Abstract
Using noninvasive in vivo imaging and experimental autoimmune uveoretinitis as a model, we show for the first time that the mechanisms controlling blood monocyte recirculation through peripheral and lymphoid tissues alter during inflammation. The recirculation of monocytes in mice with ocular inflammation but not controls was found to depend on the selectin CD62-ligand (CD62L) and on CD44. Not only was rolling efficiency ablated or markedly reduced in antibody-treated mice, but most of the labeled monocytes also disappeared from the circulation within seconds, anti-CD44–treated monocytes homing to the lymph nodes and anti–CD62L-treated monocytes homing to the spleen. Our data indicate that, although PSGL-1 has a partial role in the transmigration of monocytes into the inflamed retina, CD62L has a key role in regulating recruitment of monocytes to lymphoid tissue from the blood during inflammation and that CD44 is required to maintain CD62L+ inflammatory monocytes within the circulation during inflammation. This effect was systemic, because sequestered monocytes accumulated in mesenteric as well as draining cervical lymph nodes, and inflammation dependent, because depletion of circulating blood monocytes was much reduced or absent in normal mice and accumulations of adoptively transferred monocytes in the lymphoid tissues did not occur.
Introduction
Nondifferentiated monocytes are known to circulate in the blood and tissues for up to 3 days, and during that time they are recruited to the tissues where they differentiate into macrophages or myeloid dendritic cells.1-3 Subsets of blood monocytes with differential migratory potential have been identified, and the capacity of monocytes to preferentially migrate to sites of inflammation has been linked to expression of the selectin CD62-ligand (CD62L), CC-chemokine receptor-2 (CCR-2) and CD14, whereas subsets that are CCR-2− and CD16+ are recruited to tissues independently of inflammatory stimuli to become resident monocyte macrophages or myeloid dendritic cells (DCs).4 More recently, we have shown that differentiation and recruitment of the inflammatory subset is independent of local inflammatory stimuli, requiring monocyte conditioning or differentiation over time within the circulation. Bone marrow–derived monocytes (BM-Mo's), transferred intravenously to mice with established ocular inflammation, required 24 to 48 hours of in vivo conditioning before being able to roll on endothelium efficiently and migrate into the inflamed retina. This capacity to roll and migrate was largely lost after 72 hours in the circulation. In the retina, adoptively transferred, in vivo conditioned monocytes differentiated into CD11c+, B220+ DCs and F4/80ve macrophages, indicating that a permissive endothelium alone is not sufficient for active recruitment of monocytes from the blood.3
Understanding the processes involved in mononuclear myeloid cell trafficking has considerable importance both for targeting antigen-pulsed DCs used as vaccines and for the control of inflammatory diseases.5,6 Recruitment of mononuclear myeloid cells from the blood to the tissue and from the tissue to lymph nodes is controlled by adhesive interactions between the cell and the vascular or lymphatic endothelium. The mechanisms involved in recruitment of leukocyte subsets from the blood to the tissues and lymphoid organs have been extensively studied, primarily in in vitro model systems under defined molecular conditions. Multiple molecules, constitutively expressed or induced under inflammatory conditions, have been identified as playing a role in monocyte adhesion and diapedesis.7,8 The multistep paradigm that has emerged invokes weak interactions by selectins that allow leukocyte rolling on vascular endothelium that initiates an adhesion cascade. This is triggered by chemokines and results in firm adhesion and spreading of the monocyte on the endothelium through regulation of avidity of β1-integrins and β2-integrins.9,10 Fast rolling is mediated by CD62L11 and is regulated by vessel wall shear stress.12 Rolling through E- or P-selectin is slower, but it is also shear stress–dependent when rolling on P-selectin glycoprotein ligand-1 (PSGL-1).13 This dependence on shear stress is believed to limit leukocyte interactions in the center of vessels or in vessels with very high or very low wall shear stress, and the steady rolling generated enables the leukocyte to receive crucial activating stimuli from the endothelium. Some overlapping and redundancy appears to exist in the system,14 and mechanisms controlling monocyte trafficking to a Th1-type inflammatory lesion under physiologic conditions of flow in vivo are not known.
Using experimental autoimmune uveoretinitis (EAU) as a model inflammation, we have been able to demonstrate mechanisms controlling specific T-lymphocyte subset rolling, sticking, and transendothelial cell migration within normal and diseased neurovascular postcapillary endothelial venules where shear stress levels are relatively high (I.C., H.X., A.M., et al, manuscript submitted 2002).15-17 A reduction in shear stress in retinal veins from approximately 30 dyn/cm2 to 20 dyn/cm occurs up to 24 hours before leukocyte infiltration, and rolling and sticking efficiencies are negatively correlated with wall shear stress, providing a good model for studying leukocyte subset trafficking in vivo during inflammation. In this study we focus on the kinetics of monocyte trafficking in vivo and in particular on the role of CD44 that is implicated in primary adhesive interactions between leukocytes and endothelium18 and has a major role in leukocyte homing in EAU,19 as well as CD62L and PSGL-1 that have been shown to account for at least 90% of leukocyte rolling in vivo.20 In addition to mediating monocyte infiltration of the inflammatory site, we show for the first time that CD62L has a key role in regulating recruitment of monocytes to lymphoid tissue from the blood during inflammation and that CD44 is also required to maintain CD62L+ inflammatory monocytes within the circulation during inflammation. Unexpectedly, this effect was systemic because distant as well as draining lymph nodes were involved and inflammation specific, because no sequestration of the adoptively transferred, in vivo conditioned monocytes to lymphoid tissues was observed in normal mice.
Methods
Animals and retinal inflammation model
Eight- to 12-week-old wild-type C57BL/6 mice and homozygous C57BL/6 mice expressing enhanced green fluorescent protein (EGFP) under the control of a chicken β-actin promoter and cytomegalovirus enhancer were maintained in the Medical Research Facility at Aberdeen University. EAU was induced in wild-type C57BL/6 mice as described.21 Retinal inflammation occurred at day 16 to 18 after infection and peaked at day 21 to 28 after infection. All procedures were approved by the Home Office Regulations for Animal Experimentation, United Kingdom.
In vivo monocyte trafficking using scanning laser ophthalmoscopy
Lymphocyte-depleted BM-Mo's were prepared from EGFP+ bone marrow cells as described.3 In vivo monocyte trafficking was studied using our scanning laser ophthalmoscopy (SLO) technique as described.22,23 This nonsurgical technique minimizes any leukocyte trafficking artifacts. The retinal vasculature is imaged through the intact cornea, and adoptively transferred leukocytes may be tracked as they enter through the retinal artery, traverse the capillary network, and exit through the retinal vein. Mice are unharmed by the procedure and can be rescanned over several days if necessary. Briefly, mice were anesthetized with an intramuscular injection of 0.4 mL/kg Hypnorm (Janssen-Cilag, Antwerp, Belgium) and 1 mL/kg diazepam (Phoenix Pharmaceuticals, Gloucester, United Kingdom) intraperitoneally. EGFP+ BM-Mo cells (8 × 106) in 150 μL phosphate-buffered saline were injected into the tail vein. After 48 hours to allow in vivo conditioning to an inflammatory phenotype, SLO images were recorded simultaneously on Videotape (S-VHS) and digitally at 25 frames per second. For each eye, 3 regions of interest containing 1 to 3 veins/venules were recorded for at least 30 minutes. Video analysis was performed off-line as described.17,24 This protocol excludes any neutrophils present in the EGFP+ BM-Mo transfers because neutrophils released from the bone marrow die rapidly and turnover time within the circulation is normally no more than 24 hours. Rolling leukocytes and those not interacting with the endothelium were counted in each venule. Rolling cells were defined as those cells with a velocity below the critical velocity. The rolling efficiency was calculated as the percentage of rolling fluorescent cells among the total number of fluorescent cells entering a venule. The sticking efficiency was determined as the percentage of labeled monocytes that remained adherent for at least 20 seconds. In antibody blocking experiments, baseline measurements were recorded for up to 15 minutes before intravenous injection of 30 μg isotype control Ig (rat IgG) or 30 μg anti–mouse monoclonal antibodies (mAbs) CD44 (rat IgG2b IM7),25 CD162 PSGL-1 (rat IgG1 2PH1),26 CD11a, LFA-1 (rat IgG2a M17/4),27 CD62L, L-selectin (rat IgG MEL-14)28 all from BD Biosciences (San Jose, CA).
Ex vivo tracking of monocytes in retina and lymphoid tissue
To test the effect of mAb treatment on monocyte infiltration of the EAU retina and other tissues, groups of 6 immunized mice (21-24 days after infection) were injected intravenously with 8 × 106 EGFP BM cells and then treated for 3 days with 30 μg/mouse per day of mAb or control rat anti–mouse IgG. These concentrations have been optimized in previous studies and had no effect on the recirculation of monocytes in control groups (data not shown).29 To label retinal vessels, 50 μL of 2% Evans blue (Sigma Chemical, Poole, United Kingdom) was injected into the tail vein and allowed to bind for 5 to 10 minutes before asphyxia with CO2. Tissues were then harvested and fixed in 2% (wt/vol) paraformaldehyde (Agar Scientific, Cambridge, United Kingdom). Retinal whole mounts were prepared as described elsewhere.19 Other tissues were cryoembedded in optimal cutting temperature compound, and frozen sections were prepared. Both retinal whole mounts and tissue sections were observed using a confocal scanning laser microscope (LSM510 META; Carl Zeiss, Jena, Germany). For tissue sections both 488-nm and 543-nm wavelengths were used to distinguish between autofluorescence and EGFP+ cells. Three sections were obtained from each tissue, and 3 images were taken randomly from each using a 20× objective lens. Images were analyzed using Image Pro Plus system (Media Cybernetics, Bethesda, MD), and data were expressed as means and SEMs of the number of EGFP+ cells per square millimeter. Unfixed, frozen tissue sections were labeled with Mel-14, IM7, or anti-EGFP antibody ab290 (rabbit polyclonal; AbCam, Cambridge, United Kingdom) using the alkaline phosphatase anti–alkaline phosphatase (APAAP) technique as described.21
Flow cytometry
Single-cell suspensions were blocked with 1% normal rat serum and immunostained with CD44 (IM7), CD162 PSGL-1 (2PH1), CD62L, L-selectin (MEL-14), CD11b (M1/70), CD11c (HL3), CD11a (M17/4), B220 (RA3-6B2), or isotype control IgG (BD Biosciences, Coley, United Kingdom), or F4/80 (CI:A3-1; Serotec, Oxford, United Kingdom). Samples were analyzed by LSR flow cytometry (BD Biosciences). Antibodies were conjugated to fluorecein isothiocyanate (FITC), phycoerythrin (PE), allophycocyanin (APC), peridinin chlorophyll protein (PerCP), PerCP–cyanin (Cy) 5.5, or biotin as required. Biotin-labeled antibodies were detected by the addition of streptavidin (SA)–APC or SA-PE (1:400; BD Biosciences). Negative isotype controls and single positive controls were performed to allow accurate breakthrough compensation. Gates and instrument settings were set according to forward and side scatter characteristics, and populations were gated to exclude dead or clumped cells. Data were collected from at least 3 individual animals in each group and expressed as means and SEMs and compared using the unpaired Student t test.
Results
In vivo kinetics of monocyte recirculation through the inflamed EAU retina and effect of blocking mAb to CD62L, PSGL-1, CD44, and LFA-1
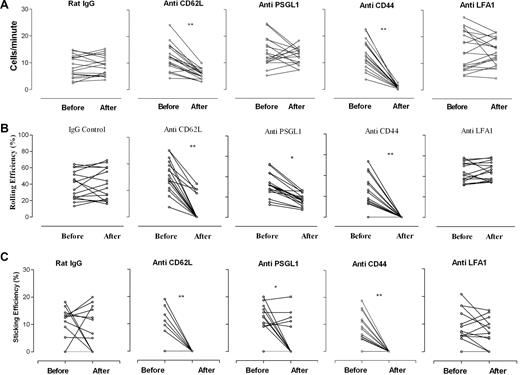
The kinetics of monocyte recirculation measured by SLO imaging of inflamed eyes is shown in Figure 1. Data were recorded from mice with EAU 48 hours after monocyte transfer, and the effects of mAb treatment on recirculation, rolling efficiency, and sticking efficiency were measured. Analysis of SLO images showed that the normal rate of EGFP+ monocyte recirculation through inflamed vessels was 11.9 (± 5.6) cells/min. The rolling efficiency of these monocytes on the endothelium varied considerably, depending on the extent of inflammation (as assessed by vessel leakage of low concentration fluorescein dye coinjected with cells), but the average rolling efficiency (ie, percentage of transferred cells passing through vessel that rolled on endothelium) was 37.8% (± 15.7%), similar to our previous observations in this model.3 The average sticking efficiency of monocytes adhering to endothelium was 8.7% (± 6.6%). LFA-1 is essential for T-cell trafficking but was shown not to be involved in monocyte adhesion and diapedesis in vitro8 ; we therefore included CD11a blocking mAb M17/4 as an additional irrelevant mAb control group for these studies.
Effect of monoclonal antibodies on monocyte trafficking in retinal vessels in EAU. Blocking antibodies to CD62L, CD44, and PSGL-1 significantly reduced rolling and sticking of monocytes in inflamed vessels. Freshly isolated EGFP bone marrow–derived monocytes (8 × 106) were injected intravenously into mice immunized 21 to 24 days previously with peptide to induce EAU. After 48 hours, cell trafficking in the retinal vasculature was analyzed by SLO. Retinal images were recorded for 15 minutes, and then mice were injected intravenously with 30 μg/mouse of rat anti–mouse antibody, and recording continued for a further 20 minutes. Data were then compared before and after antibody treatment. (A) Recirculation of adoptively transferred cells was expressed as the number of transferred EGFP monocytes detected in the same section of the retinal vessel before and after antibody infusion. (B) Rolling efficiency, expressed as the percentage of rolling fluorescent cells among the total number of fluorescent cells entering a venule before and after antibody infusion. (C) Sticking efficiency, expressed as the percentage of fluorescent monocytes within the same venule that remained adherent for at least 20 seconds. *P < .05; **P < .01; Student paired t test; n was at least 16 vessels from 3 mice.
Effect of monoclonal antibodies on monocyte trafficking in retinal vessels in EAU. Blocking antibodies to CD62L, CD44, and PSGL-1 significantly reduced rolling and sticking of monocytes in inflamed vessels. Freshly isolated EGFP bone marrow–derived monocytes (8 × 106) were injected intravenously into mice immunized 21 to 24 days previously with peptide to induce EAU. After 48 hours, cell trafficking in the retinal vasculature was analyzed by SLO. Retinal images were recorded for 15 minutes, and then mice were injected intravenously with 30 μg/mouse of rat anti–mouse antibody, and recording continued for a further 20 minutes. Data were then compared before and after antibody treatment. (A) Recirculation of adoptively transferred cells was expressed as the number of transferred EGFP monocytes detected in the same section of the retinal vessel before and after antibody infusion. (B) Rolling efficiency, expressed as the percentage of rolling fluorescent cells among the total number of fluorescent cells entering a venule before and after antibody infusion. (C) Sticking efficiency, expressed as the percentage of fluorescent monocytes within the same venule that remained adherent for at least 20 seconds. *P < .05; **P < .01; Student paired t test; n was at least 16 vessels from 3 mice.
After baseline measurements were recorded, the role of specific adhesion molecules was tested by infusing blocking mAb intravenously and imaging was continued for a further 15 to 20 minutes. CD62L and CD44, the hyaluronan (HA) receptor, are 2 major adhesion molecules on monocytes, and treatment with 30 μg/mouse of anti-CD62L mAb MEL-14 or anti-CD44 mAb IM7 had an immediate and dramatic effect (P < .01) on the numbers of circulating monocytes passing through the retinal vessels. CD44 mAb removed virtually all cells from the circulation, and rolling and sticking efficiencies were effectively reduced to nil. In some CD62L mAb–treated mice a few EGFP+ cells continued to circulate (5.8 ± 1.9 cells/min) but with significantly reduced rolling efficiency (5.1% ± 7.9%; P < .01) and sticking efficiency (P < .01). In contrast, rat IgG isotype control Ab or LFA-1 mAb had no significant effect on EGFP+ monocyte numbers in the circulation or on their rolling and sticking efficiency within the inflamed vessels (Figure 1).
CD62P (P-selectin) is up-regulated on retinal venules, the principal sites of leukocyte adhesion and diapedesis in EAU, at the time of blood-retina barrier breakdown and leukocyte infiltration of the retina.16 We therefore also examined the effect of blocking PSGL-1, a ligand for both P- and E-selectin, which was shown to mediate monocyte or platelet aggregations, secondary tethering, and integrin activation (Figure 1).30 Treatment with PSGL-1 mAb had no significant effect on circulating EGFP+ cell numbers, but rolling efficiency was significantly reduced (P < .05). In this group PSGL-1 mAb also had an overall significant effect on sticking efficiency (P < .05), but this was an “all or none” effect. This could reflect a threshold effect, indicating that in some mice PSGL-1–independent molecular receptors were involved, possibly linked to hemodynamic parameters (less severe disease and higher shear stress within the vessel) or tyrosine sulfation of the L-selectin ligand or both.31
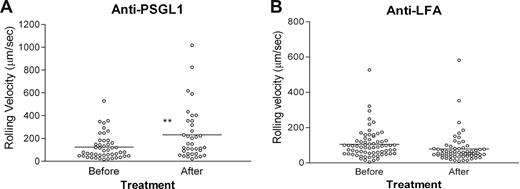
In groups in which mAb treatment did not deplete circulating EGFP+ monocytes, rolling velocity was measured on inflamed endothelium (Figure 2). The rolling velocity was recorded for 15 minutes before infusion of 30 μg of PSGL-1 mAb or LFA-1 mAb. Recording in the same vessels was continued for another 20 minutes. Average rolling velocity of EGFP+ monocytes in untreated or LFA-1 mAb–treated inflamed vessels was 124.8 (± 16.3) μm/s, and this was significantly increased in PSGL-1–treated mice (231.4 ± 38.4 μm/s; P < .01; Figure 2A), consistent with dominant PSGL-1–dependent interaction between monocytes and endothelium with residual, possibly CD62L dependent, faster rolling interactions occurring when PSGL-1 N-terminus is blocked.32,33 Our results are also consistent with previous in vitro observations of significantly faster CD62L-dependent rolling of neutrophils compared with P- or E-selectin–dependent rolling.11 These experiments were performed under high shear stress (20-30 dyn/cm2) equivalent to that found in EAU venules17 ; however, the velocity of PSGL-1–blocked monocyte rolling observed in the inflamed retinal venules here (231.4 ± 38.4 μm/sec) is approximately twice the velocity of CD62L-dependent neutrophil rolling observed in vitro by Puri et al.11
Monocyte rolling in inflamed retinal vessels is increased by blocking antibody to PSGL-1 but not LFA-1. Freshly isolated EGFP bone marrow–derived monocytes (8 × 106; A,B) were injected intravenously into mice immunized 21 to 24 days previously with peptide to induce EAU. After 48 hours, cell trafficking in the retinal vasculature was analyzed by SLO. Retinal images were recorded for 15 minutes, and then mice were injected intravenously with 30 μg/mouse of rat anti–mouse antibody to PSGL-1 (A) or LFA-1 (B) and recording continued for a further 20 minutes. Rolling velocity of transferred EGFP-expressing monocytes or T cells expressed as micrometers per second was calculated as described in “In vivo monocyte trafficking using scanning laser ophthalmoscopy” and for monocytes; data were compared before and after antibody treatment. The horizontal bar indicates the median value. *P < .05; **P < .01; Student paired t test; n was at least 36 randomly chosen rolling cells in venules of 3 mice.
Monocyte rolling in inflamed retinal vessels is increased by blocking antibody to PSGL-1 but not LFA-1. Freshly isolated EGFP bone marrow–derived monocytes (8 × 106; A,B) were injected intravenously into mice immunized 21 to 24 days previously with peptide to induce EAU. After 48 hours, cell trafficking in the retinal vasculature was analyzed by SLO. Retinal images were recorded for 15 minutes, and then mice were injected intravenously with 30 μg/mouse of rat anti–mouse antibody to PSGL-1 (A) or LFA-1 (B) and recording continued for a further 20 minutes. Rolling velocity of transferred EGFP-expressing monocytes or T cells expressed as micrometers per second was calculated as described in “In vivo monocyte trafficking using scanning laser ophthalmoscopy” and for monocytes; data were compared before and after antibody treatment. The horizontal bar indicates the median value. *P < .05; **P < .01; Student paired t test; n was at least 36 randomly chosen rolling cells in venules of 3 mice.
Circulating monocyte depletion by CD62L mAb MEL-14 and CD44 mAb IM7 is inflammation dependent
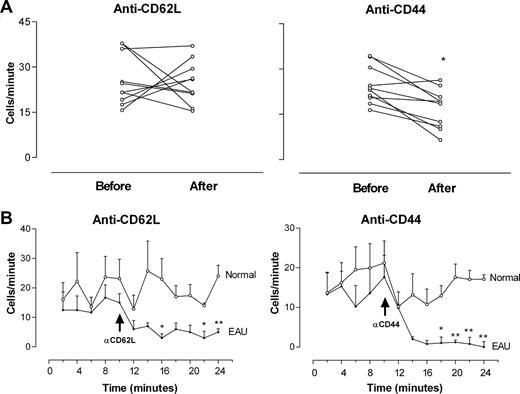
Inflammatory processes impose activating phenotypes on both endothelium and leukocytes; so to identify antibody-dependent clearance mechanisms as a reason for the sudden loss of circulating EGFP+ monocytes after treatment with MEL-14 and IM7 mAbs, we examined the effect of these antibodies on the recirculation of monocytes through the retina in normal mice in comparison to EAU mice. Figure 3A,B shows that adoptively transferred EGFP monocytes recirculated through the normal retina at approximately the same rate (25.7 ± 2.7 cells/min) as in inflamed EAU retinas (Figure 1). This value was not reduced after infusion of CD62L mAb (24.8 ± 2.23 cells/min); however, in CD44 mAb–treated normal mice a significant reduction in recirculating monocytes was observed 2 to 5 minutes after infusion (from 24.2 ± 2 to 16.4 ± 2.1 cells/min; P < .05). Infusion of mAb can temporarily sequester target cells within tissues such as the lung, and, when SLO measurements of recirculating cells were recorded over time,a sudden dip in EGFP monocytes passing through the retina was seen in both control and EAU mice 2 minutes after mAb infusion (Figure 3C,D). This was a temporary effect in normal mice, because the rate of recirculating EGFP monocytes increased back to control levels after 12 minutes. In EAU mice the numbers of recirculating monocytes in both CD62L and CD44 mAb–treated EAU mice continued to fall and remained profoundly reduced compared with controls (P < .01 in both groups), showing that the depletion of circulating monocytes observed in EAU mice was inflammation specific.
Depletion of circulating EGFP monocytes in the retinal vasculature by blocking mAb CD62L and CD44 is inflammation dependent. Freshly isolated EGFP bone marrow–derived monocytes (8 × 106) were injected intravenously into control mice or mice immunized 21 to 24 days previously with peptide to induce EAU. After 48 hours, cell trafficking in the retinal vasculature was analyzed by SLO. Retinal images were recorded for 10 minutes. Mice were then injected intravenously with 30 μg/mouse of rat anti–mouse antibody and recording continued for a further 20 minutes. Cells per minute passing through the same section of retinal vessel were compared before and after antibody treatment in control mice (A). (B) Recirculation of adoptively transferred cells was analyzed at 2-minute intervals (expressed as the number of transferred EGFP monocytes detected in the same section of retinal vessel) of control and EAU mice before and after antibody infusion. *P < .05; **P < .01; Student paired t test; n was at least 16 vessels from 3 mice in each group. Error bars represent SEM.
Depletion of circulating EGFP monocytes in the retinal vasculature by blocking mAb CD62L and CD44 is inflammation dependent. Freshly isolated EGFP bone marrow–derived monocytes (8 × 106) were injected intravenously into control mice or mice immunized 21 to 24 days previously with peptide to induce EAU. After 48 hours, cell trafficking in the retinal vasculature was analyzed by SLO. Retinal images were recorded for 10 minutes. Mice were then injected intravenously with 30 μg/mouse of rat anti–mouse antibody and recording continued for a further 20 minutes. Cells per minute passing through the same section of retinal vessel were compared before and after antibody treatment in control mice (A). (B) Recirculation of adoptively transferred cells was analyzed at 2-minute intervals (expressed as the number of transferred EGFP monocytes detected in the same section of retinal vessel) of control and EAU mice before and after antibody infusion. *P < .05; **P < .01; Student paired t test; n was at least 16 vessels from 3 mice in each group. Error bars represent SEM.
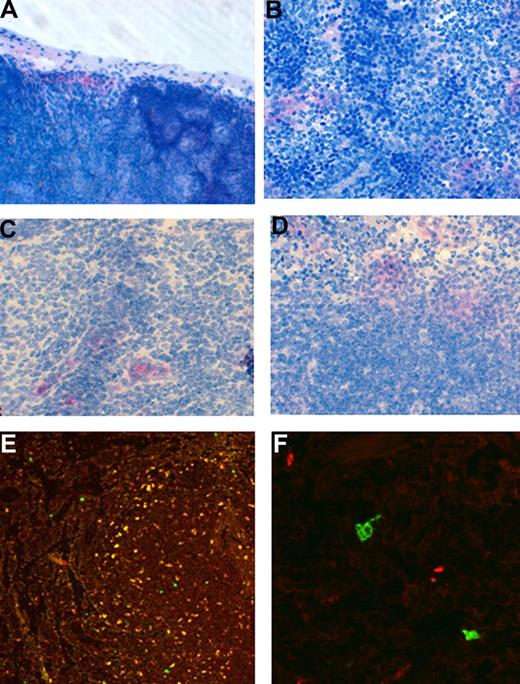
Differential trafficking of monocytes to lymphoid tissues in EAU but not control mice treated with CD62L mAb MEL-14 or CD44 mAb IM7
To determine the fate of adoptively transferred monocytes, organs and tissues of groups of recipient EAU and normal control mice were examined with flow cytometry and microscopy 30 minutes and 24 hours after a single infusion of mAb and at 72 hours after 3 infusions of mAb. Figure 4A to C shows that 30 minuets after infusion with MEL-14 anti-CD62L antibody adoptively transferred monocytes were found dispersed throughout the venous sinuses of the spleen but were restricted to the subcapsular sinuses of lymph nodes, whereas in mice treated with IM7 anti-CD44 antibody monocytes had entered the node and were present within the medullary sinus and in clusters around vessels. In the spleen, EGFP+ monocytes were also found throughout the venous sinuses and red pulp and notably in large numbers clustered around and in the marginal zones of the lymphoid follicles (Figure 4C). This rapid accumulation of transferred monocytes within the lymphoid tissues, particularly in IM7-treated mice, provides an explanation for the sudden loss of circulating fluorescent cells in the retina observed by SLO (Figures 1,3). After 24 hours these relatively large accumulations of EGFP-expressing cells were no longer evident; only a few scattered cells were detectable using the APAAP technique. Using flow cytometry, we detected a drop from 88% (± 2.5%) to 55% (± 18%) in CD11b+ monocytes in the blood of the anti-CD44 mAb–treated group at 30 minutes after infusion as predicted from SLO data (Figures 1,3), but this was not reflected in statistically significant increases or decreases of CD11b or EGFP+CD11b monocytes in the spleen, (IgG control, 0.08% ± 0.04%; CD62L, 0.07% ± 0.04%; CD44, 0.04% ± 0.005%).
Localization of adoptively transferred monocytes in lymph node and spleen differs with antibody treatment. Freshly isolated EGFP bone marrow–derived monocytes (8 × 106) were injected intravenously into control mice or mice immunized 21 to 24 days previously with peptide to induce EAU. Mice were then treated with mAb for up to 3 days. Groups of mice were killed at 30 minutes and 24 hours after a single treatment or after 3 treatments. Tissue samples were then snap frozen, cryosectioned, and immunostained using the APPAP technique (A-D) for the presence of EGFP+ cells using a specific antibody, or examined by confocal microscopy (E,F). Original magnification ×400 (A-D); ×200 (E); ×640 (F).
Localization of adoptively transferred monocytes in lymph node and spleen differs with antibody treatment. Freshly isolated EGFP bone marrow–derived monocytes (8 × 106) were injected intravenously into control mice or mice immunized 21 to 24 days previously with peptide to induce EAU. Mice were then treated with mAb for up to 3 days. Groups of mice were killed at 30 minutes and 24 hours after a single treatment or after 3 treatments. Tissue samples were then snap frozen, cryosectioned, and immunostained using the APPAP technique (A-D) for the presence of EGFP+ cells using a specific antibody, or examined by confocal microscopy (E,F). Original magnification ×400 (A-D); ×200 (E); ×640 (F).
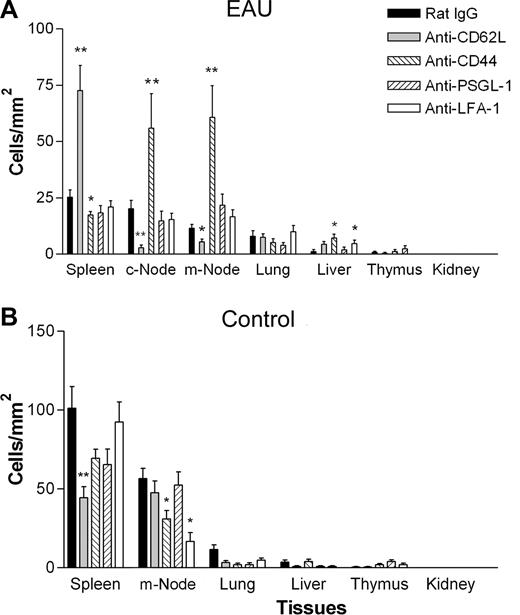
Using a more-sensitive detection technique of confocal microscopy to follow the fate of EGFP+ cells in the tissues, we were able to show significant accumulations of adoptively transferred monocytes in lymphoid tissues of mice with EAU after 3 days of treatment with CD62L or CD44 mAb compared with control IgG, LFA-1, or PSGL-1 mAbs (Figure 4D,E and Figure 5). Sectioning of lymph nodes, spleen, and other tissues showed that in EAU mice treated with CD62L mAb, EFGP+ cells had preferentially homed to the spleen (72.6 ± 13 cells/mm2; P < .01), with significantly reduced numbers being found in the cervical lymph nodes (c-LNs; 2.8 ± 1.2 cells/mm2; P < .01) and mesenteric lymph nodes (m-LNs; 5.6 ± 1.32 cells/mm2; P < .05) compared with IgG controls. Conversely, in mice treated with CD44 mAb IM7, EFGP+ cells preferentially homed to the lymph nodes (c-LN, 55.9 ± 17.3 cells/mm2; P < .05; m-LN, 60.7 ± 16.2 cells/mm2; P < .01), with reduced numbers found in the spleen (17.5 ± 1.7 cells/mm2; P < .05) compared with IgG controls. In normal mice, (Figure 5B) treatment with blocking mAb actually reduced the numbers of monocytes in the lymphoid tissues. These observations are consistent with a role for CD62L and CD44 in directing circulating monocytes to other nonlymphoid tissues such as bone marrow in the absence of inflammation.34 Very few cells were found in the lungs or livers in any of the groups, indicating that cellular clearance through the reticuloendothelial system was not a significant event with any of the mAbs tested.
Adoptively transferred monocytes in anti-CD62L–treated mice home to the spleen, and in anti-CD44–treated mice monocytes home to the lymph nodes in EAU but not control mice. Effect of monoclonal antibody treatment on trafficking of adoptively transferred monocytes into secondary lymphoid and other tissues in control and EAU mice by confocal microscopy. Freshly isolated EGFP bone marrow–derived monocytes (8 × 106) were injected intravenously into mice immunized 21 to 24 days previously with peptide to induce EAU (A) or control mice (B). Mice were then injected intravenously with 30 μg of control IgG or blocking antibody per mouse per day for 3 days. Tissue samples were then snap frozen and cryosectioned, and the numbers of EGFP+ cells present in the tissues were enumerated (A,B). c-Node indicates cervical lymph node; m-Node, mesenteric lymph node. *P < .05; **P < .01; Student paired t test; n was at least 12 randomly chosen times 20 fields of view in tissue sections from 3 mice per group. Error bars represent SEM.
Adoptively transferred monocytes in anti-CD62L–treated mice home to the spleen, and in anti-CD44–treated mice monocytes home to the lymph nodes in EAU but not control mice. Effect of monoclonal antibody treatment on trafficking of adoptively transferred monocytes into secondary lymphoid and other tissues in control and EAU mice by confocal microscopy. Freshly isolated EGFP bone marrow–derived monocytes (8 × 106) were injected intravenously into mice immunized 21 to 24 days previously with peptide to induce EAU (A) or control mice (B). Mice were then injected intravenously with 30 μg of control IgG or blocking antibody per mouse per day for 3 days. Tissue samples were then snap frozen and cryosectioned, and the numbers of EGFP+ cells present in the tissues were enumerated (A,B). c-Node indicates cervical lymph node; m-Node, mesenteric lymph node. *P < .05; **P < .01; Student paired t test; n was at least 12 randomly chosen times 20 fields of view in tissue sections from 3 mice per group. Error bars represent SEM.
Effect of mAb treatment during 72 hours on monocyte number, phenotype, and migration into inflamed retina
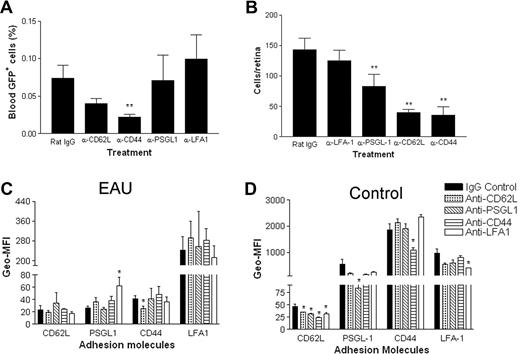
Monocytes as well as retinal antigen-specific T cells are required for full expression of EAU, and mechanisms for monocyte migration into peripheral inflamed tissues rather than lymphoid tissues are likely to differ.14 We therefore examined the effect of blocking mAb on the migration of adoptively transferred monocytes into inflamed retina. Mice with EAU were treated with 30 μg Ig/mouse per day for 3 days after infusion of donor EGFP+ monocytes. After 72 hours, blood samples were taken for fluorescence-activated cell sorting (FACS) analysis, the mice were killed, and retinal whole mounts were prepared for confocal microscopy. Figure 6A shows that 72 hours after injection, small numbers of transferred EGFP+ cells continued to circulate despite significant sequestration of cells in the lymphoid tissues of EAU mice treated with CD62L or CD44 mAbs. EGFP+ monocyte numbers in the blood of CD62L- and CD44 mAb–treated mice were depleted compared with control Ig–treated mice, but this was significant only for the CD44 mAb–treated group (P < .01). Treatment with CD62L, CD44, and PSGL-1 mAbs also significantly reduced the numbers of infiltrating EGFP+ monocytes in the retinas of EAU mice compared with isotype- and LFA-1 mAb–treated control groups (P < .01), confirming that CD11a also has no role in inflammatory monocyte trafficking in vivo (Figure 6B).
Blocking antibodies to CD62L, CD44, and PSGL-1 but not LFA-1 significantly reduce the numbers of adoptively transferred monocytes entering inflamed retina. Freshly isolated EGFP bone marrow–derived monocytes (8 × 106) were injected intravenously into mice immunized 21 to 24 days previously with peptide to induce EAU. Mice were injected intravenously with 30 μg of control IgG or blocking antibody per mouse for 3 days. Numbers of circulating cells remaining in the blood were analyzed by flow cytometry (A). Transferred cells that had infiltrated the retinas were then counted by confocal microscopy of retinal whole mounts (B); **P < .01 compared with IgG control; Student t test; n was 12. Density of expression of adhesion molecules analyzed in both EAU (C) and control mice (D) after mAb treatment expressed as the geometric mean fluorescent index (Geo-MFI). *P < .05; **P < .01 compared with IgG control; Student t test; n was 3. Error bars represent SEM.
Blocking antibodies to CD62L, CD44, and PSGL-1 but not LFA-1 significantly reduce the numbers of adoptively transferred monocytes entering inflamed retina. Freshly isolated EGFP bone marrow–derived monocytes (8 × 106) were injected intravenously into mice immunized 21 to 24 days previously with peptide to induce EAU. Mice were injected intravenously with 30 μg of control IgG or blocking antibody per mouse for 3 days. Numbers of circulating cells remaining in the blood were analyzed by flow cytometry (A). Transferred cells that had infiltrated the retinas were then counted by confocal microscopy of retinal whole mounts (B); **P < .01 compared with IgG control; Student t test; n was 12. Density of expression of adhesion molecules analyzed in both EAU (C) and control mice (D) after mAb treatment expressed as the geometric mean fluorescent index (Geo-MFI). *P < .05; **P < .01 compared with IgG control; Student t test; n was 3. Error bars represent SEM.
Anti-CD44 treatment with a mAb such as IM7 blocks HA binding function and was shown to be effective in blocking T-cell traffic and ameliorating inflammation in a number of animal models, including EAU.19 However, given the importance of soluble (shed) as well as membrane-bound CD44 in regulating cell function, inhibition of leukocyte recirculation and infiltration of the inflammatory site may involve more than simple blocking of HA function. Other mechanisms proposed include regulation of function through activation and sulfation of the adhesion molecule or changes in cell-surface expression through down-regulation or shedding. Previous studies have indicated that T-cell trafficking is controlled by surface expression of CD62L and CD44. High surface expression of CD62L, characteristic of naive T cells, is lost during inflammation as CD44 is up-regulated. Equally, CD62L is less important in inflammatory T-cell recruitment than in lymph node homing. We therefore analyzed the expression of CD62L, CD44, PSGL-1, and LFA-1 on circulating EGFP monocytes recovered from the circulation of EAU mice (Figure 6C) and normal control mice (Figure 6D) after 3 days of mAb treatment. CD44 expression (Geo-MFI) was very much lower on monocytes from all groups of EAU mice than with normal mouse groups, suggesting that monocyte transmembrane CD44 was lost during inflammation. Treatment with the mAbs had relatively minor effects on the surface expression of the adhesion molecules examined. Statistical analysis showed some significant differences. Treatment with CD62L mAb reduced CD44 expression and treatment with PSGL-1 mAb increased LFA-1 expression on monocytes from EAU but not control mice. However, whether these changes were sufficient to exert physiologic effects is in doubt because Geo-MFI changes did not mirror in vivo functional changes observed in earlier experiments.
Discussion
Differential trafficking is known to be required for the localization of effector leukocyte subsets, either to sites of inflammation or to lymphoid tissues, and tissue-specific chemokine expression and receptor signaling was identified as providing specificity to endothelial traffic signals, raising the concept of molecular codes specific for particular disease processes.5 Previously, we have shown that a permissive endothelium alone is not sufficient to recruit monocytes from the circulation,3 but data we present here support the concept of differential monocyte trafficking in vivo in steady state and inflammation, with specific roles identified for CD62L and CD44 in maintaining monocytes within the circulation during inflammation. Blockade of CD62L caused retention of monocytes within the spleen, whereas blockade of CD44 caused retention of monocytes within the lymph nodes. Surprisingly, this effect was inflammation specific because blockade of CD62L and CD44 had no apparent effect on the ability of monocytes to recirculate in normal mice. These observations are also consistent with a role for CD62L and CD44 in directing circulating monocytes to other nonlymphoid tissues such as bone marrow in the absence of inflammation.34 Identifying signals controlling monocyte differentiation and function in inflammation versus steady state will be important for identifying disease-associated therapeutic targets that do not affect normal protective immunity.
For monocytes, 2 major subsets have been identified with differing chemokine and adhesion molecule expression that govern trafficking potential to inflammatory sites.6 Recently, we showed that acquisition of the inflammatory monocyte phenotype is a time-limited property of monocytes independent of external inflammatory signals. Bone marrow–derived monocytes adoptively transferred into normal mice recirculated freely for at least 72 hours, and viable monocytes could be found in both lymph nodes and spleen throughout the sampling period.3 When injected into mice with EAU, adoptively transferred monocytes did not migrate into the inflamed retinal tissue until 24 to 48 hours after transfer, despite repeated trafficking through inflamed retinal vessels. This time frame coincided with the acquisition of maximum rolling efficiency and maximal expression of CCR-2 and LFA-1 by monocytes. These data indicate that an inflamed endothelium and associated chemokine and cytokine microenvironment are not sufficient to recruit circulating monocytes, and new monocytes leaving the bone marrow require in vivo conditioning over a defined time period before switching to a phenotype that enables homing to inflammatory sites. In the experiments described here, we have included a 48-hour in vivo conditioning period to allow inflammatory monocyte maturation or differentiation before taking SLO measurements or administering mAb treatments to examine mechanisms controlling monocyte recirculation and homing to an inflammatory site in vivo.
The main physiologic function of selectins is to allow high-affinity rolling interactions between leukocytes and the endothelium under flow, mediating signal transduction and firm adhesion.20,35 For these experiments we examined the role of CD62L because it is expressed by most monocytes and has been implicated in mediating inflammatory monocyte migration.6 PSGL-1 was also chosen as the interaction between P- and E-selectin and PSGL-1 induces changes in integrin function and increased adhesion of monocytes to endothelium in vitro,30 but to date there is no record of its role in mediating monocyte function in delayed-type hypersensitivity (DTH) inflammation in vivo. CD44 was also included in this study because both CD44 and its ligand hyaluronan are up-regulated in the EAU eye,16 and, although implicated in lymphocyte function in both in the uveitic eye and other DTH models, no specific role for CD44 in monocyte trafficking has been identified. Antibodies to CD11a were included to confirm that LFA-1 has no role in monocyte adhesion and diapedesis in vivo in our model, and it provided a useful additional negative control for our assays. VLA-4 has been implicated in monocyte trafficking in other models, but inclusion of this integrin was considered beyond the scope of this study because VLA-4 was shown not be involved in monocyte attachment under flow,36 and is not specifically up-regulated in retinal venules in EAU.16
Our experiments show that, although PSGL-1 has a partial role in regulating monocyte migration into the inflamed retina, CD62L and CD44 were absolutely required for effective monocyte trafficking to the retina in EAU. The accumulation of monocytes in the spleens of CD62L mAb–treated EAU but not control mice is unexplained. Immunostaining of lymph nodes and spleens from normal and EAU mice showed no obvious differences in the expression of CD44 or CD62L (data not shown). This is perhaps not surprising because in this organ-specific inflammatory model, the focus of inflammation is limited to the retina of the eye. The restriction of EGFP+ cells to the subcapsular region of the lymph nodes in MEL-14–treated mice is consistent with a defect in the ability of these cells to traverse high endothelial venule (HEVs), particularly during inflammation. In contrast, the spleen has no HEVs so in MEL-14–treated animals monocytes were able traffic freely through this tissue.
MEL-14 mAb at high concentrations (100 μ/mouse) were reported to affect cell trafficking, particularly of lymphocytes,29 but a dose of 30 μg/mouse per day appeared to have no significant effect on monocyte trafficking in our control mice. Sialomucin (CD43) as well as CD62L is involved in monocyte migration into lymph nodes from the blood by HEVs,14 and our data would indicate that CD62L is not necessary or largely redundant in monocyte trafficking in the normal mouse, consistent with data from L-selectin–deficient mice.37 The inflammation-specific nature of sequestering would imply that monocyte-expressed CD62L or its endothelial cell–expressed ligands have undergone modification in response to the EAU inflammatory response in the host, allowing the adhesive interactions necessary for monocyte adhesion and migration.35 Our previous observation that rolling is an acquired characteristic of adoptively transferred CD62L+ monocytes in mice with EAU and that inflamed endothelium alone does not permit monocyte rolling3 would suggest that it is monocyte-expressed ligand changes as well as endothelial cell–expressed ligand changes that are critical for rolling. Flow cytometric analysis of CD62L expression on monocytes retrieved from the circulation of normal and untreated EAU mice showed no significant differences in CD62L expression (percentage and geo-MFI; data not shown), indicating that factors other than receptor density may determine L-selectin–dependent monocyte rolling.
CD44-deficient mice also show no obvious immunologic defects until challenged with infection or inflammation,38 and posttranslational modification of CD44 can alter function at different stages of disease.18 Our data are therefore consistent with previous studies that have suggested that CD44 is not important for normal T-cell trafficking to lymph nodes and that physiologic changes associated with inflammation alter hierarchy of adhesion molecule function to allow selective recruitment of T cells to lymphoid or inflammatory site, and is the first indication that CD44 also has a specific role in monocyte trafficking during inflammation. Although distribution of monocytes in the MEL-14–treated mice can be rationalized as an effect on MEL-14–dependent trafficking at the HEVs, the reduced numbers of EGFP+ cells in the spleens are more difficult to understand. CD44 clearly has a role in trafficking, particularly during inflammation, and monocytes were able to enter the spleen rapidly after adoptive transfer in IM7-treated mice. The localization of large numbers of these cells both with the red pulp and around the marginal zone at 30 minutes after transfer and later loss in inflammation could have 2 explanations: (1) the cells were targeted for clearance; (2) the cells became sequestered in another compartment (such as the bone marrow). We believe the second explanation is more likely because IM7 is widely used as a blocking antibody in functional studies, cells were not found in the lungs or liver in any number, and the simple clearance of antibody opsonized cells would be independent of inflammation.
In conclusion, we demonstrate for the first time that the mechanisms controlling monocyte recirculation through peripheral and lymphoid tissues by the blood alters during inflammation, and we also show that the effect is systemic. Differential trafficking is known to be required for the selective recruitment of naive or effector T cells either to sites of inflammation or to lymphoid tissues.37,38 Our data suggest that differential trafficking of monocytes also occurs during inflammation and that regulation of ligand density or posttranslational modification of CD44 and CD62L may be involved. Thus, posttranslational modification of CD44 is required to maintain monocytes within the circulation during inflammation and mediate homing to the inflammatory site, and CD62L may be less important during inflammation but necessary for recruitment to the lymph node. PSGL-1 was also shown to be involved in monocyte rolling and recruitment to an inflammatory site. The SLO data also support our earlier hypothesis that monocytes must undergo maturation and differentiation in the circulation before they acquire the ability to roll on inflamed endothelium and that changes in monocyte receptor function are crucial events that enable inflammatory monocytes to traffic to the site of inflammation. To date there is little information on monocyte trafficking in vivo during inflammation. This study highlights the dynamic relation between CD44 and CD62L in controlling monocyte trafficking in vivo. Understanding the mechanisms controlling differential trafficking of immature and inflammatory monocytes as well as naive and effector T cells will be required for effective control of chronic inflammation in the future.
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by The Wellcome Trust
Wellcome Trust
Authorship
Contribution: J.L. directed the research and wrote the paper; A.M. operated the SLO analysis; H.X. carried out the SLO analysis, prepared the figures, and contributed to the manuscript; I.C. assisted with experimental design and contributed to the manuscript; R.D. prepared and scored the tissue sections and provided technical support.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Janet Liversidge, University of Aberdeen Institute of Medical Sciences, Foresterhill, Aberdeen, AB25 2ZD United Kingdom; e-mail: j.liversidge@abdn.ac.uk.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal