Abstract
VAF347 is a low-molecular-weight compound that inhibits allergic lung inflammation in vivo. This effect is likely the result of a block of dendritic cell (DC) function to generate proinflammatory T-helper (Th) cells because VAF347 inhibits interleukin (IL)–6, CD86, and human leukocyte antigen (HLA)–DR expression by human monocyte-derived DC, 3 relevant molecules for Th-cell generation. Here we demonstrate that VAF347 interacts with the aryl hydrocarbon receptor (AhR) protein, resulting in activation of the AhR signaling pathway. Functional AhR is responsible for the biologic activity of VAF347 because (1) other AhR agonists display an identical activity profile in vitro, (2) gene silencing of wild-type AhR expression or forced overexpression of a trans-dominant negative AhR ablates VAF347 activity to inhibit cytokine induced IL-6 expression in a human monocytic cell line, and (3) AhR-deficient mice are resistant to the compound's ability to block allergic lung inflammation in vivo. These data identify the AhR protein as key molecular target of VAF347 and its essential role for mediating the anti-inflammatory effects of the compound in vitro and in vivo.
Introduction
Dendritic cells (DCs) play an obligatory role in the initiation and maintenance of immune responses by providing help to naive T cells to develop into effector T-helper (Th) cells. The development of Th cells occurs in secondary lymphoid organs through physical interaction of naive T-cell precursors with antigen-carrying DCs. At least 3 signals are necessary to fully activate naive T cells. The primary signal originates from binding of the T-cell receptor to antigenic peptides presented in the context of major histocompatibility complex (MHC) molecules on DC. A second costimulatory signal is provided by B7 molecules, such as CD80 or CD86, expressed on DCs with counter-receptors, such as CD28 on T cells.1 Third, DC-derived cytokines strongly determine the type and function of Th cells being produced. Th2 type cells are generated in the presence of interleukin (IL)–4 and are the main drivers of immune responses on encounter with allergens or parasites.2 Allergic immune reactions have been implicated in the development of diseases, such as asthma or atopic dermatitis. In contrast, IL-12 will direct Th1 cell generation, which is required for mounting antipathogen immune responses.3 More recently, Th17 cells were identified as critical effector cells in a number of autoimmune reactions. These cells can be produced in the presence of IL-6 and transforming growth factor-β1.4
Recently, a novel low-molecular-weight compound with potent anti-inflammatory activity was reported. VAF347 acts as immunomodulator by blocking the function of DC to generate functional Th cells in vitro. This phenotype translated into inhibition of pulmonary allergic inflammation in a mouse model in vivo as evidenced by a blockade of lung eosinophilia, serum IgE, and goblet cell hyperplasia.5 Mechanistically, this effect may be the result of reduced expression of IL-6, CD86, and human leukocyte antigen (HLA)–DR by DC because all 3 molecules have been implicated in DC/T-cell communication. However, the molecular target(s) of VAF347 mediating these effects has not been identified.
The aryl hydrocarbon receptor (AhR) is a ligand-induced transcription factor that interacts with a wide spectrum of structurally diverse compounds of natural or manmade origin.6-8 In the absence of ligand, it resides in the cytoplasm as part of a multiprotein complex, including heat shock protein 90 (hsp90),9 XAP-2,10 and p23.11 On ligand binding, AhR translocates into the nucleus where it heterodimerizes with the AhR nuclear translocator (Arnt) protein (another closely related bHLH-PAS transcription factor family member) after dissociating from the hsp90 protein complex. The AhR/Arnt heterodimers specifically interact with cis-regulatory sequences (xenobiotic response elements [XREs]) in the promoter regions of various target genes and directly modulate their transcription. The best studied example is that of the cytochrome P4501A1 (CYP1A1) gene.12 In addition, the AhR protein may modulate gene expression through its interaction with other factors, such as nuclear factor–κB (NF-κB) p65.13
This study aimed at the identification of the molecular target of VAF347 and its mode of action. The data indicate that VAF347 binds to the AhR protein and consequently induces AhR driven signal transduction similar to the prototype AhR agonist 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD). Importantly, down-regulation of wild-type AhR protein expression or overexpression of a trans-dominant negative AhR ablates the biologic activity of VAF347 to inhibit cytokine-induced IL-6 expression in human monocytic cells. Most importantly, no anti-inflammatory activity of the compound was observed in AhR-deficient mice. Accordingly, our results identify the AhR protein as key molecular target mediating the anti-inflammatory phenotype of VAF347 in vitro and in vivo.
Methods
Cell culture
MonoMac1 (MM1) cells were cultured at 37°C with 5% CO2 in RPMI 1640 medium supplemented with 10% heat inactivated fetal bovine serum (Invitrogen, Carlsbad, CA), glutamine (2 mM), penicillin (100 U/mL), streptomycin (100 μg/mL), sodium bicarbonate (1.5 g/L), glucose (4.5 g/L), N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid (HEPES) (10 mM), sodium pyruvate (1 mM), and 1% nonessential amino acids. Phoenix cells (a kind gift of Gary Nolan) were cultured at 37°C with 5% CO2 in Dulbecco modified Eagle medium (DMEM) supplemented with 10% heat inactivated fetal calf serum (Invitrogen), glutamine (2 mM), penicillin (100 U/mL), and streptomycin (100 μg/mL).
Retroviral infection of MonoMac1 cells
Phoenix cells were seeded at 1.7 × 106 cells per 25 cm2 cell culture flask (Corning Life Sciences, Acton, MA) in 4 mL DMEM, supplemented with 10% fetal bovine serum (Invitrogen) and glutamine (2 mM). After 24 hours, cells were transfected with 9 μg of PMX plasmid using Lipofectamine 2000 (Invitrogen) following the protocols recommended by the manufacturer. On the next day, the medium was removed and replaced with 4 mL of fresh DMEM for 24 hours. Finally, the supernatant was collected, filtered through a 0.45-μm filter (Millipore, Billerica, MA) and polybrene (Sigma-Aldrich, St Louis, MO) was added to a final concentration of 1 μg/mL. Infection of MonoMac1 cells was achieved by adding 2 mL of viral supernatant to 5 × 105 MonoMac1 cells in 24-well plates (Corning Life Sciences) followed by centrifugation at 310g for 3 hours at room temperature. After a 24-hour incubation step, the medium was removed and replaced with fresh MonoMac1 medium. After 2 to 3 days, cells expressing high levels of green fluorescent protein (GFP) were sorted using a FACSVantage SE machine (BD Biosciences, Franklin Lakes, NJ). Sorted GFP-positive MonoMac1 cells were cultured in medium supplemented with 1 μg/mL puromycin (Sigma-Aldrich).
Western blot analyses
Whole cell lysates were resolved on 4% to 20% SDS-polyacrylamide gels (Invitrogen) and transferred to a nitrocellulose membrane (Invitrogen). The membrane was blocked with 2.5% nonfat milk in Tris-buffered saline containing 0.1% Tween 20 and incubated with a 1:500 dilution of anti-AhR antibody (clone N-19) (Santa Cruz Biotechnology, Santa Cruz, CA) for 2 hours at room temperature. Specific signals were made visible after incubation with a donkey anti–goat IgG antibody (Santa Cruz Biotechnology; 1:5000 dilution) coupled to horseradish peroxidase using the SuperSignal kit (Pierce Chemical, Rockford, IL).
Compounds
VAF347 ([4-(3-chloro-phenyl)-pyrimidin-2-yl]-(4-trifluoromethyl-phenyl)-amine), VAG005 4-(2-chloro-pyridin-4-yl)-2-(4-chloro-3-trifluoromethyl-phenoxy)-pyrimidine, and VAG539 [4-(3-chloro-phenyl)-pyrimidin-2-yl]-(4-trifluoromethyl-phenyl)-carbamic acid 2-[(2-hydroxy-ethyl)-methyl-amino]-ethyl ester were synthesized at Novartis (Vienna, Austria), TCDD was purchased from Crescent Chemical (Islandia, NY).
Generation of monocyte-derived dendritic cells
Human peripheral blood monocytes were prepared by elutriation or by negative selection of peripheral blood mononuclear cells using a monocyte isolation kit (Miltenyi Biotec, Auburn, CA). Monocytes (typically > 97% positive for CD14) were differentiated into immature DCs by adding 40 ng/mL IL-4 and 15 ng/mL or 50 ng/mL granulocyte-macrophage colony-stimulating factor (GM-CSF) for 6 to 8 days in the absence or presence of increasing concentrations of VAF347. Maturation of DCs was induced by activation with 20 ng/mL of GM-CSF (Novartis), 100 U/mL of IFN-γ (Bender MedSystems, Vienna, Austria), 20 U/mL TNF-α (Bender MedSystems), 4 μg/mL of anti-CD40 mAb, and 1 μg/mL of goat antimouse IgG (Dianova, Hamburg, Germany) for 48 hours. The levels of IL-6 in the cell supernatants were measured by enzyme-linked immunosorbent assay (ELISA; R&D Systems, Minneapolis, MN). DC-mediated autologous T-cell proliferation was carried out exactly as described.5
Microarray analysis
Hybridization of biotinylated cRNA to GeneChip Human Genome 133 2.0 Plus Expression Arrays (Affymetrix, Santa Clara, CA) was carried out at 45°C for approximately 18 hours. The arrays were stained on a GeneChip Fluidics Workstation 450 and scanned on a GeneArray Scanner 3000 according to the manufacturer's technical manual (Affymetrix). The obtained signal intensities (CEL files) were uploaded into GeneSpring (Agilent Technologies, Palo Alto, CA) and preprocessed using gcRMA. Probe sets were required to have a minimum raw value of at least 50 in at least one condition. VAF347-treated samples were normalized against untreated samples, for each of the 2 donors separately. Fold changes were calculated as geometric and arithmetic means.14
RNA isolation and reverse-transcription–polymerase chain reaction
Total RNA was isolated using the Absolutely RNA Miniprep kit (Stratagene, La Jolla, CA) according to the instructions of the manufacturer. cDNA was synthesized by reverse transcription (RT) using iScript reverse transcriptase (Bio-Rad, Hercules, CA) according to the instructions of the manufacturer.
The expression levels of CYP1A1 and IL-6 were analyzed with the commercially available SYBR Green RT-polymerase chain reaction (PCR) kit (Applied Biosystems, Foster City, CA) in an ABI7900 machine with the following PCR parameters: 1 cycle of 2 minutes at 50°C and 10 minutes at 95°C followed by 40 cycles of 15 seconds at 95°C and 1 minute at 62°C. All templates were analyzed in duplicates, and the average was used for further calculation. The average cycle threshold (Ct) values of the CYP1A1 or IL-6 samples were normalized to the average Ct values of the housekeeping gene elongation factor 1-α (eF-1α) using the following equation: 2(Ct“EF-1 α” − Ct“test gene”) × 1000. The following primer pairs were used: CYP1a1, 5′-CAGCTGACTTCATCCCTATTC-3′ and 5′-AGCTGGACATTGGCGTTCTCA-3′; IL-6, 5′-GTACATCCTCGACGGCATCTC-3′ and 5′-GGCAAGTCTCCTCATTGAATC-3′ and eF-1α, 5′-TTTGAGACCAGCAAGTACTATGTGACT-3′ and 5′-TCAGCCTGAGATGTCCCTGTAA-3′. Specificity of this amplicon was verified by using the VIC-labeled probe 5′-TCATTGATGCCCCAGGACACAGAGAC-3′.
Recombinant plasmids
cDNA of MonoMac1 cells was used to amplify an AhR cDNA fragment corresponding to amino acids 1-515 using the upstream PCR primer 5′-GTCAGCGGCCGCCTGGGCACCATGAACAGCAGC-3′ and the reverse primer 5′-CTGACTCGAGGTCAATTTGCTCATGTTTCAGGAT-3′. The PCR fragment was cloned into the retroviral vector pMX-pie (containing an internal ribosome entry site [IRES]-GFP element; kindly provided by T. Kitamura, University of Tokyo, Tokyo, Japan). The plasmid sequence (AhR515) was verified by DNA sequencing. A lentiviral vector backbone encoding AhR shRNAmir was purchased from Open Biosystems (Huntsville, AL). The coding sequences for shAHR were subcloned into the retroviral backbone MSCV-LMP (Open Biosystems, Heidelberg, Germany) using the EcoRI and XhoI restriction sites.
Lung inflammation in mice
C57BL/6 (B6) mice (age 6-8 weeks) were obtained from The Jackson Laboratory (Bar Harbor, ME) or the National Cancer Institute (Frederick, MD). B6.AhRtm1Bra (AhR−/−) were bred as previously described15 and have been backcrossed over 10 times onto a C57Bl/6 background. All mice were housed in microisolator cages in a specified pathogen-free facility and were provided food and water ad libitum. All animal treatments were conducted with approval of Institutional Animal Care and Use Committee. VAG539 was dissolved in 5.4% glucose, diluted to 3 mg/mL, and a dose of 30 mg/kg body weight was administered (per os) twice daily for 5 consecutive days and then once on days 6 and 7. Control animals received the 5.4% glucose solution by gavage. Mice were sensitized intraperitoneally with ovalbumin (OVA, 100 μg) adsorbed with 1 mg Imject Alum in PBS (Pierce Chemical) on days 1 and 5. On day 12, mice were challenged by inhalation of OVA aerosol in an exposure chamber for 60 minutes. Aerosolized OVA (1% in PBS) was generated using a Shuco 3000 nebulizer. Recruitment of cells to the airways, cytokine levels, and serum levels of OVA-specific Ig were determined 48 hours after challenge. Leukocytes were obtained from lung airways by bronchoalveolar lavage (BAL) into cold RPMI 1640 containing 1% BSA, and 10 mM HEPES, and the BAL fluid from the first wash was retained for cytokine analysis. BAL cells from 3 consecutive washes were combined, and the number of viable cells was determined using a hemacytometer. Eosinophils were counted on May-Gruenwald/Giemsa-stained cytocentrifuge preparations. The levels of mouse IL-5 in the BAL fluid were determined by the Luminex technology (R&D Systems) as recommended by the instructions of the manufacturer. Serum IgE levels were determined by ELISA.
Statistical analysis
Statistical analysis was done with 2-tailed Student t test.
AhR ligand binding assay
Aliquots of guinea pig hepatic cytosol (2 mg/mL), prepared as previously described,16 were incubated with 2 nM [3H]TCDD in the presence of dimethylsulfoxide (DMSO; 1%), 2,3,7,8-tetrachlorodibenzofuran (TCDF; 200 nM), or the indicated solvent or sample extract for 2 hours in a room temperature water bath. [3H]TCDD binding in aliquots of the incubation (200 μL) was determined by HAP binding as previously described.16 The total amount of [3H]TCDD specific binding was obtained by subtracting the nonspecific binding ([3H]TCDD and TCDF) from the total binding ([3H]TCDD). The ability of a chemical(s) in a sample extract to bind to the AhR was indicated by its ability to competitively reduce [3H]TCDD specific binding.
Electrophoretic mobility shift assay
Complementary synthetic oligonucleotides containing the DRE3 AhR DNA binding site (5′-GATCTGGCTCTTCTCACGCAACTCCG-3′ and 5′-GATCCGGAGTTGCGTGAGAAGAGCCA-3′ were prepared, reannealed, and end-labeled with [32P]ATP as described.16 Guinea pig hepatic cytosol (8 mg/mL), prepared as previously described,16 was incubated for 2 hours in a room temperature water bath with DMSO (2% final concentration), TCDD (20 nM final concentration in DMSO), or VAF347 (200nM). An aliquot of the reaction was mixed with poly[dI·dC] and [32P]-DRE (100 000 cpm), and AhR:DRE complexes were resolved by gel retardation analysis16 and visualized by autoradiography of the dried gels.
Results
VAF347 induces an AhR signaling signature in human monocyte-derived DC
In an attempt to better understand which pathways may be regulated by VAF347, immature monocyte-derived DCs were activated with anti-CD40 antibodies for 8 hours in the absence or presence of 50 nM VAF347. The genome wide gene expression profile of both samples was determined by RNA chip analysis. Table 1 lists the top 15 genes that were either induced or repressed by VAF347 in these experiments. As expected, the IL-6–positive control gene5 was among the top 15 down-regulated genes, thus validating the chip analysis. The c-myc gene was most prominently diminished by 4-fold, whereas the most dramatic induction was that of the thrombospondin-1 gene whose expression was induced more than 180-fold by VAF347.
Examining these genes to identify those known to be regulated by a common pathway revealed that 3 of the top 15 induced genes are up-regulated in an AhR-dependent fashion-aryl hydrocarbon receptor repressor (AhRR), cytochrome P450 1B1 (CYP1B1), and TCDD inducible poly(ADP-ribose) polymerase (TiPARP). This raised the possibility that VAF347 may work as an activator of AhR signal transduction.
VAF347 binds to the AhR and acts as signaling agonist
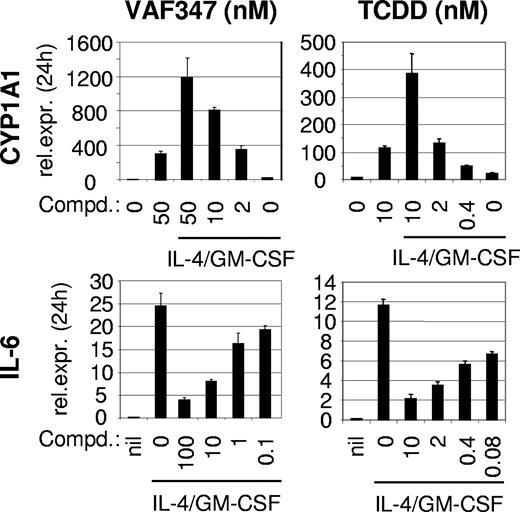
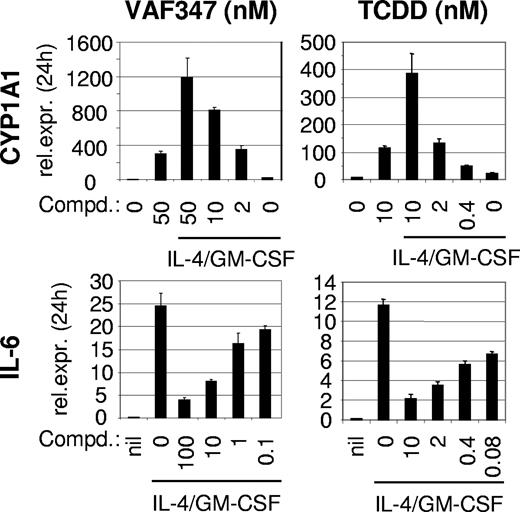
To address this possibility, we examined the ability of VAF347 to compete with [3H]TCDD for binding to the AhR using hepatic cytosol.16 Figure 1A demonstrates that VAF347 was able to compete with [3H]TCDD for AhR binding in a concentration dependent fashion. Indeed, as little as 10 nM VAF347 could competitively displace approximately 50% of [3H]TCDD from the receptor. VAG005, a closely related derivative of VAF347, which was found inactive on human B cells to inhibit IgE production5 (data not shown), was a weak competitive ligand with a binding potency of at least 100-fold less than that of VAF347. To confirm whether VAF347 is an AhR agonist, electrophoretic mobility shift assays with a radiolabeled oligonucleotide containing an XRE motif were performed. Addition of VAF347 to AhR-containing guinea pig hepatic cytosol induced the formation of a specific protein-DNA complex which migrated to the same position at that induced by TCDD, demonstrating that VAF347 stimulated AhR/Arnt binding to XRE-containing DNA (Figure 1B). Final confirmation that VAF347 acts as an agonist of the AhR signaling pathway was obtained from experiments in which the induction of CYP1A1 gene expression was measured. Human peripheral monocytes were incubated with 50 nM VAF347, 50 nM VAG005 or 20 nM TCDD for 4 hours and steady-state CYP1A1 mRNA levels were determined by quantitative RT-PCR. VAF347 and TCDD both induced CYP1A1 transcript levels in primary monocytes, whereas VAG005 had no effect (Figure 2A). In parallel samples, cells were also stimulated with IL-4 and GM-CSF in combination with the compounds. Interestingly, cytokine treatment alone significantly increased levels of CYP1A1 mRNA, and this effect was further enhanced by VAF347 and TCDD but not by VAG005 (Figure 2A). The extent of CYP1A1 induction by VAF347 or TCDD was similar in cytokine-treated or untreated cells. The enhancing effect of IL-4 and GM-CSF on CYP1A1 expression in monocytes is reminiscent of data obtained in human B cells in which IL-4 was able to induce CYP1A1 gene expression.17 Thus, it appears as if IL-4 is a more general activator of CYP1A1. Collectively, these data indicated that VAF347 acts as bona fide agonist of AhR signaling.
Binding to and activation of the AhR by VAF347. (A) Guinea pig hepatic cytosol (2 mg/mL) was incubated with 2 nM of [3H]TCDD in the absence or presence of 100-fold excess TCDF or increasing concentrations of VAF347 or VAG005 for 2 hours at room temperature. [3H]TCDD specific binding was determined using the hydroxyapatite binding assay, as described in “Methods.” Data are presented as a mean plus or minus SD percentage displacement of [3H]TCDD specific binding from at least triplicate incubations. (B) Guinea pig hepatic cystosol (8 mg/mL in HEDG) was incubated with DMSO (2.0%), TCDD (20 nM), or VAF347 (200 nM) for 2 hours at room temperature. Aliquots of each reaction were incubated with [32P]XRE and run by electrophoretic mobility shift analysis to resolve protein-DNA complexes. The arrow indicates the position of the induced ligand/AhR/ARNT/XRE complex and the free (unbound) XRE probe.
Binding to and activation of the AhR by VAF347. (A) Guinea pig hepatic cytosol (2 mg/mL) was incubated with 2 nM of [3H]TCDD in the absence or presence of 100-fold excess TCDF or increasing concentrations of VAF347 or VAG005 for 2 hours at room temperature. [3H]TCDD specific binding was determined using the hydroxyapatite binding assay, as described in “Methods.” Data are presented as a mean plus or minus SD percentage displacement of [3H]TCDD specific binding from at least triplicate incubations. (B) Guinea pig hepatic cystosol (8 mg/mL in HEDG) was incubated with DMSO (2.0%), TCDD (20 nM), or VAF347 (200 nM) for 2 hours at room temperature. Aliquots of each reaction were incubated with [32P]XRE and run by electrophoretic mobility shift analysis to resolve protein-DNA complexes. The arrow indicates the position of the induced ligand/AhR/ARNT/XRE complex and the free (unbound) XRE probe.
VAF347 induces AhR signaling. (A) VAF347 and TCDD, but not VAG005, induce the expression of CYP1A1 mRNA in peripheral blood monocytes. RNA was analyzed by qRT-PCR after a 4-hour incubation period with the compounds alone or in the additional presence of IL-4 and GM-CSF. The expression level of CYP1A1 relative to the housekeeping gene eF-1α is shown. The error bars indicate SD. P values indicate statistical significance determined by a paired Student t test. (B) VAF347 and TCDD, but not VAG005, inhibit IL-6 production by maturing DCs. Monocyte-derived DCs were activated with a cocktail containing GM-CSF, IFN-γ, TNF-α, anti-CD40 mAb, and goat anti–mouse IgG to crosslink the anti-CD40 mAb. The levels of IL-6 were measured in the supernatants by ELISA. (C) VAF347 and TCDD, but not VAG005, inhibit DC-mediated T-cell proliferation. Immature monocyte-derived DCs were pulsed with KLH and subsequently cocultured with autologous CD4+ T cells. The primed T cells were restimulated for 4 additional days with fresh antigen-pulsed DC before T-cell proliferation was measured by tritiated thymidine incorporation.
VAF347 induces AhR signaling. (A) VAF347 and TCDD, but not VAG005, induce the expression of CYP1A1 mRNA in peripheral blood monocytes. RNA was analyzed by qRT-PCR after a 4-hour incubation period with the compounds alone or in the additional presence of IL-4 and GM-CSF. The expression level of CYP1A1 relative to the housekeeping gene eF-1α is shown. The error bars indicate SD. P values indicate statistical significance determined by a paired Student t test. (B) VAF347 and TCDD, but not VAG005, inhibit IL-6 production by maturing DCs. Monocyte-derived DCs were activated with a cocktail containing GM-CSF, IFN-γ, TNF-α, anti-CD40 mAb, and goat anti–mouse IgG to crosslink the anti-CD40 mAb. The levels of IL-6 were measured in the supernatants by ELISA. (C) VAF347 and TCDD, but not VAG005, inhibit DC-mediated T-cell proliferation. Immature monocyte-derived DCs were pulsed with KLH and subsequently cocultured with autologous CD4+ T cells. The primed T cells were restimulated for 4 additional days with fresh antigen-pulsed DC before T-cell proliferation was measured by tritiated thymidine incorporation.
To determine whether activation of the AhR had consequences on DC function, we examined the effect of VAF347 and TCDD on monocyte samples that were differentiated into immature DCs by treatment with IL-4 and GM-CSF for 6 days. Maturation of these DC was induced by treatment with a cocktail containing GM-CSF, IFN-γ, tumor necrosis factor-α (TNF-α), anti-CD40 mAb, and goat anti–mouse IgG to crosslink the anti-CD40 mAb. After 48 hours, IL-6 production by mature monocyte-derived DCs was measured by ELISA. TCDD and VAF347 inhibited IL-6 synthesis, whereas VAG005 had no effect (Figure 2B). Similarly, VAF347 and TCDD were potent inhibitors of DC-mediated autologous T-cell proliferation, whereas VAG005 again was inactive (Figure 2C). These data indicated that TCDD had a very similar biologic profile on human DC compared with VAF347. The inability of VAG005, which did not activate AhR signaling, to modulate DC functions, further confirms the relationship between AhR activation and blockade of DC functionality.
VAF347 inhibits IL-4+ GM-CSF induced IL-6 production in MM1 cells
To further explore the role of the AhR as a functionally important molecular target for VAF347, the human monocytic cell line MM1 was used. Treatment of MM1 cells with IL-4 and GM-CSF induced IL-6 mRNA expression which was inhibited by VAF347 and TCDD in a concentration-dependent fashion (Figure 3). The IC50 for VAF347 was approximately 5 nM, a concentration very similar to that calculated for IL-6 blockade in monocyte-derived DCs.5 The potency of TCDD was considerably stronger, with an IC50 approximately 80 pM. Both compounds also induced CYP1A1 expression in MM1 cells in a dose-dependent manner. Although the presence of IL-4 plus GM-CSF had minimal effects on CYP1A1 expression, it enhanced the magnitude of VAF347- and TCDD-dependent CYP1A1 induction, an effect similar to that observed with primary monocytes (Figures 2A,3).
VAF347 inhibits cytokine induced IL-6 production in MonoMac1 cells. Top row: induction of CYP1A1 mRNA expression measured by qRT-PCR on treatment with VAF347 and TCDD in the absence or presence of IL-4 and GM-CSF; bottom row: inhibition of IL-4 plus GM-CSF–induced IL-6 transcription by the 2 AhR agonists. The expression levels of CYP1A1 and IL-6 relative to the housekeeping gene eF-1α are depicted. Error bars represent SD.
VAF347 inhibits cytokine induced IL-6 production in MonoMac1 cells. Top row: induction of CYP1A1 mRNA expression measured by qRT-PCR on treatment with VAF347 and TCDD in the absence or presence of IL-4 and GM-CSF; bottom row: inhibition of IL-4 plus GM-CSF–induced IL-6 transcription by the 2 AhR agonists. The expression levels of CYP1A1 and IL-6 relative to the housekeeping gene eF-1α are depicted. Error bars represent SD.
A functional AhR is necessary for the biologic phenotype of VAF347 in MM1 cells
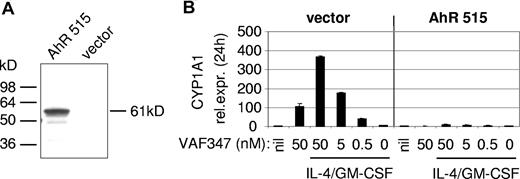
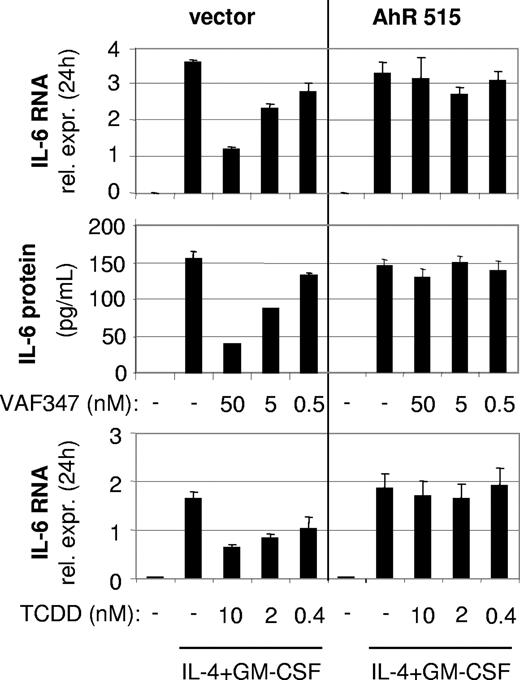
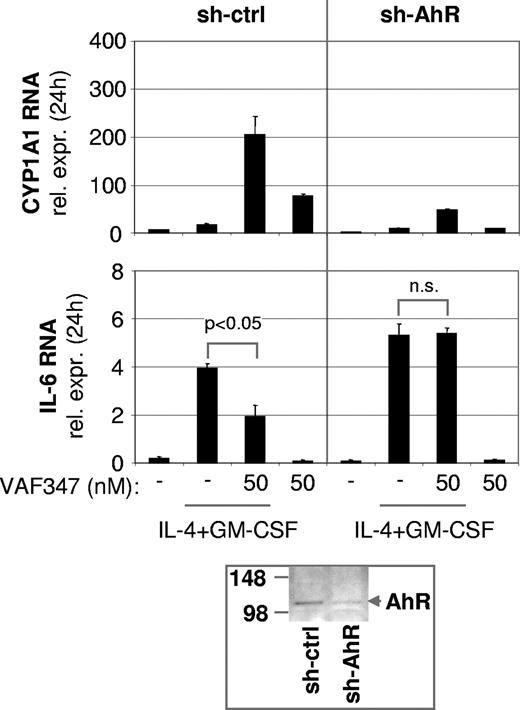
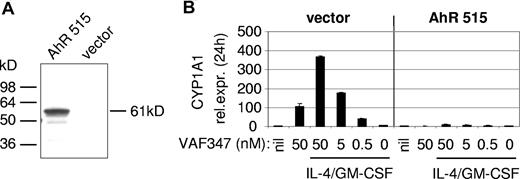
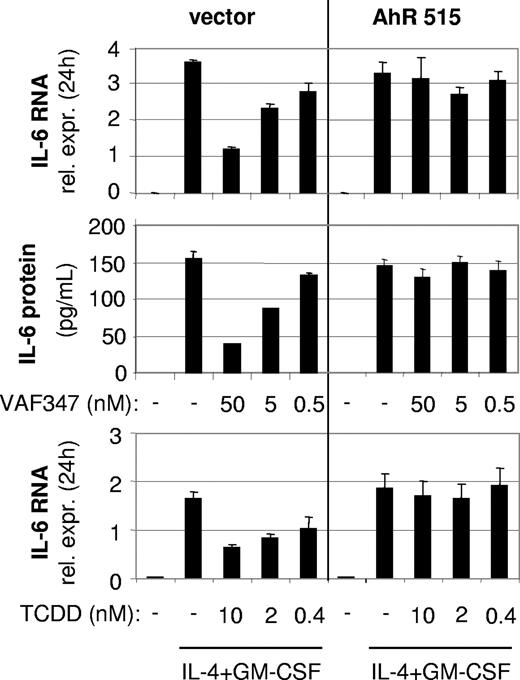
To directly demonstrate an involvement of the AhR in the mode of action of VAF347, MM1 cells were infected with a recombinant retroviral expression vector encoding a truncated AhR protein lacking the transactivation domain at the carboxyl terminus (AhR515). This shorter AhR was shown previously to act in a trans-dominant negative fashion.18 Western blot analysis confirmed overexpression of the recombinant protein in transduced cells (Figure 4A). The AhR515 protein indeed acted as a trans-dominant negative repressor because induction of CYP1A1 expression by VAF347 was completely abrogated in MM1 cells overexpressing the truncation mutant but not in cells infected with the empty vector (Figure 4B). Identical results were obtained using TCDD as the AhR agonist (data not shown). Interestingly, although VAF347 did not inhibit IL-4 plus GM-CSF–induced IL-6 transcription in AhR515-expressing cells, VAF347-dependent inhibition of IL-6 expression was observed in empty vector infected cells at concentrations comparable with that in uninfected MM1 cells (Figure 5). These data were confirmed at the protein level by measuring IL-6 in the cell supernatants by ELISA. Identical results were obtained when TCDD was used as IL-6 inhibitor (Figure 5). Essentially identical results were obtained when wild-type AhR expression was inhibited in MM1 cells using a gene silencing approach. MM1 cells infected with a retroviral vector encoding a short hairpin (sh) AhR sequence produced significantly less AhR protein than control shRNA infected cells resulting in complete abrogation of CYP1A1 expression by VAF347 in sh-AhR infectants (Figure 6). Importantly, VAF347 was unable to inhibit IL-4 plus GM-CSF–induced IL-6 production in sh-AhR cells, whereas robust inhibition was observed in control shRNA infected cells (Figure 6). Taken together, these data demonstrated that the AhR protein plays a key role in the mechanism by which VAF347 inhibits IL-6 synthesis.
A trans-dominant negative AhR ablates CYP1A1 induction in MonoMac1 cells. (A) Ectopic overexpression of a truncated AhR (AhR515) in MonoMac1 cells. A Western blot analysis is shown. At the left, the position of molecular weight standards is indicated. (B) AhR515 acts as in a trans-dominant negative fashion by blocking the induction of CYP1A1 expression by VAF347. vector indicates cells infected with the parental retroviral vector.
A trans-dominant negative AhR ablates CYP1A1 induction in MonoMac1 cells. (A) Ectopic overexpression of a truncated AhR (AhR515) in MonoMac1 cells. A Western blot analysis is shown. At the left, the position of molecular weight standards is indicated. (B) AhR515 acts as in a trans-dominant negative fashion by blocking the induction of CYP1A1 expression by VAF347. vector indicates cells infected with the parental retroviral vector.
A trans-dominant negative AhR protein blocks the function of VAF347 in MM1 cells. Loss of biologic activity of VAF347 and TCDD to block IL-6 synthesis in AhR515 overexpressing MonoMac1 cells. Top row: qRT-PCR of IL-4+GM-CSF–induced IL-6 RNA after 24 hours in control (vector) or AhR515-expressing cells; middle row: IL-6 protein was measured in the supernatants from the same cells; bottom row: similar effects as for VAF347 are shown with TCDD on IL-6 mRNA. Error bars represent SD.
A trans-dominant negative AhR protein blocks the function of VAF347 in MM1 cells. Loss of biologic activity of VAF347 and TCDD to block IL-6 synthesis in AhR515 overexpressing MonoMac1 cells. Top row: qRT-PCR of IL-4+GM-CSF–induced IL-6 RNA after 24 hours in control (vector) or AhR515-expressing cells; middle row: IL-6 protein was measured in the supernatants from the same cells; bottom row: similar effects as for VAF347 are shown with TCDD on IL-6 mRNA. Error bars represent SD.
VAF347 has no biologic effects in MM1 cells expressing reduced AhR levels. The graph shows CYP1A1 (top panel) or IL-6 (middle panel) RNA expression in cells infected with a control shRNA (sh-ctrl) or a sh-AhR retroviral construct. At the bottom, an immunoblot is shown demonstrating reduced AhR protein expression in sh-AhR–infected cells. The size of molecular weight protein standards is given in kilodaltons.
VAF347 has no biologic effects in MM1 cells expressing reduced AhR levels. The graph shows CYP1A1 (top panel) or IL-6 (middle panel) RNA expression in cells infected with a control shRNA (sh-ctrl) or a sh-AhR retroviral construct. At the bottom, an immunoblot is shown demonstrating reduced AhR protein expression in sh-AhR–infected cells. The size of molecular weight protein standards is given in kilodaltons.
The AhR mediates the anti-inflammatory phenotype of VAG539 in a mouse model of allergic lung inflammation
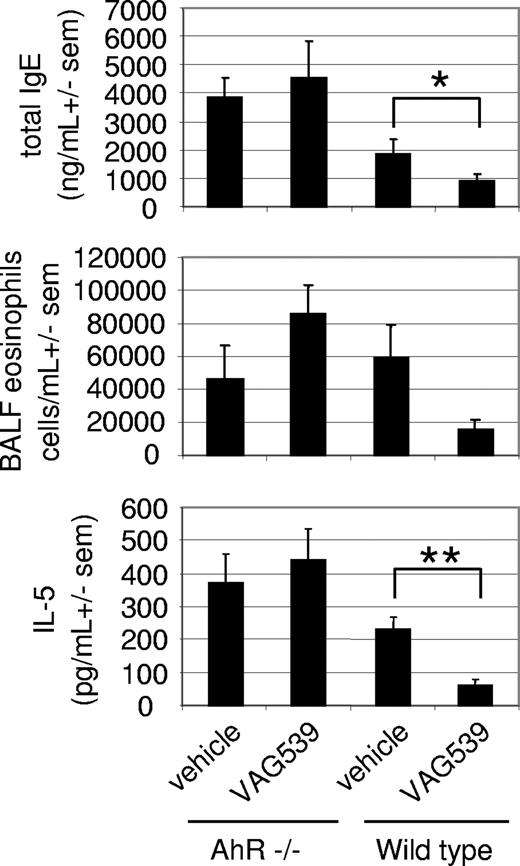
To explore if AhR was also critical for mediating the anti-inflammatory effects of the compound in vivo, a mouse model of allergic lung inflammation was established in homozygous AhR-deficient mice identical to the model described recently.5 Immunization of mice with OVA in Al(OH)3 followed by challenge with aerosolized OVA induces allergic airway disease, characterized by lung eosinophilia, IL-5 secretion into the bronchoalveolar space and elevated serum IgE levels. Mice were treated with 30 mg/kg VAG539 on days 0 to 7. VAG539 is a close derivative of VAF347 and is efficiently converted to VAF347 in vivo. The compound is equipotent to VAF347 in vitro and in vivo (data not shown). In wild-type mice, VAG539 treatment led to a strong reduction of total serum IgE levels compared with vehicle-treated animals (Figure 7), similar to that observed with VAF347.5 IL-5 levels in the bronchoalveolar fluid were inhibited to a comparable degree. In addition, although the influx of eosinophils into the lung was substantially reduced, the values were not statistically different in this experiment. In contrast, none of the 3 parameters was reduced by VAG539 in AhR-deficient animals (Figure 7). From these data, it was concluded that the AhR protein plays a critical role in mediating the action of the 2 compounds and thus represents a key molecular target for VAF347 and VAG539 mediating the anti-inflammatory phenotype of the 2 compounds in this mouse model of allergic pulmonary inflammation.
Loss of anti-inflammatory potency of VAG539 in AhR-deficient mice. Allergic lung inflammation was induced in wild-type or AhR−/− mice in the absence or presence of VAG539 treatment and serum IgE levels (top), eosinophilia (middle), and IL-5 (bottom) in the bronchoalveolar fluid were measured. sem indicates standard error of the mean (*P < .05. **P < .01).
Loss of anti-inflammatory potency of VAG539 in AhR-deficient mice. Allergic lung inflammation was induced in wild-type or AhR−/− mice in the absence or presence of VAG539 treatment and serum IgE levels (top), eosinophilia (middle), and IL-5 (bottom) in the bronchoalveolar fluid were measured. sem indicates standard error of the mean (*P < .05. **P < .01).
Discussion
VAF347 has been shown recently to inhibit major hallmarks of allergic immune responses in vivo by blocking elevated serum IgE levels, the influx of eosinophils into lung tissue and goblet cell hyperplasia.5 The available data indicated that VAF347 exerted its anti-inflammatory effects on DC by blocking their appropriate helper function for the development of Th cells, which orchestrate the allergic immune response. In particular, VAF347 inhibited the expression of IL-6, CD86, and HLA-DR by DCs, all of which are important molecules in productive DC/T-cell collaboration. Reduced expression of these factors may account for the immunomodulated phenotype of VAF347-treated DCs. The concept of DC immunomodulation by VAF347 has also been demonstrated in vivo. Application of VAF347 treated bone marrow–derived DCs to syngeneic mice inhibited rejection of transplanted allogeneic islets by inducing long-lasting tolerance.19
This study was conducted to identify functionally relevant molecular targets for VAF347. A comparison of gene expression in human moDCs in the absence or presence of VAF347 revealed a number of interesting genes whose expression was changed by the compound. The thrombospondin-1 gene (tsp-1) was most prominently induced by VAF347. tsp-1 has been described to be induced on maturation of immature DCs. Functionally, tsp-1 is known as potent anti-inflammatory molecule. It can act as autocrine negative regulator of human DC activation.20 Alternatively, tsp-1 directly inhibits the generation of Th1 cells21,22 and induces the development of regulatory T cells.23 Finally, it activates transforming growth factor-β, which is a known immunoregulatory cytokine.24 Thus, induction of tsp-1 expression by VAF347 could explain at least partly the immunomodulated DC phenotype observed with this compound. Studies are ongoing to address this possibility.
Strikingly, VAF347 induced an AhR-dependent gene expression signature in moDCs as evidenced by induction of AhR-repressor, TiPARP, and CYP1B1 gene expression, all of which are known to be up-regulated by activated AhR. This raised the possibility that VAF347 may act as agonist of AhR signaling. This hypothesis was verified at several levels. First, VAF347 could directly bind to the AhR protein. The interaction appears to involve a similar region on the protein as that used by the prototypical AhR agonist TCDD (ie, the ligand binding pocket) because VAF347 potently competed with TCDD for AhR binding. The VAF347/AhR interaction induced activation of AhR signaling was proven by the appearance of a VAF347-dependent AhR/DNA complex and up-regulation of CYP1A1 expression. Compound-mediated activation of AhR was a necessary step for induction of DC immunomodulation in vitro because the prototype AhR agonist TCDD inhibited IL-6 secretion and autologous T-cell help by DCs similar to VAF347. In contrast, VAG005 lacked effects on DCs and was a poor AhR agonist. In addition, inhibition of AhR expression or overexpression of a trans-dominant negative AhR in MM1 cells was sufficient to block the biologic activity of the compound on IL-6 expression. Most importantly, AhR-deficient mice were resistant to treatment with VAG539, a close homolog of VAF347. In a model of allergic lung inflammation VAG539 had no anti-inflammatory efficacy on 3 disease relevant parameters. Collectively, these data established the AhR protein as critical molecular target involved in the mode of action of VAF347 in vitro and in vivo.
The antiallergic phenotype of VAF347 in vivo is reminiscent of that reported for M50367. This compound, although structurally unrelated to VAF347, also exerts its anti-inflammatory effects by activating AhR signaling.25 M50367 appears to directly block the differentiation of naive T cells into Th2 effector cells via suppression of GATA-3, a key transcription factor for Th2 differentiation. Our experiments designed to demonstrate direct biologic effects of VAF347 on T cells have been unsuccessful so far5 (data not shown). Thus, despite using the same molecular target, VAF347 and M50367 may act on different cell types to block allergic immune responses in mice. Similar discrepancies have been noted in the study of immune modulation by TCDD. Depending on the antigen and model system used, AhR activation by TCDD suppresses T-cell activation by direct26,27 and indirect28 mechanisms. Mechanistically, immunomodulation of DCs by VAF347 resulting in defective Th differentiation will ultimately translate into the same antiallergic phenotype compared with a direct inhibitory effect on T cells by M50367. It is possible that binding of different agonists to the AhR will result in different conformations of the receptor allowing for different downstream mechanistic events. Clearly, a more detailed analysis is needed to confirm this hypothesis.
Immunomodulation of DCs by AhR agonists has been described in several studies. In vivo application of TCDD to naive or immunized mice led to increased expression of costimulatory markers on DC and elevated proliferation of allogeneic T cells in coculture experiments. However, fewer DCs were recovered from TCDD-treated mice, which led to the hypothesis that the initial DC activation signal provided by AhR activation resulted in premature deletion of the cells.29 This, in turn, may alter the survival of the T cells on DC contact and might lead to deregulation of the immune response.30 Benzo(α)pyrene, another AhR agonist, was shown to inhibit cytokine secretion by LPS-stimulated mouse bone marrow–derived DCs as well as DC-mediated T-cell proliferation.31 These effects were likely the result of AhR activation and the resultant AhR-responsive genes because they were inhibited in the presence of the AhR antagonist α-naphthoflavone. No effect on the expression of cell surface markers, such as MHC class II, CD86, or CD11c, was observed. In the human system, slightly different results were obtained. Benzo(α)pyrene blocked the differentiation of monocyte-derived DCs in response to IL-4 and GM-CSF as measured by the failure to induce CD1a and costimulatory molecule expression on maturation with LPS. Furthermore, the compound blocked the capacity of DC to take up antigen, secrete IL-10 and IL-12 after LPS maturation, and impaired the T-cell stimulatory activity of the DCs.32 In the same study, TCDD had no effect on the expression levels of CD1a and costimulatory markers during DC differentiation. It is not clear which effects of these compounds are the result of activation of the AhR and which are caused by unrelated mechanisms. The block of DC-mediated T-cell proliferation by benzo(α)pyrene is similar to the effects seen with VAF347. Similarly, both compounds inhibit IL-12 production by DCs (VAF347 data not shown). On the other hand, no reduction in cell surface expression of CD1a or the capacity to take up antigen by DC was observed for VAF347.5 Thus, VAF347 has an overlapping but not identical biologic phenotype on human monocyte-derived DC function with benzo(α)pyrene. One could speculate that the overlapping activities are the result of activation of the AhR, whereas the unique effects of benzo(α)pyrene on CD1a expression and antigen uptake may be the result of other AhR-independent or related mechanisms (ie, metabolism of benzo(α)pyrene by constitutive or inducible cytochrome P450 enzymes (CYPs) to a modulatory chemical).
Similar to VAF347, other AhR agonists have been shown to inhibit IL-6 expression. TCDD and 7,12-dimethylbenz[a]anthracene both blocked LPS-mediated induction of IL-6 synthesis in a mouse bone marrow stromal cell line. This effect may be related to reduced binding of the transcription factor NF-κB to its recognition site in the IL-6 promoter.33 In another study, inhibition of IL-6 by the AhR agonist β-naphthoflavone in C6 glioma cells was demonstrated, leading to a block in astrocyte differentiation for which IL-6 is an important autocrine factor.34
Given the complex biology of the AhR, several scenarios can be envisioned to explain the mode of action of VAF347 on DC to inhibit IL-6 production. One possibility is the presence of negatively acting AhR binding sites in the IL-6 promoter. The existence of such negatively cis-acting XRE elements has been described for a number of genes whose expression is repressed by AhR agonists, such as the c-fos, pS2, and cathepsin D gene.35-38 However, examination of the IL-6 promoter has not revealed the presence of bona fide XRE motifs (data not shown), making this possibility unlikely. Another possibility may be that VAF347 induces the expression of a factor that limits IL-6 production in an autocrine fashion, such as tsp-1 as described also in “Discussion.” A third attractive option may come from the fact that activated AhR interacts not only with DNA but also with other signaling molecules, thereby modulating gene expression in a transcription-independent manner.13 One prime candidate factor may be NF-κB p65.33 Indeed, direct interaction of AhR with NF-κB p65 has been shown in response to TCDD in a mouse DC line. As consequence, DC maturation induced by tumor necrosis factor-α (TNF-α) and anti-CD40 antibodies, which requires the action of NF-κB family members, was significantly reduced.39,40 Moreover, NF-κB factors have been described to be intimately involved in IL-6 gene regulation.41,42 Thus, one could hypothesize that VAF347-activated AhR inhibits IL-6 production by functional inactivation of NF-κB via AhR/NF-κB heterodimerization. This possibility is currently under investigation.
In conclusion, we have identified the AhR as a functionally relevant molecular target for VAF347. Interaction of the compound with the AhR protein is essential for the induction of an immunomodulated state in DC that appears to be the basis for the potent anti-inflammatory phenotype in vivo. Although the toxicity of particular AhR ligands is well studied, the discovery that the biologic action of the AhR can be harnessed for therapeutic purposes suggests a new tool for the treatment of inflammatory or immunologic disorders.
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Nicole Hartmann and Markus Jaritz for performing and analyzing the gene chip experiments, Mark Bauter for assistance during experiments with AhR-deficient mice, and Toshio Kitamura for providing the retroviral expression vector PMX-pie.
This work was supported in part by the following research grants from the National Institutes of Health: R01-ES10619 (B.P.L.), K02-ES012409 (B.P.L.), and R01-ES012498 (M.S.D).
National Institutes of Health
Authorship
Contribution: B.P.L. designed experiments and wrote parts of the paper; M.S.D. designed experiments and wrote parts of the paper; H.N., B.A.V., N.H., W.N., and C.R. performed experiments; and M.W. supervised the project, designed experiments, and wrote the paper.
Conflict-of-interest disclosure: H.N., N.H., W.N., C.R., and M.W. are employed by Novartis Pharma AG; B.P.L., B.A.V., and M.S.D. declare no competing financial interests.
Correspondence: Maximilian Woisetschläger, Department of Autoimmunity and Transplantation, Novartis Institute for Biomedical Research, Vienna, Brunnerstrasse 59, A-1230 Vienna, Austria; e-mail: maximilian.woisetschlaeger@novartis.com.

![Figure 1. Binding to and activation of the AhR by VAF347. (A) Guinea pig hepatic cytosol (2 mg/mL) was incubated with 2 nM of [3H]TCDD in the absence or presence of 100-fold excess TCDF or increasing concentrations of VAF347 or VAG005 for 2 hours at room temperature. [3H]TCDD specific binding was determined using the hydroxyapatite binding assay, as described in “Methods.” Data are presented as a mean plus or minus SD percentage displacement of [3H]TCDD specific binding from at least triplicate incubations. (B) Guinea pig hepatic cystosol (8 mg/mL in HEDG) was incubated with DMSO (2.0%), TCDD (20 nM), or VAF347 (200 nM) for 2 hours at room temperature. Aliquots of each reaction were incubated with [32P]XRE and run by electrophoretic mobility shift analysis to resolve protein-DNA complexes. The arrow indicates the position of the induced ligand/AhR/ARNT/XRE complex and the free (unbound) XRE probe.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/112/4/10.1182_blood-2007-08-109645/6/m_zh80080818340001.jpeg?Expires=1768464578&Signature=Xm86EaPK4Z3Wlp23GdYkKZ2YFF4NH4Fkx4rH9h38zwjzU6AKyM3RuqdLh4G8ztSZyZo6bilZqHyPyMH2yhRP-F2kYG2m2MpFvG-7PmIGEFPnD4VsrtW3P5WV3XevJeQftDH7oVRL~qSrbHyl-MKiEmTvSQa1z7StRLKtYXH0UymiQnbWcrh1rvKnDHIzIIa8SkZbgjK~ieSpPN701umy04uukaOxP0POlJGj3sNNtCT8xqLRSYqUlz92--45K~nm~uX-lPVXLHBojsd5HniWCsqvnI6WoTYcpZUjQex26vc3EN-1VdDnZHBn-50aQeDzBFdJkn8uvW--PhwrjPEFSg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)



![Figure 1. Binding to and activation of the AhR by VAF347. (A) Guinea pig hepatic cytosol (2 mg/mL) was incubated with 2 nM of [3H]TCDD in the absence or presence of 100-fold excess TCDF or increasing concentrations of VAF347 or VAG005 for 2 hours at room temperature. [3H]TCDD specific binding was determined using the hydroxyapatite binding assay, as described in “Methods.” Data are presented as a mean plus or minus SD percentage displacement of [3H]TCDD specific binding from at least triplicate incubations. (B) Guinea pig hepatic cystosol (8 mg/mL in HEDG) was incubated with DMSO (2.0%), TCDD (20 nM), or VAF347 (200 nM) for 2 hours at room temperature. Aliquots of each reaction were incubated with [32P]XRE and run by electrophoretic mobility shift analysis to resolve protein-DNA complexes. The arrow indicates the position of the induced ligand/AhR/ARNT/XRE complex and the free (unbound) XRE probe.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/112/4/10.1182_blood-2007-08-109645/6/m_zh80080818340001.jpeg?Expires=1768464579&Signature=uisqQyhsXJBE1b4sCYpjdPvhve714tTos4cREjCWi~MtTQc5Qq~ysPZetsGJ~pLIqrGIB6tOXgxEqs3LPKdFcgh7UV7HVRuvx4gipl8QtNu7O4llegzK-JLr9jPA04~8H~Waaww6op78N6Q~6r0qZoX-QF6enpWQJAxCHL4QtLZCP9c5oRJZubg5IHjV9WTSfNxdHybLiCDXoNyvznip3TzPBieiURkpydjeVCQxhxNq2ZY2dxbw-rIsAoFHsrNhTh1xLB33AKCPHs2JEJ-XYDDE7C58YIy7tYRv5l3~EiGxglMXF-Vix3EN63PuV45JNcrAz8rWzR5uTf90~Uoi~Q__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)