Abstract
X-linked severe combined immunodeficiency (XSCID) is caused by mutations of the common gamma chain (γc) and usually characterized by the absence of T and natural killer (NK) cells. Here, we report an atypical case of XSCID presenting with autologous T and NK cells and Omenn syndrome-like manifestations. The patient carried a splice-site mutation (IVS1+5G>A) that caused most of the mRNA to be incorrectly spliced but produced normally spliced transcript in lesser amount, leading to residual γc expression and development of T and NK cells. The skin biopsy specimen showed massive infiltration of revertant T cells. Those T cells were found to have a second-site mutation and result in complete restoration of correct splicing. These findings suggest that the clinical spectrum of XSCID is quite broad and includes atypical cases mimicking Omenn syndrome, and highlight the importance of revertant mosaicism as a possible cause for variable phenotypic expression.
Introduction
Omenn syndrome (OS) is a peculiar immunodeficiency characterized by erythroderma, lymphadenopathy, hepatosplenomegaly, eosinophilia, hypogammaglobulinemia, elevated serum IgE, and activated/oligoclonal T cells.1,2 The genes responsible for OS include RAG1, RAG2, Artemis, RMRP, and IL7RA.3-8 Clinical manifestations resembling OS were demonstrated in cases of severe combined immunodeficiency (SCID) with maternal T-cell engraftment and of atypical DiGeorge syndrome.9,10 In addition, we have recently reported an X-linked SCID (XSCID) patient with massive skin infiltration of natural killer (NK) cells, resulting in OS-like manifestations.11 Here, we describe another case of XSCID mimicking OS. The patient carried a splice site mutation that allowed development of peripheral T and NK cells, and showed revertant T-cell mosaicism caused by a second-site mutation predominately in the skin. Both of these findings may have contributed to his phenotypic variation of XSCID
Methods
Patient
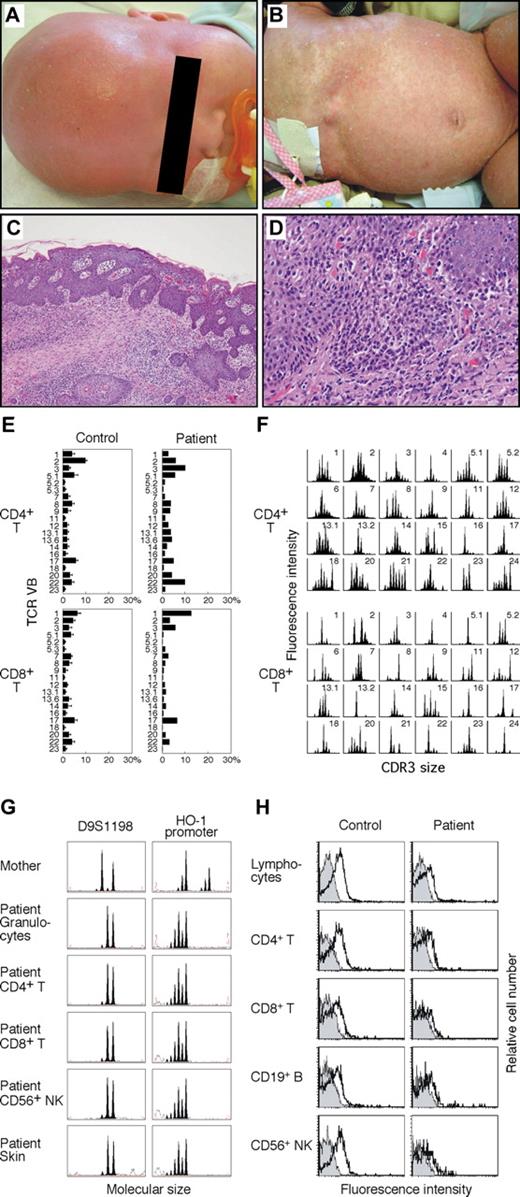
A 5-month-old Japanese boy from nonconsanguineous parents was hospitalized because of failure to thrive, protracted diarrhea, and hypoproteinemia. He developed generalized erythematous rash and alopecia at 1 month of age (Figure 1A,B), followed by persistent cough, fever, hepatosplenomegaly, and lymphadenopathy. Laboratory studies revealed leukocytosis (60.0 × 109/L) with eosinophilia, liver dysfunction, and a low level of serum immunoglobulin G (IgG; 0.17 g/L). Serum IgE levels were significantly elevated at 1300 kIU/L. Immunophenotypic analysis of lymphocytes showed an increased percentage of CD3+ T cells (93.1%), the majority expressing the activation markers CD45RO and HLA-DR. The ratio of CD4+ to CD8+ T cells was 1.1. The patient's CD20+ B and CD56+ NK cells were detectable at 3.8% and 3.2%, restrictively. The level of soluble interleukin-2 receptor was markedly elevated at 10 895 kIU/L. He had a normal thymus shadow. A skin biopsy exhibited massive lymphocytic infiltration and mild spongiosis (Figure 1C,D). His maternal half-brother died of interstitial pneumonia at infancy.
Skin lesions, skin biopsy specimen, T-cell receptor repertoire, microsatellite markers, and γc expression. (A) Alopecia. (B) Generalized scaling erythroderma. (C) Hematoxylin and eosin, original magnification ×40. (D) Hematoxylin and eosin, original magnification ×100. Micrographs were acquired using an Olympus BX51 microscope (Olympus, Melville, NY) fitted with 10× eyepieces, Olympus UPlanApo 4×/0.10 numeric aperture and 10×/0.40 numeric aperture objectives, a CoolSNAP cf CCD camera (Photometrics, Tucson, AZ), and Openlab image acquisition software version 3.1 (Improvision, Waltham, MA). Images were processed in Photoshop CS2 (Adobe Systems, San Jose, CA). (E) Expression profile of TCR variable β (VB) subfamilies. Peripheral blood samples were stained with monoclonal antibodies (mAbs) for individual TCR VB together with anti-CD4 and anti-CD8 mAbs. The percentage of each TCR VB expression within CD4+ or CD8+ T cells was analyzed by FACS. Error bars represent SD. (F) CDR3 spectratyping. Each TCR VB fragment was amplified from cDNA with one of the VB-specific primers. The size distribution of PCR products was determined by an automated sequencer and GeneScan software. (G) Microsatellite analysis. Two different markers were amplified with FAM-labeled specific primers and subjected to GeneScan analysis. HO-1 indicates heme oxygenase-1. (H) Analysis of γc expression. Shown are the results of γc expression on lymphocytes and lymphocyte subsets. Solid peaks indicate control Ab; open peaks represent anti-CD132 mAb.
Skin lesions, skin biopsy specimen, T-cell receptor repertoire, microsatellite markers, and γc expression. (A) Alopecia. (B) Generalized scaling erythroderma. (C) Hematoxylin and eosin, original magnification ×40. (D) Hematoxylin and eosin, original magnification ×100. Micrographs were acquired using an Olympus BX51 microscope (Olympus, Melville, NY) fitted with 10× eyepieces, Olympus UPlanApo 4×/0.10 numeric aperture and 10×/0.40 numeric aperture objectives, a CoolSNAP cf CCD camera (Photometrics, Tucson, AZ), and Openlab image acquisition software version 3.1 (Improvision, Waltham, MA). Images were processed in Photoshop CS2 (Adobe Systems, San Jose, CA). (E) Expression profile of TCR variable β (VB) subfamilies. Peripheral blood samples were stained with monoclonal antibodies (mAbs) for individual TCR VB together with anti-CD4 and anti-CD8 mAbs. The percentage of each TCR VB expression within CD4+ or CD8+ T cells was analyzed by FACS. Error bars represent SD. (F) CDR3 spectratyping. Each TCR VB fragment was amplified from cDNA with one of the VB-specific primers. The size distribution of PCR products was determined by an automated sequencer and GeneScan software. (G) Microsatellite analysis. Two different markers were amplified with FAM-labeled specific primers and subjected to GeneScan analysis. HO-1 indicates heme oxygenase-1. (H) Analysis of γc expression. Shown are the results of γc expression on lymphocytes and lymphocyte subsets. Solid peaks indicate control Ab; open peaks represent anti-CD132 mAb.
Cellular and molecular studies
Cell isolation, fluorescence-activated cell sorting (FACS) analysis for the common gamma chain (γc) and T-cell receptor (TCR) repertoire, spectratyping, microsatellite analysis, and mutation analysis of γc were performed as described elsewhere.11-15 The purity of the sorted CD4+ T, CD8+ T, CD19+ B, and CD56+ NK cells was 98.1%, 99.3%, 86.4%, and 98.3%, respectively. T-cell and B-cell lines from the patient were established by transformation with Herpes virus saimiri and Epstein-Barr virus, respectively. Approval for the study was obtained from the Human Research Committee of Kanazawa University Graduate School of Medical Science, and informed consent was obtained in accordance with the Declaration of Helsinki.
Results and discussion
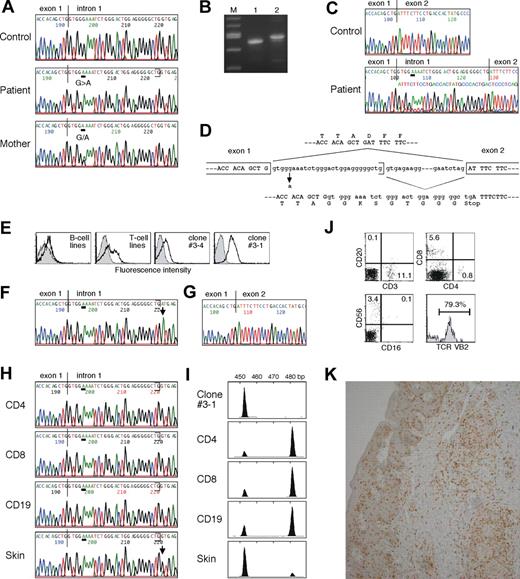
The patient's clinical findings were reminiscent of OS. However, he showed neither mutation in the RAG genes (data not shown) nor severely restricted TCR repertoire (Figure 1E,F). Microsatellite analysis ruled out maternal T-cell engraftment (Figure 1G). Because of possible X-linked inheritance in this family and our previous experience,11 we assessed γc expression. FACS analysis clearly demonstrated defective γc expression on his lymphocytes and lymphocyte subpopulations, leading to the diagnosis of XSCID (Figure 1H). The patient had a G to A substitution at the fifth nucleotide of intron 1 of the IL2RG (IVS1+5G>A) that was previously listed in the IL2RG database (http://research.nhgri.nih.gov/apps/scid/IL2RGbase.shtml), and that caused most of the mRNA to be incorrectly spliced but produced, in lesser amount, normally spliced wild-type transcript (Figure 2A-D). When we analyzed such sequences in subcloned RT-PCR products obtained from his peripheral blood mononuclear cells, the wild-type sequence was detected in 15% of 20 clones. Accordingly, his lymphocytes showed the residual γc protein that likely permitted T- and NK-cell development to occur. Similar results were obtained from the study in another case of atypical XSCID with poorly functioning peripheral T cells.16
Characterization of inherited IL2RG gene mutation, second-site suppressor mutation, and skin-infiltrating lymphocytes. (A) The IL2RG gene was amplified from DNA extracted from normal, the patient's, and the mother's peripheral blood mononuclear cells (PBMCs). Direct sequence was performed using an automated sequencer. Bars show the locations of the mutation. (B) RT-PCR analysis for IL2RG mRNA using PBMCs obtained from normal control (lane 1) and the patient (lane 2). Lane M contains a 100-bp molecular size marker. (C) Direct sequence analysis of IL2RG cDNA using PBMCs from normal control and the patient. (D) Schematic representation of effect of the inherited splice-site mutation. (E) Analysis of γc expression. Shown are the results of γc expression on B-cell and T-cell lines, and cloned T cells #3-4 and #3-1 established from the patient. (F) Direct sequence analysis of IL2RG gene using DNA obtained from the clone #3-1. A bar shows the locations of the inherited mutation and an arrow highlights the second-site mutation. (G) Direct sequence analysis of IL2RG cDNA from the clone #3-1. (H) Direct sequencing analysis of IL2RG gene using DNA obtained from the patient's primary CD4+ and CD8+ T cells and CD19+ B cells, and his skin. (I) GeneScan analysis of IL2RG cDNA amplified from the clone #3-1, the primary lymphocytes including CD4+ and CD8+ T cells and CD19+ B cells, and the skin of the patient. A peak of the size of approximately 454 nucleotides represents wild-type mRNA and a second peak of approximately 482 nucleotides is generated by aberrant splicing. (J) FACS analysis of skin-infiltrating lymphocytes. The percentage of cells gated in each region is shown. (K) Immunohistochemical staining of the skin biopsy specimen. The samples were stained with anti-CD8 mAb.
Characterization of inherited IL2RG gene mutation, second-site suppressor mutation, and skin-infiltrating lymphocytes. (A) The IL2RG gene was amplified from DNA extracted from normal, the patient's, and the mother's peripheral blood mononuclear cells (PBMCs). Direct sequence was performed using an automated sequencer. Bars show the locations of the mutation. (B) RT-PCR analysis for IL2RG mRNA using PBMCs obtained from normal control (lane 1) and the patient (lane 2). Lane M contains a 100-bp molecular size marker. (C) Direct sequence analysis of IL2RG cDNA using PBMCs from normal control and the patient. (D) Schematic representation of effect of the inherited splice-site mutation. (E) Analysis of γc expression. Shown are the results of γc expression on B-cell and T-cell lines, and cloned T cells #3-4 and #3-1 established from the patient. (F) Direct sequence analysis of IL2RG gene using DNA obtained from the clone #3-1. A bar shows the locations of the inherited mutation and an arrow highlights the second-site mutation. (G) Direct sequence analysis of IL2RG cDNA from the clone #3-1. (H) Direct sequencing analysis of IL2RG gene using DNA obtained from the patient's primary CD4+ and CD8+ T cells and CD19+ B cells, and his skin. (I) GeneScan analysis of IL2RG cDNA amplified from the clone #3-1, the primary lymphocytes including CD4+ and CD8+ T cells and CD19+ B cells, and the skin of the patient. A peak of the size of approximately 454 nucleotides represents wild-type mRNA and a second peak of approximately 482 nucleotides is generated by aberrant splicing. (J) FACS analysis of skin-infiltrating lymphocytes. The percentage of cells gated in each region is shown. (K) Immunohistochemical staining of the skin biopsy specimen. The samples were stained with anti-CD8 mAb.
The presence of circulating T cells offered us a unique opportunity to establish T-cell lines from XSCID. Unexpectedly, FACS analysis showed a significant fraction of cells with normal γc expression among his T-cell lines (Figure 2E). Therefore, sequence analysis was performed using the cloned T-cell lines. We found the IVS1+5G>A in DNA from the γc-defective clone (#3-4), whereas DNA from the γc-expressing clone (#3-1) showed coexistence of the IVS1+5G>A and a second-site mutation (IVS1+29G>A), which was located at position +1 of the cryptic 5′ splice site (Figure 2F). Analysis of 100 normal chromosomes demonstrated the absence of the IVS1+5G>A and the IVS1+29G>A. The IVS1+29G>A was found to suppress the effect of the IVS1+5G>A and result in complete restoration of correct splicing that was consistent with the restored γc expression on the cell surface (Figure 2G). To determine whether the IVS1+29G>A was present in vivo, direct sequencing was performed with his various DNA samples. A peak of the IVS1+29G>A was only detectable in his skin specimen, but was absent among circulating CD4+ and CD8+ T and CD19+ B cells (Figure 2H). When we analyzed the sequence in subcloned PCR products obtained from the skin, the IVS1+29G>A was detected in 12.9% of 31 clones. RT-PCR and GeneScan analysis demonstrated that a peak of the normal-sized transcript but not the mutated one was evident in the skin. Because IL2RG mRNA is expressed only in hematopoietic cells, these findings suggest that the majority of skin-infiltrating lymphocytes carry the IVS1+29G>A, thus indicating revertant cells (Figure 2I).
Although revertant γc expression would confer selective advantage in vivo to T- and NK-cell progenitors,17 we could not detect the γc-expressing T cells in his circulation. This lack of in vivo selective advantage is in contrast to a previously described XSCID patient with revertant T-cell mosaicism in whom a reasonably diversified TCR repertoire was generated from a single T-cell progenitor, resulting in a mild clinical course.18,19 The reasons underlying these findings are presently unclear. It is possible that the substantial numbers of activated peripheral T cells with defective γc expression that were not seen in the previous case19 might have competed with the revertants, reduced the abilities of selective advantage of γc expression, and provided insufficient time for the reversion to reach the detection threshold in our patient. On the other hand, selective proliferation of revertant CD8+ T cells with TCR VB2 in the skin (Figure 2J,K) may be due to a clonal expansion in response to infections or autoantigens and be involved in pathogenesis of his skin lesions. However, his complicated condition including the presence of autologous γc-defective T and NK cells in the peripheral blood and the expansion of revertant γc-expressing T cells in the skin does not allow us to draw clear conclusion about which aspects are responsible for the development of OS phenotype. Because recent studies in OS patients and murine models have indicated that impaired immune tolerance and defective immune regulation play important roles in the pathophysiology of OS,2,20 the highly elevated numbers of activated, γc-defective T cells with a moderate restricted TCR repertoire may have more contributed to the OS phenotype in our patient. If this is the case, it is possible that any SCID diseases having a profound but incomplete block in T-cell development could result in OS.
In summary, our studies indicate that the clinical spectrum of XSCID is broad and includes immunodeficient patients mimicking OS. In addition, the detection of the revertant T cells caused by the second-site suppressor mutation makes our case a second example of genetic reversion in XSCID and highlights the importance of somatic revertant mosaicism as a possible cause for atypical clinical presentations.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Ms Harumi Matsukawa and Ms Emi Tamamura for excellent technical assistance.
This work was supported by a Grant-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology of Japan; and a grant from the Ministry of Health, Labour, and Welfare of Japan, Tokyo.
Authorship
Contribution: T.W. and A.Y. participated in designing and performing the research; T.T., Y.N., M.N., M.S., M.O., Y.K., and S.K. analyzed data; M.Y., M.I., and K.K. made clinical contributions and provided critical paper review; T.W. wrote the paper; and all authors checked the final version of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Taizo Wada, MD, PhD, Department of Pediatrics, Graduate School of Medical Science, Kanazawa University, 13-1 Takaramachi, Kanazawa 920-8641, Japan; e-mail: taizo@ped.m.kanazawa-u.ac.jp.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal