumor models to hematologic malignancies. In the first study, Sauer and colleagues used immunohistochemistry methods to examine ED-B FN expression in a large panel of hematologic malignancies.4 They found ED-B, which was absent in normal lymph nodes and bone marrow biopsies, to be expressed in the vast majority of Hodgkin and non-Hodgkin lymphoma (NHL) cases. Importantly, these investigators used a 131I-labeled L19 SIP therapeutically to treat patients with relapsed lymphoma and reported achievement of partial remissions in 2 patients with Hodgkin lymphoma. Furthermore, using single photon emission computed tomography (SPECT) imaging, the authors convincingly showed selective ED-B FN targeting of malignant lesions.
In a different approach, Schliemann and colleagues conjugated a high-affinity L19derivative to ED-B with interleukin (IL)-2.5 This novel immunocytokine conjugate can bind to lymphoma vasculature and deliver IL-2 to the tumor microenvironment, which in turn recruits natural killer cells and macrophages to tumor sites. When given with rituximab, the conjugate showed dramatic and prolonged efficacy in xenograft models of NHL. The idea of combining systemic cytokines with monoclonal antibodies to enhance the antibody-dependent cell-mediated cytotoxicity has been previously evaluated in clinical trials in patients with lymphoma with mixed results. However, this approach has not been widely adopted to clinical practice, because of either the toxicity induced by the systemic administration of these cytokines, or the lack of proven superiority to the antibody therapy alone. The ability to deliver cytokines directly to tumor sites through selective targeting may reduce toxicity associated with the high doses of cytokines required for many previous trials.
In recent years, several naked and conjugated antibodies have been approved by the FDA for the treatment of hematologic malignancies, including rituximab, the radio-labeled antibodies tositumomab and 90-Y ibritumomab tiuxetan, and the immunotoxin conjugate gemtuzumab ozogamicin. Other antigens and receptors are also being evaluated as potential therapeutic targets for lymphoma with promising early results, including C30, CD22, CD40, CD80, CD19, and TRAIL death receptors.6 The identification of ED-B FN as a marker of lymphoma neovasculature adds additional potential therapeutic strategy for this disease. A potential advantage of targeting the tumor vasculature and the microenvironment is that these targets could have a broad therapeutic applicability across various tumor types. Collectively, these two experiments suggest that L19-based therapy is a promising new treatment strategy for patients with lymphoma and warrants evaluating these molecules in clinical trials.
Conflict-of-interest disclosure: The authors declare no competing financial interests. ■

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal