Abstract
CD40 signaling is critical for innate and adaptive immunity against pathogens, and the cytoplasmic domain of CD40 is highly conserved both within and between species. A novel missense single nucleotide polymorphism (SNP) in the cytoplasmic domain of CD40 at position 227 (P227A) was identified, which resides on a conserved ancestral haplotype highly enriched in persons of Mexican and South American descent. Functional studies indicated that signaling via human (h) CD40-P227A stably expressed in several B-cell lines led to increased phosphorylation of c-Jun, increased secretion of the pro-inflammatory cytokines interleukin (IL)–6 and TNF-α, and increased Ig production, compared with wild-type hCD40. Cooperation between hCD40-P227A signaling and B-cell receptor (BCR)– or Toll-like receptor 9 (TLR9)–mediated signaling was also enhanced, resulting in elevated and synergistic production of IL-6 and Ig. We have thus identified a novel genetic variant of hCD40 with a gain-of-function immune phenotype.
Introduction
CD40 is a member of the tumor necrosis factor (TNF) receptor (TNFR) family of genes1 and is expressed on B cells and antigen-presenting myeloid cells. Interactions between CD40 and its ligand, CD154, are required for T-cell dependent (T-dependent) B-cell responses, including efficient germinal center formation, class switching and memory B-cell generation.2 In dendritic cells, CD40 ligation leads to enhanced survival and tumoricidal activity, and increased production of cytokines and nitrous oxide.3 Genetic mutations in CD154 cause human X-linked immunodeficiency with hyper-IgM, type 1 (HIGM1), a disease associated with severe immune deficiency and increased susceptibility to bacterial and opportunistic infections.4 Complete deficiencies of CD40 (HIGM3) are also described and show a similar phenotype to HIGM1.5
CD40 is located on chromosome 20q13.1. Genetic linkage with this region has been reported for autoimmune diseases including systemic lupus erythematosus (SLE),6 juvenile rheumatoid arthritis,7 and Graves disease (GD),8 making CD40 an attractive candidate contributor to these conditions. Initial reports demonstrated genetic linkage of GD with a region of chromosome 20q11.2-13.1 that contains the CD40 locus.8 Further evaluation revealed the C allele or CC genotype of a Kozak sequence polymorphism in the 5′-UTR at position −1 of CD40 to be associated with GD in white cohorts from the United States,9 Korea,10 and Japan.11 However, 2 large GD cohorts from the United Kingdom failed to demonstrate this association.12,13 Functional evaluations in immune cells from CC genotype subjects show higher surface CD40 and enhanced CD40 signaling. Enhanced translational efficiency from DNA strands with the rs1883832 C allele has been proposed as underlying elevated CD40 protein expression.
Chadha et al used a family-based haplotype tagging approach in a cohort of 408 European-White SLE families from the United Kingdom to test the hypothesis of association between CD40 and SLE incidence.14 The results of this study failed to provide convincing evidence of association with the occurrence of SLE and single nucleotide polymorphisms (SNPs) or SNP CD40 haplotypes. However, the possibility that CD40 contributes to particular disease symptoms or severity was not explored in either this or the GD studies.
Our group recently published a fine-mapping study of the chromosome 20q13.1 region.6 This study, although not specifically designed to target the CD40 locus, identified modest evidence of association with SNPs in and around the CD40 locus in a cohort of 230 SLE families. Interestingly, the evidence for association in the region of CD40 weakened when only white families were considered, suggesting that a portion of the effect was attributable to non-white pedigrees, which comprised only 20% of the total sample.
Here, we describe genetic and functional characterization of a novel missense SNP in exon 9 of the CD40 gene resulting in a proline-to-alanine amino acid substitution at position 227 of CD40. Our data demonstrate an unusual population enrichment of this variant on an ancestral haplotype present in Mexican and South American populations. Functional analyses using well-characterized mouse and human B-cell lines reveal that hCD40-P227A signals resulted in enhanced ability to activate B-cell function, amplified cooperation with the B-cell receptor (BCR) and Toll-like receptor 9 (TLR9), and increased activation of a subset of early signaling pathways. This “gain of function” suggests a possible role for the P227A polymorphism in increasing efficacy of immune responses to pathogens endemic to Mexico and South America. It may also predispose persons to a general susceptibility to lymphoid hyperreactivity.
Methods
Human DNA samples and SNP genotyping
The Human Genome Diversity Panel (HGDP), a DNA collection representing 1064 donors from 52 world populations,15 was used to characterize the worldwide prevalence of P227A. HGDP samples were genotyped for rs11086998 using MassARRAY (Sequenom, San Diego, CA) according to manufacturer-recommended protocols.
Cells
The human B-cell line T5-116 and the mature mouse B-cell lines CH12.LX17 and M12.4.118 have been described previously; both were chosen as responsive to signals from both CD40 and TLRs. Cells were maintained in RPMI 1640 with 10% heat-inactivated fetal calf serum (FCS; Atlanta Biologicals, Norcross, GA), 10 μM 2-ME (GIBCO, Grand Island, NY), penicillin and streptomycin (B-cell medium [BCM]). Stable transfectants expressing wild-type (WT) hCD40 or a chimeric molecule consisting of the hCD40 extracellular and transmembrane domains and the LMP1 intracellular domain (hCD40-LMP1) have been described and were maintained in 400 μg/mL G418 disulfate (Research Products International, Mt Prospect, IL).19 In all experiments, 3 or more individual stably transfected subclones with similar levels of endogenous and transfected hCD40 or mouse (m) CD40 were tested, and signaling via each transfected molecule was compared with signaling via the clone's endogenous CD40, to ensure that results obtained were not attributable to clonal variation. Hi5 insect cells infected with WT or hCD154-expressing baculoviruses have been described.20 Hi5 cells grow at 25°C and lyse at 37°C, providing a source of membrane-bound trimeric hCD154 without overgrowing cultures. Whereas agonistic α-CD40 mAbs recapitulate most CD40 effects on B cells, including recruitment of TNF receptor-associated factor (TRAF) 2 and TRAF3, activation of MAPK/SAPK and NF-κB, and secretion of TNF-α and Ig, membrane-bound CD154 is required for optimal TRAF6 recruitment and interleukin (IL)–6 production.21 Although Hi5 cells expressing baculovirus-encoded mCD154 cells are available, mCD154 not only optimally activates mCD40, but can also occasionally modestly activate hCD40. However, hCD154 doesn't stimulate mCD40, so results obtained with hCD154 are not attributable to stimulation of mCD40.21 Therefore, species-specific agonistic Abs were used to evaluate the effectiveness of the internal control, mCD40.
Stable transfection of B-cell lines
hCD40-P227A cDNA was subcloned into the plasmid pRSV5.neo* for stable expression in CH12.LX and M12.4.1 cells, which were transfected by electroporation.18 Chimeric molecules consisting of the extracellular and TM domain of mCD40 and intracellular portions of either hCD40 or hCD40-P227A were constructed by overlap-extension polymerase chain reaction (PCR) to generate mCD40-hCD40 and mCD40-hP227A.22 These cDNAs were subcloned into pRSV5.neo* for expression in T5-1 human B cells by electroporation at 250V, 30ms with a BTX Electro Square Porator ECM 830 (BTX Harvard Apparatus; Holliston, MA). Stable clones were maintained in 400 μg/mL G418 (for CH12.LX and M12.4.1; Sigma-Aldrich, St Louis, MO) or 1200 μg/mL G418 for T5-1 clones. Surface expression of endogenous and transfected CD40 was determined by immunofluorescence flow cytometry as described for hCD40WT.19 Clones with similar levels of both endogenous and transfected CD40 were used for experiments, with 2 or more individual clones tested for each B-cell function.
Abs and chemicals
Stimulatory Abs used include 1C10 (rat anti–mCD40 IgG2a), G28-5 (mouse anti–hCD40 IgG1), and the isotype controls mAB72 (rat anti–human α-L-fucosidase IgG2a) and MOPC21 (mouse IgG1). The 1C10 hybridoma was a kind gift of Dr Frances Lund (Trudeau Institute, Saranac Lake, NY). The G28-5, mAB72, and MOPC21 hybridomas were purchased from ATCC (Manassas, VA). Hybridoma supernatants were purified by saturated ammonium sulfate precipitation and the mAbs quantified by isotype-specific sandwich enzyme-linked immunosorbent assay (ELISA). Goat anti–mouse μ chain–specific F(ab)′2 was used to engage the BCR (Jackson ImmunoResearch Laboratories, West Grove, PA).
The following Abs were used for immunoblotting. Rabbit anti-TRAF1 (N19), rabbit anti-TRAF3 (H122), rabbit anti-JNK1/2 (FL), and mouse anti-YY1 (H-10) were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Rabbit anti-TRAF2 and chicken anti-TRAF6 were purchased from Medical and Biological Laboratories (Watertown, MA). Polyclonal sheep anti-GST-hCD40 Ab was prepared by Elmira Biologicals (Iowa City, IA) as described previously.23 Rabbit anti-pJNK1/2, rabbit anti-pIκBα, and rabbit anti-IκBα were purchased from Cell Signaling Technology (Danvers, MA). Rabbit anti–phosphorylated c-Jun (Ser63) and HRP-conjugated anti–sheep Ab were purchased from Upstate Biotechnology (Charlottesville, VA). Rabbit anti-phosphorylated c-Jun (Ser73) was purchased from Biosource/Invitrogen (Carlsbad, CA). Mouse anti-actin (C4) was purchased from Chemicon (Temecula, CA). Mouse anti-GAPDH (6C5) was purchased from Abcam (Cambridge, MA). HRP-conjugated goat anti–mouse, goat anti–rabbit, and donkey anti–chicken Abs were purchased from Jackson ImmunoResearch Laboratories.
The Class-B(K) stimulatory CpG oligodeoxynucleotide 2084 5′-TCCTGACGTTGAAGT-3′ and the nonstimulatory oligodeoxynucleotide 2087 5′-TCCTGAGCTTGAAGT-3′ have been described previously24 and were purchased from Integrated DNA Technologies (Coralville, IA). The TLR7/8 agonist R848 was purchased from Alexis Biochemicals (San Diego, CA).
Sheep erythrocytes for IgM secretion assays were purchased from Elmira Biologicals. Guinea pig complement was purchased from Invitrogen Life Technologies (Carlsbad, CA).
IgM and cytokine secretion assays
CH12.LX and its subclones produce IgM reactive against phosphatidylcholine, a component of erythrocyte cell membranes.25 Ig-secreting cells were quantified by direct plaque-forming cell (Pfc) assay on a lawn of sheep red blood cells (SRBCs) as described.25 Quantitative ELISAs for IL-6 and TNF-α have been described.25
Signaling and TRAF degradation assays
Immunoprecipitation of hCD40
TRAF recruitment and coimmunoprecipitation was performed as described by lysis in 1% Brij-58.27 The Brij-soluble fraction corresponds to the non-raft portion of the cell lysate, and the Brij-insoluble fraction corresponds to the lipid rafts. Samples were diluted in 2× sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) loading buffer, heated to 95°C for 5 minutes, and analyzed by SDS-PAGE.
Immunoblots
Proteins were resolved on 10% SDS-PAGE and transferred to Immulon-P membranes (Millipore, Billerica, MA). Membranes were blocked with 10% nonfat dry milk for 1 hour at 25°C, incubated with primary Ab overnight at 4°C, washed 3 times, and incubated with secondary Ab for 1 hour at 25°C. Blots were developed with enhanced chemiluminescence (ECL; Pierce Biotechnology, Rockford, IL), read on a low-light digital camera (LAS-1000; Fujifilm Medical Systems USA, Stamford, CT), and quantified using Image Gauge (Fujifilm).
Results
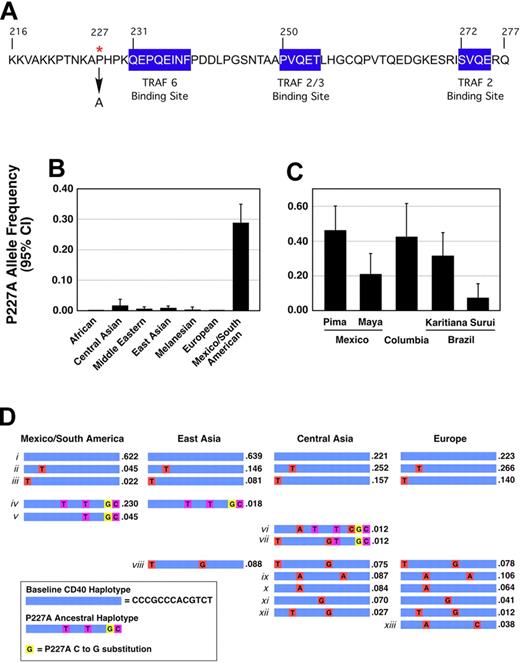
CD40 signaling is critical for coordination of T cell–dependent B-cell responses and myeloid cell activation. Naturally occurring coding sequence variation in the CD40 gene is rare, with only 2 nonsynonymous SNPs recorded in the public domain. The infrequency of coding region variation in the CD40 locus likely indicates the importance of the WT sequence in proper function of the immune system. We therefore hypothesized that coding region variation in the CD40 locus at a frequency high enough to be catalogued in public databases might lead to properties that confer a selective advantage for a given population. Because of its proximity to CD40 intracellular signaling sequences, the rs11086998 variant was evaluated for functional effects. SNP rs11086998 results in a C-to-G transversion in exon 9, resulting in a proline-to-alanine missense substitution at amino acid 227 (P227A) in the cytoplasmic domain of CD40 (Figure 1A). This domain is 62aa long and contains 3 TRAF binding sites that link the ligand-aggregated receptor to downstream NF-κB and JNK signaling pathways. The P227A polymorphism is in a proline-rich spacer region located between the membrane-spanning portion of CD40 and the proximal TRAF6 binding site (Figure 1A).
The G allele of rs11086998 changes the amino acid sequence of CD40 at position 227 from proline to alanine and is enriched in persons from Mexico and South America. (A) Amino acid sequence of the CD40 intracellular domain. The position of proline 227 (red asterisk) and the location of the downstream TRAF binding sites (blue boxes) are noted. (B) SNP rs11086998 was genotyped in the Human Genome Diversity Panel (HGDP) representing 7 geographic regions worldwide. Samples typed were from people from equatorial Africa (n = 150), Central Asia (n = 67), the Middle East (n = 345), East Asia (n = 226), Melanesia (n = 39), Europe (n = 119), and Mexico and South America (n = 108). (C) Results from the Central and South American samples: Pima (n = 25), Mayan (n = 24), Colombian (n = 13), Karitiana Brazilian (n = 24), and Surui Brazilian (n = 21). (D) Thirteen markers across CD40 were genotyped in Central and South American (n = 108), European (n = 119), Central Asian (n = 43), and East Asian (n = 59) donors to the HGDP. The predicted ancestral haplotype is shown in blue, with haplotype-tagging SNPs shown in red; the P227A SNP is shown in yellow, with adjacent haplotype-tagging SNPs shown in purple. Haplotypes were analyzed by Haploview.50
The G allele of rs11086998 changes the amino acid sequence of CD40 at position 227 from proline to alanine and is enriched in persons from Mexico and South America. (A) Amino acid sequence of the CD40 intracellular domain. The position of proline 227 (red asterisk) and the location of the downstream TRAF binding sites (blue boxes) are noted. (B) SNP rs11086998 was genotyped in the Human Genome Diversity Panel (HGDP) representing 7 geographic regions worldwide. Samples typed were from people from equatorial Africa (n = 150), Central Asia (n = 67), the Middle East (n = 345), East Asia (n = 226), Melanesia (n = 39), Europe (n = 119), and Mexico and South America (n = 108). (C) Results from the Central and South American samples: Pima (n = 25), Mayan (n = 24), Colombian (n = 13), Karitiana Brazilian (n = 24), and Surui Brazilian (n = 21). (D) Thirteen markers across CD40 were genotyped in Central and South American (n = 108), European (n = 119), Central Asian (n = 43), and East Asian (n = 59) donors to the HGDP. The predicted ancestral haplotype is shown in blue, with haplotype-tagging SNPs shown in red; the P227A SNP is shown in yellow, with adjacent haplotype-tagging SNPs shown in purple. Haplotypes were analyzed by Haploview.50
Population prevalence of rs11086998
To evaluate the worldwide population frequency of rs11086998, we genotyped the P227A SNP in the Human Genome Diversity Panel (HGDP), a set of 1064 DNA samples representing 52 world populations.15 The results demonstrated a high prevalence of rs11086998 in populations of Mexico and South America (mean allele frequency 29%) compared with other populations (Figure 1B). The Mexican Pima population demonstrated the highest allele frequency (46%; Figure 1C). In sharp contrast, the rs11086998 G allele was rare in Central, East Asian and Middle Eastern populations (mean allele frequency ≤ 1%), and wasn't present on any of the African, Melanesian, or European chromosomes tested.
To test whether this global pattern of diversity is unusual, we estimated how often alleles common in the Americas are present at low frequencies elsewhere in the world, using 404 microsatellite markers genotyped in the HGDP DNA samples.28 Of 486 alleles present at a frequency of 20% to 40% in the Americas, only 1 showed a frequency of less than or equal to 5% in all other HGDP populations (data not shown), suggesting that population stratification of the G allele of rs11086998 is rare.
Overall CD40 haplotype structure was evaluated by typing 13 haplotype-tagged SNPs in selected HGDP populations (Americas, East Asia, Central Asia, and Europe). This analysis identified 3 common haplotypes (Figure 1Di-iii) that account for approximately two-thirds of chromosomes (Americas, 68.9%; East Asia, 86.6%; Central Asia, 63.0%; Europe, 62.9%). The rs11086998 C-to-G substitution is carried on a unique ancestral haplotype that contains additional tightly linked SNPs (Figure 1Div) and shows 3 recombinant variants (Figure 1Dv-vii). In this sample, the rs11086998 ancestral haplotype was present at a frequency of 27.5% in the Americas, compared with 2.4% in Central Asia and 1.8% in East Asia. The rs11086998 G allele was not found in European samples, consistent with Chadha et al.14 No lower frequency haplotypes (Figure 1Dviii-xiii) in the other 3 populations were observed in samples from the Americas.
Because rs11086998 G is a proline-to-alanine substitution near important signaling motifs, we hypothesized that the G allele might lead to altered function of CD40. To examine functional effects of hCD40-P227A on B-cell activation, cDNA constructs were first stably expressed in mouse B-cell lines CH12.LX and M12.4.1, which are frequently used models for studying CD40-mediated B-cell activation.17,18 Previous work has shown that mouse and hCD40 signal indistinguishably in this system, as measured by recruitment of TRAF 1, 2, 3, and 6,29 activation of MAPK/SAPK family members,29 activation of NF-κB and AP-1 transcription factors,30 induction of proinflammatory cytokines,25 and IgM secretion.18 A strong advantage of this system is that it allowed us to use the endogenous mCD40 as an internal control for clone-to-clone variation in absolute responses to various signals, because mAbs specific to mouse or hCD40 do not cross-react and hCD154 does not stimulate mCD40.18,21,25,30 This experimental system also allowed us to study the cooperation of CD40 signals with other immune receptors, including the BCR and TLR9.
CD40-mediated IgM secretion in cells expressing hCD40-P227A
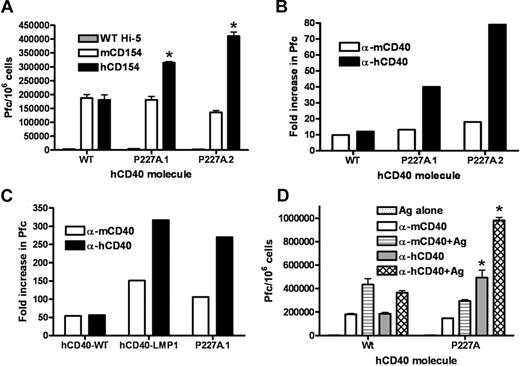
A critical role of the CD40-CD154 interaction is Ig production by B cells,2 so we first tested the effect of hCD40-P227A signaling on Ig secretion. When compared with expression-matched levels of stably expressed hCD40WT or to endogenous mCD40, hCD40-P227A signaling resulted in significantly increased Ig secretion (Figure 2). This trend was observed whether cells were stimulated with membrane-bound hCD154 (Figure 2A) or with agonistic α-CD40 Abs (Figure 2B).
IgM secretion stimulated by hCD40-P227A versus WT hCD40. (A) CH12.LX cells stably expressing hCD40WT (WT) or hCD40-P227A (P227A.1, P227A.2) were stimulated for 72 hours with insect cells infected with WT baculovirus (WT-Hi5), mCD154 expressing baculovirus (mCD154), or hCD154 expressing baculovirus (hCD154). IgM-secreting cells in replicate cultures are enumerated (± SE) as described.25 Results are representative of 2 similar experiments. (B) Cells were stimulated with 2 μg/mL agonistic α-mCD40 mAb, α-hCD40 mAb, or isotype control mAbs for 72 hours. Data represent fold increase in Pfc: mean Pfc of replicate cultures stimulated through CD40 divided by mean Pfc of replicate cultures of cells stimulated with isotype control mAbs, and are the mean values of 3 separate experiments. (C) CH12.LX cells stably expressing hCD40WT, hCD40-P227A (P227A.1) or a chimeric molecule consisting of the extracellular and transmembrane domains of hCD40 and the intracellular domain of LMP-1 (hCD40-LMP1) were stimulated as in panel B. Data represent mean fold increase in Pfc over cells stimulated with isotype control antibodies, as in panel B and are representative of 3 similar experiments. (D) Cells were stimulated as indicated with 2 μg/mL agonistic α-mCD40 or α-hCD40 mAb plus or minus 0.1% SRBC (Ag). IgM production was measured as in panel A and is representative of 2 similar experiments. Similar results were observed with a second subclone expressing hCD40-P227A. Asterisk denotes statistical difference (*P < .05) between hCD40WT and hCD40-P227A by 2-tailed Student t test.
IgM secretion stimulated by hCD40-P227A versus WT hCD40. (A) CH12.LX cells stably expressing hCD40WT (WT) or hCD40-P227A (P227A.1, P227A.2) were stimulated for 72 hours with insect cells infected with WT baculovirus (WT-Hi5), mCD154 expressing baculovirus (mCD154), or hCD154 expressing baculovirus (hCD154). IgM-secreting cells in replicate cultures are enumerated (± SE) as described.25 Results are representative of 2 similar experiments. (B) Cells were stimulated with 2 μg/mL agonistic α-mCD40 mAb, α-hCD40 mAb, or isotype control mAbs for 72 hours. Data represent fold increase in Pfc: mean Pfc of replicate cultures stimulated through CD40 divided by mean Pfc of replicate cultures of cells stimulated with isotype control mAbs, and are the mean values of 3 separate experiments. (C) CH12.LX cells stably expressing hCD40WT, hCD40-P227A (P227A.1) or a chimeric molecule consisting of the extracellular and transmembrane domains of hCD40 and the intracellular domain of LMP-1 (hCD40-LMP1) were stimulated as in panel B. Data represent mean fold increase in Pfc over cells stimulated with isotype control antibodies, as in panel B and are representative of 3 similar experiments. (D) Cells were stimulated as indicated with 2 μg/mL agonistic α-mCD40 or α-hCD40 mAb plus or minus 0.1% SRBC (Ag). IgM production was measured as in panel A and is representative of 2 similar experiments. Similar results were observed with a second subclone expressing hCD40-P227A. Asterisk denotes statistical difference (*P < .05) between hCD40WT and hCD40-P227A by 2-tailed Student t test.
Signaling through latent membrane protein-1 (LMP1), an oncogenic mimic of CD40 encoded by the B-lymphotropic Epstein-Barr virus (EBV), also results in abnormally increased Ig secretion by B cells.22 Expression of LMP1 in CD40-deficient mice restores humoral immunity, but results in B-cell hyperactivity and autoreactivity,22 as well as exacerbated collagen-induced arthritis.31 We thus wished to compare directly the magnitude of elevated Ig secretion induced by hCD40-P227A to that induced by a chimeric molecule consisting of the extracellular and transmembrane portion of hCD40 and the intracellular domain of LMP1 (hCD40-LMP1) that we have previously exploited to study LMP1 signaling.19 The magnitude of Ig secretion stimulated by hCD40-P227A signaling was similar to the elevated levels stimulated by hCD40-LMP1 (Figure 2C).
Previous work shows that simultaneous engagement of the BCR and CD40 leads to synergistic production of IgM, IL-6, and TNF-α,25 so we investigated whether BCR-CD40 cross-talk was enhanced by hCD40-P227A signaling. Figure 2D shows both endogenous mCD40 and hCD40WT synergized in IgM production when stimulated simultaneously with BCR and CD40 agonists. However, synergistic production of IgM was greatly enhanced by hCD40-P227A compared with hCD40WT.
IL-6 production via hCD40-P227A signaling
IL-6 is a proinflammatory cytokine induced by CD40 signaling that contributes to Ig production and plasma cell differentiation.32 IL-6 is elevated in autoimmune patient sera and contributes directly to disease pathogenesis in autoimmune mouse models.33 We have previously shown that, unlike most CD40 effects on B cells, the production of IL-6 requires a source of membrane-bound CD154 and cannot be induced by agonistic Abs.21 To test whether hCD40-P227A signaling resulted in increased IL-6 production, we stimulated B cells with hCD154 expressed by insect cells. Supernatants were collected at 48 hours, the peak of the response, and analyzed by quantitative ELISA.21 IL-6 production was enhanced upon hCD40-P227A signaling versus hCD40WT (Figure 3A). More IL-6 was produced at all time points of stimulation in cells expressing hCD40-P227A, although kinetics of IL-6 production were similar in response to hCD40WT (Figure 3B).
IL-6 secretion stimulated by hCD40-P227A versus WT hCD40. (A) CH12.hCD40WT (hCD40WT) or CH12.P227A (P227A.1, P227A.2, P227A.3) cells were stimulated as indicated with Hi5 cells (1:5 ratio with B cells) plus or minus 10 μg/mL α-μ chain F(ab)′2 Ig for 48 hours and IL-6 ELISA was performed. Two separate subclones of CH12.LX expressing hCD40WT were tested with similar results (not shown). Data represent means plus or minus SD of triplicate cultures. A representative experiment of 5 independent experiments with similar results is shown. Hi5-WTBV; Hi5 infected with Wt baculovirus. (B) Cells were stimulated as in panel A for the indicated times and IL-6 ELISA was performed. IL-6 was undetectable in supernatants from cells stimulated with media (BCM) or with uninfected Hi5 cells. A representative of 3 independent experiments with similar results is shown. (C) Cells were stimulated with 10 μg/mL agonistic α-mCD40, α-hCD40, or isotype control mAbs plus or minus 10 μg/mL α-μ chain F(ab)′2 Ab (α-BCR) or media (BCM) for 48 hours. IL-6 ELISA was performed. Data represent means plus or minus SD of 3 independent experiments. Two separate subclones of CH12.LX expressing hCD40WT were tested with similar results (not shown). (D) Cells were stimulated for 20 hours as indicated with Hi5 cells (1:5 ratio with B cells) plus or minus 25 nM CpG 2084 or control CpG 2087 oligonucleotides. Culture supernatants were analyzed by ELISA. Data are representative of 3 independent experiments with similar results, and a second hCD40WT subclone was tested with similar results (not shown). Asterisks denotes statistically significant difference between hCD40WT and hCD40-P227A: *P < .05, **P < .001 by 2-tailed Student t test.
IL-6 secretion stimulated by hCD40-P227A versus WT hCD40. (A) CH12.hCD40WT (hCD40WT) or CH12.P227A (P227A.1, P227A.2, P227A.3) cells were stimulated as indicated with Hi5 cells (1:5 ratio with B cells) plus or minus 10 μg/mL α-μ chain F(ab)′2 Ig for 48 hours and IL-6 ELISA was performed. Two separate subclones of CH12.LX expressing hCD40WT were tested with similar results (not shown). Data represent means plus or minus SD of triplicate cultures. A representative experiment of 5 independent experiments with similar results is shown. Hi5-WTBV; Hi5 infected with Wt baculovirus. (B) Cells were stimulated as in panel A for the indicated times and IL-6 ELISA was performed. IL-6 was undetectable in supernatants from cells stimulated with media (BCM) or with uninfected Hi5 cells. A representative of 3 independent experiments with similar results is shown. (C) Cells were stimulated with 10 μg/mL agonistic α-mCD40, α-hCD40, or isotype control mAbs plus or minus 10 μg/mL α-μ chain F(ab)′2 Ab (α-BCR) or media (BCM) for 48 hours. IL-6 ELISA was performed. Data represent means plus or minus SD of 3 independent experiments. Two separate subclones of CH12.LX expressing hCD40WT were tested with similar results (not shown). (D) Cells were stimulated for 20 hours as indicated with Hi5 cells (1:5 ratio with B cells) plus or minus 25 nM CpG 2084 or control CpG 2087 oligonucleotides. Culture supernatants were analyzed by ELISA. Data are representative of 3 independent experiments with similar results, and a second hCD40WT subclone was tested with similar results (not shown). Asterisks denotes statistically significant difference between hCD40WT and hCD40-P227A: *P < .05, **P < .001 by 2-tailed Student t test.
As mentioned above, previous work showed that CD40 binding by agonistic α-CD40 Abs is not sufficient to induce IL-6; rather, a membrane-bound form of CD154 is required to elicit IL-6 production.21 However, B cells expressing hCD40-LMP1 or splenocytes isolated from mCD40-LMP1 transgenic mice secrete IL-6 in response to agonistic α-CD40 Abs.22 Like hCD40-LMP1, hCD40-P227A signaling triggered via agonistic α-hCD40 Abs, but not α-mCD40 Abs, resulted in detectable levels of IL-6 (Figure 3C), indicating that hCD40-P227A required a lower signaling threshold to elicit IL-6 production.
Because hCD40-P227A induced enhanced synergistic IgM and IL-6 contributes to IgM secretion, we tested for enhanced synergistic IL-6 secretion by hCD40-P227A signaling. hCD40-P227A or hCD40WT cells were stimulated for 48 hours with hCD154 or α-CD40 Abs plus or minus goat α-mouse IgM F(ab)′2 (α-BCR). Enhanced synergistic IL-6 production was observed in hCD40-P227A-expressing cells stimulated with hCD154 plus α-BCR (Figure 3A) and α-hCD40 Ab plus α-BCR (Figure 3C). Synergistic production of IL-6 was not observed in cells expressing hCD40WT, even at lower doses of hCD154 (data not shown).
CD40 also cooperates with TLR9 to enhance IL-6 production,34 and TLR9 cooperates with the BCR to increase B-cell proliferation and Ig production.24 TLR9 recognizes unmethylated CpG motifs present in both self-and bacterial DNA and contributes to autoimmune disease pathogenesis.35 To test if hCD40-P227A demonstrated enhanced cooperation with TLR9 in IL-6 production, cells were stimulated for 20 hours with hCD154 with or without stimulatory CpG 2084 or control CpG 2087. When combined with a TLR9 stimulus, hCD40-P227A signaling resulted in markedly enhanced synergistic IL-6 production (Figure 3D). hCD40WT also induced synergistic IL-6 production in combination with a TLR9 agonist, but the effect was only slightly more than additive.
Previous work showed that hCD40 and mCD40 bind TRAFs similarly, activate similar downstream signaling pathways, and lead to the same functional outcomes when expressed in either mouse or human B cells. This is not surprising, as the cytoplasmic domains of mouse and hCD40 are highly conserved, as are the TRAF molecules to which they bind.29 Thus, the well-characterized mouse cell lines used here provided an appropriate and valid model for the assessment of hCD40-P227A function with endogenous mCD40 as an internal control. However, we wished to confirm that hCD40-P227A also delivers enhanced signals to human as well as mouse B cells. We stably expressed chimeric constructs with the extracellular domain of mCD40 and the intracellular domain of either hCD40WT or hCD40-P227A (mCD40-hCD40 and mCD40-hP227A, respectively) in the human B-cell line T5-1. Cells were stimulated for 48 hours with mCD154 plus or minus 1 μg/mL R848, a TLR7/8 agonist, and supernatants were analyzed by quantitative human IL-6 ELISA. As observed with hCD40-P227A signaling in mouse B cells (Figure 3D), mCD40-hP227A signaling resulted in enhanced synergistic production of IL-6 in cooperation with TLR7/8 stimulation (Figure 4). This verifies the use of mouse B-cell lines as a valid model for hCD40-P227A signaling, similar to the well-established comparable WTCD40 signaling in mouse versus human B cells.
IL-6 secretion induced by mCD40-hP227A versus mCD40-hCD40 in the human B-cell line, T5-1. T5-1.mCD40-hCD40 (MH40WT-n24.7, MHWT-n24.12) and T5-1.mCD40-hP227A (MHP227A-n22.7, MHP227A-n22.12) were stimulated with Hi5-mCD154 plus R848 for 48 hours and supernatants analyzed by ELISA for human IL-6. Data are presented as means plus or minus SD of background-corrected IL-6 in replicate cultures from 3 separate experiments. Asterisk denotes statistically significant difference (*P < .05) between MH40WT and MHP227A by 2-tailed Student t test.
IL-6 secretion induced by mCD40-hP227A versus mCD40-hCD40 in the human B-cell line, T5-1. T5-1.mCD40-hCD40 (MH40WT-n24.7, MHWT-n24.12) and T5-1.mCD40-hP227A (MHP227A-n22.7, MHP227A-n22.12) were stimulated with Hi5-mCD154 plus R848 for 48 hours and supernatants analyzed by ELISA for human IL-6. Data are presented as means plus or minus SD of background-corrected IL-6 in replicate cultures from 3 separate experiments. Asterisk denotes statistically significant difference (*P < .05) between MH40WT and MHP227A by 2-tailed Student t test.
TNF-α production induced by CD40-P227A signaling
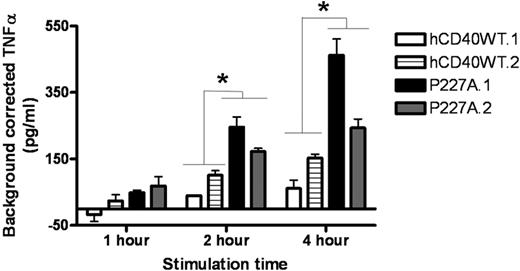
TNF-α is secreted by mouse and human B cells upon CD40 signaling and acts in an autocrine and paracrine fashion to promote proliferation and Ig secretion.20 TNF-α contributes to CD40-mediated Ig secretion via interaction with B cell–expressed CD120b/TNFRII.20 Although induction of TNF-α after CD40 signaling is independent of TRAFs 1, 2, or 3, a direct interaction of TRAF2 with TNFRII is required for full Ig secretion in response to CD40.20 Because hCD40-P227A signaling resulted in enhanced IgM secretion, we tested whether TNF-α itself was increased. Figure 5 shows hCD40-P227A signaling led to earlier increases in TNF-α production (1 hour) versus hCD40WT, and the increase was more pronounced at 2 hours and 4 hours. Although some reports suggest that TNF-α can also enhance IL-6 production by B cells,36 we have not seen this dependence in CD40 signaling.37 Previous work showed that addition of blocking Abs against IL-6 and TNF-α to CD40-stimulated cultures decreases Ig secretion more than blocking either IL-6 or TNF-α alone, indicating that both these cytokines contribute to CD40-mediated Ig secretion.38 When IL-6 and TNF-α are blocked after hCD40-P227A or endogenous mCD40 signaling, the increased IgM secretion observed by hCD40-P227A decreases to a similar level to endogenous mCD40 (not shown). Thus, both elevated IL-6 and TNF-α contribute to the increased IgM secretion induced by hCD40-P227A versus WThCD40.
TNF-α secretion induced by hCD40-P227A versus WT hCD40. M12.hCD40WT (hCD40WT.1, hCD40WT.2) and M12.P227A (P227A.1, P227A.2) cells were stimulated for the indicated times and TNF-α ELISA was performed. Data are presented as means plus or minus SD of background-corrected TNF-α in replicate cultures from 3 separate experiments: (mean TNF-α induced by Hi5-hCD154) − (mean TNF-α induced by MDM2 control virus-infected Hi5). Asterisk denotes statistical difference (*P < .05) between hCD40WT and hCD40-P227A by 2-tailed Student t test.
TNF-α secretion induced by hCD40-P227A versus WT hCD40. M12.hCD40WT (hCD40WT.1, hCD40WT.2) and M12.P227A (P227A.1, P227A.2) cells were stimulated for the indicated times and TNF-α ELISA was performed. Data are presented as means plus or minus SD of background-corrected TNF-α in replicate cultures from 3 separate experiments: (mean TNF-α induced by Hi5-hCD154) − (mean TNF-α induced by MDM2 control virus-infected Hi5). Asterisk denotes statistical difference (*P < .05) between hCD40WT and hCD40-P227A by 2-tailed Student t test.
Phosphorylation of JNK and c-Jun after hCD40-P227A signaling
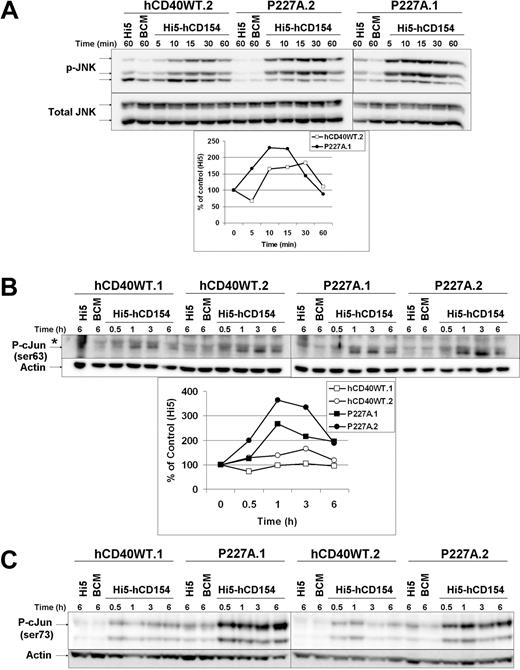
Early events after CD40 signaling include the activation of MAPK/SAPK cascades.29 Compared with CD40, JNK activity is augmented and sustained during LMP1 signaling, contributing to the enhanced IL-6 production induced by this viral mimic.27 c-Jun is a component of the AP-1 transcription factor which, in addition to NF-κB and C/EBPβ/γ, contributes to il-6 transcription in B cells after CD40 engagement.30 hCD40-P227A signaling increased IL-6 production, so we tested if JNK or its downstream target, c-Jun, had altered kinetics or magnitude of phosphorylation after CD40 engagement. JNK phosphorylation downstream of hCD40-P227A signaling was modestly increased from that induced by hCD40WT or endogenous mCD40 in magnitude, but did not display altered kinetics (Figure 6A). More striking was the more substantial amount of the JNK target c-Jun that was phosphorylated (Ser 63/73) after hCD40-P227A signaling compared with hCD40WT (Figure 6B,C). NF-κB, ERK, p38, and Akt activity after hCD40-P227A signaling were not reproducibly altered compared with hCD40WT (not shown).
Activation of JNK, NF-κB, and c-Jun phosphorylation by hCD40-P227A versus WT hCD40 signaling. (A) CH12.hCD40WT (hCD40WT.2) and CH12.P227A (P227A.1, P227A.2) cells were stimulated for the indicated times with medium (BCM), uninfected Hi5 (Hi5), or Hi5-hCD154. Total cell lysates were resolved by SDS-PAGE and immunoblotted for phosphorylated JNK, followed by total JNK as a loading control. Data are representative of 3 independent experiments with multiple clones tested. Results for P227A.2 are not included in the graph because its lower background produces a different scale. (B) CH12.hCD40WT (hCD40WT.1, hCD40WT.2) and CH12.P227A (P227A.1, P227A.2) cells were stimulated for the indicated times with media (BCM), uninfected Hi5 (Hi5), or Hi5-hCD154. Total cell lysates were analyzed via SDS-PAGE and immunoblotted for phosphorylated c-Jun (Ser63), followed by actin as a loading control. Results were quantified as described in “Immunoblots,” and are representative of 4 independent experiments. (C) M12.hCD40WT (hCD40WT.1, hCD40WT.2) and M12.P227A cells (P227A.1, P227A.2) were stimulated and total cell lysates prepared. Samples were immunoblotted for phosphorylated c-Jun (Ser73) followed by actin as a loading control. Results are representative of 5 experiments.
Activation of JNK, NF-κB, and c-Jun phosphorylation by hCD40-P227A versus WT hCD40 signaling. (A) CH12.hCD40WT (hCD40WT.2) and CH12.P227A (P227A.1, P227A.2) cells were stimulated for the indicated times with medium (BCM), uninfected Hi5 (Hi5), or Hi5-hCD154. Total cell lysates were resolved by SDS-PAGE and immunoblotted for phosphorylated JNK, followed by total JNK as a loading control. Data are representative of 3 independent experiments with multiple clones tested. Results for P227A.2 are not included in the graph because its lower background produces a different scale. (B) CH12.hCD40WT (hCD40WT.1, hCD40WT.2) and CH12.P227A (P227A.1, P227A.2) cells were stimulated for the indicated times with media (BCM), uninfected Hi5 (Hi5), or Hi5-hCD154. Total cell lysates were analyzed via SDS-PAGE and immunoblotted for phosphorylated c-Jun (Ser63), followed by actin as a loading control. Results were quantified as described in “Immunoblots,” and are representative of 4 independent experiments. (C) M12.hCD40WT (hCD40WT.1, hCD40WT.2) and M12.P227A cells (P227A.1, P227A.2) were stimulated and total cell lysates prepared. Samples were immunoblotted for phosphorylated c-Jun (Ser73) followed by actin as a loading control. Results are representative of 5 experiments.
TRAF binding and degradation after hCD40-P227A signaling
CD40 mediates intracellular signaling principally via binding to adapter molecules TRAF1, TRAF2, TRAF3, and TRAF6, although some TRAF-independent signaling events have been reported.39 Previous work has shown that distinct TRAFs are required for specific CD40 signaling events (reviewed in Bishop29 ). TRAF6 is required for CD40-mediated IL-6 secretion by B cells, which is independent of TRAFs 1, 2, and 3.32 Because the P227A mutation is 3 residues upstream of the TRAF6 binding site and signaling through hCD40-P227A enhanced c-Jun phosphorylation and IL-6 production, we tested whether the P227A mutation affected TRAF binding to CD40. When hCD40 was immunoprecipitated,27 hCD40-P227A bound TRAF1 (not shown), TRAF2, TRAF3, and TRAF6 with stoichiometry similar to that of hCD40WT (Figure 7A). hCD40-P227A–induced degradation of TRAF2 and TRAF3 was also similar to hCD40WT19 (Figure 7B).
TRAF binding and degradation upon signaling via hCD40-P227A and WT hCD40. (A) M12.hCD40WT or M12.hCD40-P227A (P227A.1, P227A.2) cells were stimulated for 30 minutes with 1 Hi5: 5 B cells and cell lysates prepared. The Brij insoluble fraction (I) represents the lipid raft fraction, whereas the Brij soluble fraction (S) represents the non-raft portion of the cell lysates. hCD40 was immunoprecipitated as described27 and subjected to SDS-PAGE and Western blot analysis for TRAF6, followed by TRAF1 (not shown), TRAF2, TRAF3, and hCD40. Data are representative of 3 independent experiments. A second hCD40WT clone was tested with similar results (not shown). (B) CH12.hCD40WT (hCD40WT.1, hCD40WT.2) and CH12.P227A (P227A.1, P227A.2) were stimulated for the indicated times and cell lysates prepared. Membranes were immunoblotted for TRAF2, then stripped and reprobed for TRAF3 followed by GAPDH as a loading control. Data are representative of at least 3 experiments.
TRAF binding and degradation upon signaling via hCD40-P227A and WT hCD40. (A) M12.hCD40WT or M12.hCD40-P227A (P227A.1, P227A.2) cells were stimulated for 30 minutes with 1 Hi5: 5 B cells and cell lysates prepared. The Brij insoluble fraction (I) represents the lipid raft fraction, whereas the Brij soluble fraction (S) represents the non-raft portion of the cell lysates. hCD40 was immunoprecipitated as described27 and subjected to SDS-PAGE and Western blot analysis for TRAF6, followed by TRAF1 (not shown), TRAF2, TRAF3, and hCD40. Data are representative of 3 independent experiments. A second hCD40WT clone was tested with similar results (not shown). (B) CH12.hCD40WT (hCD40WT.1, hCD40WT.2) and CH12.P227A (P227A.1, P227A.2) were stimulated for the indicated times and cell lysates prepared. Membranes were immunoblotted for TRAF2, then stripped and reprobed for TRAF3 followed by GAPDH as a loading control. Data are representative of at least 3 experiments.
Discussion
The present study describes a novel coding region polymorphism in the human CD40 gene, rs11086998. The minor G allele of rs11086998 results in a proline-to-alanine substitution (P227A) in the intracellular, membrane proximal region of CD40, 3 amino acid residues proximal to the TRAF6 binding site. The G allele of rs11086998 demonstrated unusual population stratification, with allele frequencies in Mexican and South American populations averaging 29% compared with other populations where the G allele frequency was less than 2%.
The unusual global allele frequency distribution of rs11086998 G could reflect positive selection after its arrival in the Americas, or demographic history such as a population bottleneck/genetic drift when the American continents were populated 15 000 to 20 000 years ago,40 or in the ensuing expansion phase. Extended haplotype homozygosity (EHH)41 represents one approach to test for positive selection. This method compares linkage disequilibrium (LD) surrounding an allele of interest to other haplotypes at the same locus. A longer stretch of LD surrounding the allele of interest suggests positive selection. Using EHH, we compared LD structure surrounding chromosomes harboring rs11086998 G versus chromosomes with the C allele in HDGP Mexican and South American subjects. A core haplotype of 20 markers around the CD40 locus from markers rs6065924 to rs1883833 was defined. Additional markers were genotyped in each direction from the core haplotype (24 markers downstream, 23 markers upstream, data not shown) and the decay of LD from the core haplotype was estimated. We were unable to detect any differences in the length of the LD signal for either allele of rs11086998 (data not shown). The failure to identify EHH for the rs11086998 G allele may reflect limitations of the method, and should not be interpreted as indicating that selective pressure was not acting on rs11086998 G in the Americas. EHH is most sensitive in detecting positive selection for variants relatively recent in population history (< 10 000 years old) and when recombination in the region of interest is low.41 Our data suggest that rs11086998 G is ancient, given that the identical haplotype is present in Asian chromosomes at low frequency (Figure 1D haplotypes iv and vi). In addition, our data support a relatively high rate of recombination in the CD40 region (data not shown). Both factors limit sensitivity of EHH to detect positive selection. Because we could not rule out a role for positive selection of this allele, it is interesting to speculate that this allele may have evolved or persisted in these populations to provide enhanced defense against pathogens endemic to Mexico and South America, including Plasmodium falciparum and Trypanosoma cruzi, the causative agents of malaria and Chagas disease, respectively. Recent evidence suggests that treatment of mice with CD154 mimetics enhances the immune response to T cruzi, promoting parasite clearance and decreasing mortality.42 Carriers of the G allele of rs11086998 may have a similar enhanced immune response to T cruzi infection and be less likely to develop complications of chronic Chagas disease, which can involve the cardiac, nervous, and digestive systems.42 Future experiments in an hCD40P227A transgenic mouse will test this possibility.
The hCD40-P227A mutation conferred a gain-of-function phenotype, as B cells expressing hCD40-P227A produced increased amounts of Ig upon CD40 engagement (Figure 2), due in large part to increased production of known contributors to Ig secretion, TNF-α and IL-6 (Figures 3,Figure 4–5). Importantly, these cytokines also contribute to B lymphocyte and myeloid cell activation in chronic inflammatory diseases.33,43 TRAF binding by hCD40-P227A was not detectably altered compared with hCD40WT (Figure 7A), consistent with the location of this mutation outside all known TRAF binding sites.29 Phosphorylation of c-Jun at JNK-specific residues Ser63/73 was consistently enhanced (Figure 6). c-Jun forms one component of AP-1, a transcription factor required for CD40-mediated il6 transcription, and thus could contribute to increased IL-6 production upon hCD40-P227A signaling.30
CD40 plays important roles in T-dependent immune and autoimmune responses including Ig secretion, GC formation, and isotype switching.2 Consistent with the hypothesis that hCD40-P227A is a gain-of-function mutation, Ig secretion in response to hCD40-P227A signaling was enhanced, and increased even further when combined with a BCR signal (Figure 2B). Previous studies have shown that a CD40 signal can rescue anergic, autoreactive B cells, reconstituting robust isotype-switched antibody responses.44 CD40 can also rescue B cells from CD95/Fas-mediated apoptosis.45 The work presented here suggests that hCD40-P227A may have an increased capacity to participate in this process.
Although stimulation with membrane-bound hCD154 is normally required for IL-6 production, hCD40-P227A signaling in response to agonistic α-hCD40 Abs was sufficient to induce IL-6 (Figure 3A). This suggests that hCD40-P227A has a lower signaling threshold required for IL-6 induction, which contributes to the increased Ig production upon hCD40-P227A engagement. Because blocking IL-6 already significantly reduces Ig production by CH12.LX in response to WT CD4038 (not shown), this approach could not provide clear information on how much the increase in hCD40-P227A–mediated Ig production depends on increased IL-6, but it makes an important contribution.
hCD40-P227A signaling induced enhanced CD40-mediated functions, including cytokine and Ig secretion, but did not show enhanced TRAF binding (Figure 7A) or altered degradation of TRAF2 or TRAF3 (Figure 7B). This could be due in part to the inability of available techniques to detect subtle changes in TRAF binding. Alternatively, hCD40-P227A may mediate its effects in a TRAF-independent manner, as TRAF-independent CD40 signaling has been reported previously.39 One candidate for mediating some of these events is Janus kinase 3 (Jak3), which is reported to associate with the proline-rich spacer region upstream of the TRAF6 binding site corresponding to the site of the hCD40-P227A mutation.46 Jak3 phosphorylation and activation of STAT3 upon CD40 signaling were reported to play a role in CD40-mediated up-regulation of CD23, ICAM-1, and LT-α in vitro.46 However, B cells from Jak3-deficient patients reveal no defects in CD40 signaling. Similarly, CD40−/− mice reconstituted with a mCD40 transgene with the proline-rich spacer region deleted (aa 222-230) show no defects in CD40-mediated isotype switching and B-cell activation.47 It is thus still an open question whether Jak3 makes important and nonredundant contributions to CD40 signals; we have not been able to detect Jak3 binding to CD40 in B cells (not shown). Because most CD40-associated proteins studied to date were first identified by the yeast 2-hybrid method, which can miss associations that require mammalian-specific posttranslational modifications, newer proteomic approaches may be profitable in identifying additional CD40-associated signaling proteins that could be involved in the enhanced signaling by hCD40-P227A.
This study focused on hCD40-P227A signaling effects in B cells, but CD40 is also expressed on other immune cells including activated T cells, dendritic cells, and plasmacytoid dendritic cells (pDCs).1 Stimulation of pDCs via CD40 and TLR9 induces synergistic production of type I interferon (IFN) and IL-12p70.35 Furthermore, pDC-derived type I IFN and IL-6 can induce plasma cell differentiation of CD40-activated human B cells.48 Future studies will examine whether hCD40-P227A also provides gain-of-function signals to myeloid-derived cells.
The enhanced functional signaling of hCD40-P227A makes rs11086998 G a compelling candidate for association with immunity to pathogens endemic to Mexico and South America or with susceptibility to lymphoid hyperreactivity. We have not identified any persons homozygous for the G allele of rs11086998, suggesting that 2 copies of this gain-of-function allele may be detrimental to the host, possibly due to CD40 effects on vascular endothelium, which also expresses CD40. Although association of rs11086998 G with SLE incidence was not seen in the Hispanic populations we tested (data not shown), there is a documented tendency to increased SLE severity, particularly lupus nephritis, in patients with Hispanic ethnicity.49 It is therefore possible that the hCD40-P227A polymorphism can exacerbate SLE in patients who have already developed the disease, which the available data cannot reveal because they only record disease incidence, not severity or specific symptoms. It is currently not feasible to gain access to sufficient clinical samples from patients who have this CD40 polymorphism for meaningful experiments on fresh human samples. However, the abundant published documentation that mCD40 and hCD40 signal using the same mechanisms, and with the same outcomes, in normal B cells, assures that the findings reported here are highly relevant to function of hCD40-P227A in human B cells. A transgenic mouse expressing a mouse CD40 molecule with this interesting gain-of-function polymorphism is currently being produced to study effects on in vivo immunity to pathogens, as well as susceptibility to exacerbated disease in autoimmune models.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
G.A.B. is supported by grants AI28847, AI49993, and CA099997 from the National Institutes of Health (NIH; Bethesda, MD) and a Career Award from the Department of Veterans' Affairs. P.M.G. is supported by grants from the NIH (AR052125, AI063274) and the Lupus Foundation of Minnesota (Bloomington, MN). A.L.P. is an MD/PhD candidate, and this work is presented as partial fulfillment of that degree; she was supported by NIH T32 AI007533.
National Institutes of Health
Authorship
Contribution: A.L.P. designed and performed research shown in Figures 2 through 7, analyzed and interpreted data, and drafted the manuscript. R.M.P. and R.R.G. performed research summarized in Figure 1; collected, analyzed, and interpreted data; and performed statistical analysis. D.M.A. and K.L.M. designed research and analyzed and interpreted data contained in Figure 1. G.A.B. performed some of the experiments shown in Figure 2. G.A.B. and P.M.G. codirected this project, designed research, and provided input during writing of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Dr Gail A. Bishop, 2193B Medical Education and Research Facility, 375 Newton Road, Department of Microbiology, University of Iowa, Iowa City, IA 52242; e-mail: gail-bishop@uiowa.edu.