The diversity of factor VIII (fVIII) C2 domain antibody epitopes was investigated by competition enzyme-linked immunosorbent assay (ELISA) using a panel of 56 antibodies. The overlap patterns produced 5 groups of monoclonal antibodies (MAbs), designated A, AB, B, BC, and C, and yielded a set of 18 distinct epitopes. Group-specific loss of antigenicity was associated with mutations at the Met2199/Phe2200 phospholipid binding β-hairpin (group AB MAbs) and at Lys2227 (group BC MAbs), which allowed orientation of the epitope structure as a continuum that covers one face of the C2 β-sandwich. MAbs from groups A, AB, and B inhibit the binding of fVIIIa to phospholipid membranes. Group BC was the most common group and displayed the highest specific fVIII inhibitor activities. MAbs in this group are type II inhibitors that inhibit the activation of fVIII by either thrombin or factor Xa and poorly inhibit the binding of fVIII to phospholipid membranes or von Willebrand factor (VWF). Group BC MAbs are epitopically and mechanistically distinct from the extensively studied group C MAb, ESH8. These results reveal the structural and functional complexity of the anti-C2 domain antibody response and indicate that interference with fVIII activation is a major attribute of the inhibitor landscape.
Introduction
Approximately 30% of patients with hemophilia A develop detectable anti–factor VIII (fVIII) antibodies in response to infusions of fVIII.1,,–4 The immune response to fVIII currently is the most significant complication in the management of patients with hemophilia A. In addition, autoimmune antibodies to fVIII can develop in nonhemophiliacs, producing acquired hemophilia A, which frequently produces life- or limb-threatening bleeding
FVIII contains a domain sequence designated A1-A2-B-ap-A3-C1-C2. It circulates as an A1-A2-B/ap-A3-C1-C2 heterodimer bound to von Willebrand factor (VWF). During the activation of fVIII by thrombin, the B domain and the light chain activation peptide, ap, are released, and cleavage between the A1 and A2 domains occurs, producing an A1/A2/A3-C1-C2 heterotrimer.5 Factor Xa also activates fVIII and catalyzes these cleavages along with additional cleavages in the A1 and A2 domains and a slow cleavage in the A3 domain. Activated fVIII (fVIIIa) functions as a cofactor for factor IXa during intrinsic pathway factor X activation on phospholipid membrane surfaces. Thus, fVIII inhibitors potentially can act by interfering with the activation of fVIII, dissociation of fVIII from VWF, inhibition of fVIIIa function, or assembly in the intrinsic fXase complex.
In either congenital or acquired hemophilia A, the majority of inhibitory antibodies are directed at either the 40-kDa A2 or the 15-kDa C2 domains of fVIII.6 Initially, anti-C2 antibodies have been identified that inhibit the binding of fVIII to negatively charged phospholipid membranes, which is critical for the interaction of fVIIIa with platelet or monocyte surfaces.7 However, the C2 domain also contributes to the binding of fVIII to VWF, thrombin, and factor Xa.8,–10 Phospholipid membranes and VWF bind to at least partially overlapping sites on fVIII, because their binding to fVIII is mutually exclusive.11,–13 Consistent with this observation, anti-C2 antibodies have been identified that inhibit the binding of fVIII to both phospholipid membranes and VWF.14,–16 However, these sites are not identical because the ap region makes a major contribution to the interaction of fVIII with VWF, but not phospholipid.17,18 In addition, although most antibodies that inhibit phospholipid binding also inhibit VWF binding, differential inhibition has been observed in some cases.19 Because VWF is not necessary for the procoagulant function of fVIII, per se, antibodies that solely inhibit the binding of fVIII to VWF might not have inhibitory activity in in vitro coagulation assays. However, they could be pathogenic by decreasing the circulatory lifetime of fVIII, which decreases when it is not bound to VWF.
Anti-C2 antibodies also have been identified that interfere with the activation of fVIII. A murine anti-C2 monoclonal antibody (MAb), ESH8, inhibits fVIII procoagulant function, but does not block the binding of fVIII to phospholipid.19 ESH8 does not inhibit any of the cleavages of fVIII catalyzed by thrombin, but slows the dissociation of cleaved fVIII from VWF.20 However, ESH8 also inhibits the cleavage of the fVIII light chain at Arg1689 by factor Xa.9 In addition, an inhibitory human anti-C2 polyclonal IgG, A-FF, has been identified that inhibits cleavage of this site by thrombin.10 Cleavage at Arg1689 is necessary for the dissociation of fVIII from VWF, which in turn is necessary for fVIII to bind to phospholipid.21,22
A 1.5-Å X-ray structure of the human fVIII C2 domain reveals a β-sandwich core with 3 hydrophobic protrusions, consisting of 2 β-hairpins containing Met2199/Phe2200 and Leu2251/Leu2252, respectively, and a loop containing Val2223.23 These solvent-exposed hydrophobic residues project from a ring of positively charged residues, suggesting that this region is the binding site for negatively charged phospholipid membranes. Consistent with this, an X-ray structure of a complex between the C2 domain and the Fab fragment of a human antihuman C2 MAb, BO2C11, which inhibits the binding of fVIII to phospholipid and VWF, showed Fab contacts with Met2199, Phe2200, Val2223, Leu2251, and Leu2252.24 In addition, site-directed mutagenesis of the β-hairpins has been associated with decreased binding of fVIII to BO2C11 and human polyclonal anti-C2 IgG,25 as well as phospholipids and VWF.26
These studies indicate that anti-C2 antibodies are functionally complex with multiple potential pathogenic mechanisms of action. However, apart from the phospholipid-binding site in the C2 domain, little is known about the epitopes recognized by other anti-C2 antibodies and the functional correlation of antibody binding to these epitopes. In the present study, 55 murine anti-C2 hybridoma antibodies and BO2C11 were studied to characterize the structural and functional diversity of C2 epitopes.
Materials and methods
Materials
DMEM/F12 (11330-032), fetal bovine serum (FBS), and penicillin/streptomycin were purchased from Invitrogen (Carlsbad, CA). Alcian blue was purchased from Sigma-Aldrich (St Louis, MO). Immobilized protein A, sulfo-NHS-LC-biotin, Tween-80, and Handee minispin columns were purchased from Pierce Biotechnology (Rockford, IL). Immulon-1B enzyme-linked immunosorbent assay (ELISA) plates were purchased from Thermo Fisher Scientific (Waltham, MA). Streptavidin–alkaline phosphate conjugate was purchased from Jackson ImmunoResearch (West Grove, PA). MAbs ESH-4 and ESH-8 were purchased from American Diagnostica (Stamford, CT). Pooled citrated normal plasma and factor VIII–deficient plasma were obtained from George King Biomedical (Overland Park, KS). Phosphatidylcholine/phosphatidylserine (PCPS) (75/25, wt/wt) vesicles were prepared as described previously.27 Human B domain–deleted (BDD) fVIII and fVIII mutants M2199L/F2200L, V2223A/K2227E, M2199L/F2200L/L2251V/L2252F, F2196L, and K2227E were prepared as described previously.25 Recombinant full-length human fVIII was a gift from Baxter Biosciences (Duarte, CA). An Epstein Barr virus–immortalized human B-cell line expressing BO2C1116 was a generous gift from Dr Marc Jacquemin at the Katholieke Universiteit (Leuven, Belgium). All other materials were reagent grade or are described in the cited literature.
Anti-fVIII MAbs from anti-fVIII hybridomas
The murine anti-fVIII C2 domain MAbs were obtained from splenic B-cell hybridomas that have been described previously.28 Briefly, 9- to 12-week-old E16 hemophilia A mice29 partially backcrossed into a C57BL/6 background received 6 weekly tail vein injections of 10 μg/kg BDD human fVIII, followed 2 weeks later by a final injection of 25 μg/kg. Spleens were obtained 3 days after the last injection and hybridomas were produced using the method of Kohler and Milstein.30 MAbs were purified from hybridoma supernatants and biotinylated as described previously.28
MAb BO2C11 IgG was prepared from cells grown in DMEM/F12 medium supplemented with 10% FBS, 100 U/mL penicillin, and 100 μg/mL streptomycin. The cells then were grown in CD medium for expression of IgG, which was isolated by immobilized protein A–Sepharose chromatography. The purity of the IgGs was judged to be greater than 95% by SDS–polyacrylamide gel electrophoresis (PAGE). IgG con-centrations were calculated using an extinction coefficient at 280 nm of 1.37 (mg/mL)−1 cm−1.
Competition ELISA for anti-C2 antibodies
A competition sandwich ELISA using immobilized anti-C2 primary MAb, human fVIII, biotinylated anti-C2 secondary MAb, and streptavidin–alkaline phosphatase conjugate for detection was performed as described previously.28
ELISA for binding of anti-C2 antibodies to fVIII mutants
Binding of MAbs to full-length human fVIII and the fVIII mutants M2199L/F2200L, V2223A/K2227E, M2199L/F2200L/L2251V/L2252F, F2196L, and K2227E also was done using a competition ELISA. FVIII constructs were captured using a murine anti-A2 MAb, 2–76, as primary antibody, followed by addition of biotinylated anti-C2 MAbs and detection with streptavidin–alkaline phosphatase conjugate.
ELISA for inhibition by anti-C2 MAbs of binding of fVIII to phospholipid
Immulon 1B plates were pretreated with 1% Alcian blue in 3% acetic acid for 30 minutes at 37°C. Plates were washed twice with 0.15 M NaCl/20 mM HEPES/5 mM CaCl2, pH 7.4 (HBS/Ca2+), and then dried at room temperature. Plates were coated with 6 μg/mL PCPS vesicles overnight at 4°C and blocked with HBS/Ca2+/1% nonfat dry milk for 1 hour at room temperature. Dilutions of BDD human fVIII and anti-fVIII MAbs were made using HBS/Ca2+ containing 0.5% ovalbumin to prevent nonspecific adsorption. FVIII (1 nM) was mixed 1:1 with anti-C2 antibodies at concentrations ranging from 0 to 20 μg/mL and incubated for 2 hours at 37°C. After washing with HBS/Ca2+/0.05% Tween-80, samples or BDD human fVIII standards at concentrations ranging from 0 to 1 nM were added. FVIII binding to PCPS was quantitated using biotinylated anti-fVIII A1 domain antibody (MAb 2–16), streptavidin–alkaline phosphatase conjugate, and p-nitrophenylphosphate.
ELISA for inhibition by anti-C2 MAbs of binding of fVIII to VWF
Immulon 1B plates were coated overnight at room temperature with 6 μg/mL VWF in HBS/0.05% sodium azide. Plates were blocked with HBS/Ca2+/0.05% sodium azide/0.05% Tween-80/0.25% BSA for 1 hour at room temperature. Dilutions of BDD human fVIII and anti-fVIII MAbs were made using HBS/Ca2+ containing 0.25% BSA to prevent nonspecific adsorption. FVIII (1nM) was mixed 1:1 with anti-C2 antibodies at concentrations ranging from 0 to 50 μg/mL and incubated for 2 hours at 37°C. After washing with HBS/Ca2+ containing 0.05% Tween-80, samples or BDD human fVIII standards at concentrations ranging from 0 to 1 nM were added. FVIII binding to VWF was quantitated using biotinylated anti-fVIII A1 domain MAb 2–116, streptavidin–alkaline phosphatase conjugate, and p-nitrophenylphosphate.
Bethesda assay
FVIII inhibitor titers were measured with the Bethesda assay,31 using the modifications previously described.32 Pooled normal human plasma was used as the source of fVIII activity. One Bethesda unit (BU)/mL is defined as the dilution of inhibitor that produces 50% inhibition of fVIII activity. At least 8 MAb concentrations were tested in duplicate, and the resulting inhibition curve was fitted using the 4-parameter logistic equation to estimate the concentration of MAb producing 50% inhibition.
Intrinsic fXase assays for measurement of fVIIIa activity
A chromogenic substrate assay for fVIIIa activity was used based on factor X activation by an enzymatic complex consisting of factor IXa, PCPS, calcium, and limiting amounts of fVIIIa as described previously.27,33 Factor Xa formation was measured using the chromogenic substrate Spectrazyme Xa as described previously34 using a VersaMax kinetic plate reader (Molecular Devices, Sunnyvale, CA).
Results
The diversity of epitopes recognized by anti-C2 MAbs
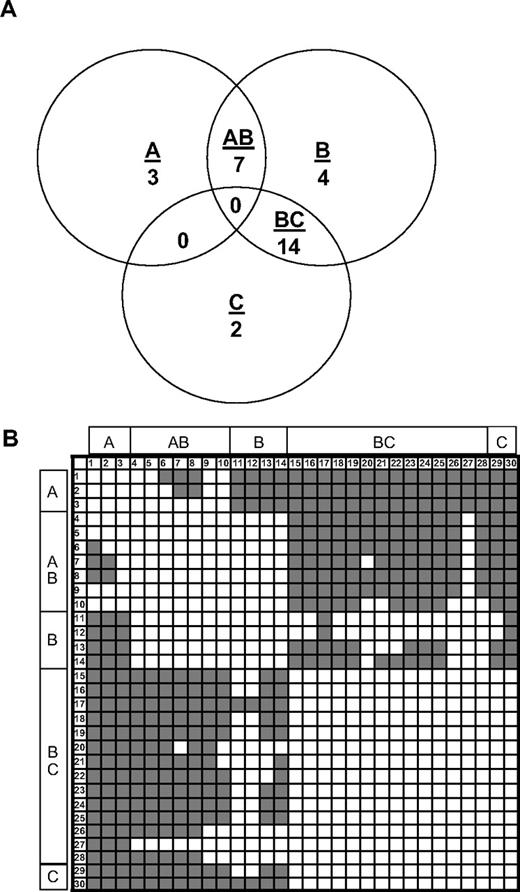
A competition ELISA was used to evaluate overlapping epitopes of a panel of 29 murine antihuman C2 MAbs and the human anti-C2 MAb, BO2C11. Full-length fVIII was bound to an immobilized primary (capture) anti-C2 MAb, followed by addition of biotinylated secondary anti-C2 MAb and detection of secondary MAb using streptavidin–alkaline phosphatase conjugate. Binding of the secondary MAb indicates that the antibody pair recognizes nonoverlapping epitopes. Competing and noncompeting MAb pairs were scored by visual inspection of the microtiter wells.28 Each MAb was used in both primary and secondary configurations. The results of this analysis yielded a basis set of 3 MAbs, ESH4, 1B5, and ESH8, which is defined as a set of MAbs that do not compete with each other for binding to human fVIII but as a group compete with the remaining 27 MAbs.
Nine (33%) of the 27 non–basis set MAbs competed with ESH4, 24 (87%) of 27 competed with 1B5, and 15 (56%) of 27 competed with ESH8. These results defined 5 groups of MAbs (Figure 1A). Group A consists of ESH4 and MAbs that compete only with ESH4. Group B consists of 1B5 and MAbs that compete only with 1B5. Group C consists of ESH8 and MAbs that compete only with ESH8. Group AB consists of MAbs that compete with 1B5 and ESH4. Group BC consists of MAbs that compete with 1B5 and ESH8. No MAbs competed with both ESH4 and ESH8, suggesting that their epitopes are physically separated on the C2 surface.
Competition ELISA of C2 MAbs. (A) Venn diagram showing the overlap patterns of 30 anti-C2 MAbs in a competition ELISA. Groups A, B, and C are defined based on overlaps with the basis set MAbs, ESH4, 1B5, and ESH8, respectively. (B) Competition matrix showing the interaction of the 30 anti-C2 antibodies. The rows represent primary MAbs, and the columns represent biotinylated secondary MAbs. Noncompeting and competing MAbs are shown in gray and white, respectively. MAb numbering is defined in Table 1.
Competition ELISA of C2 MAbs. (A) Venn diagram showing the overlap patterns of 30 anti-C2 MAbs in a competition ELISA. Groups A, B, and C are defined based on overlaps with the basis set MAbs, ESH4, 1B5, and ESH8, respectively. (B) Competition matrix showing the interaction of the 30 anti-C2 antibodies. The rows represent primary MAbs, and the columns represent biotinylated secondary MAbs. Noncompeting and competing MAbs are shown in gray and white, respectively. MAb numbering is defined in Table 1.
A matrix showing the competition pattern of all 30 MAbs is shown in Figure 1B. Noncompeting and competing MAbs are depicted as color (gray)– or noncolor (white)–producing elements, respectively. Because each antibody was used in primary and secondary configurations, the matrix is square. As expected, the diagonal elements are white, indicating that each MAb competed with itself for binding. The matrix is symmetrical (the off-diagonal elements produce a mirror image), demonstrating that the same results were obtained regardless of the configuration of the MAb pairs. Thus, there was no loss of binding due to biotinylation of the antigen-binding site or other anomalous behavior.
The matrix was interpreted assuming that the inability of the secondary (biotinylated) MAb to bind is simply due to physical overlap of its epitope with that of the primary MAb. This produced a Venn diagram that was consistent with all pairwise overlaps and nonoverlaps, yielding 18 unique epitopes (Figure 2A). Several of these epitopes were recognized by more than one MAb. Conceivably, these apparently identical epitopes could result from the trivial case of hybridomas derived from clones of the same plasma cell. However, this was not the case because 24 of the 30 MAbs either recognized unique epitopes or recognized the same epitope but were from different mice. In one case, 8 MAbs from 5 mice shared one epitope.
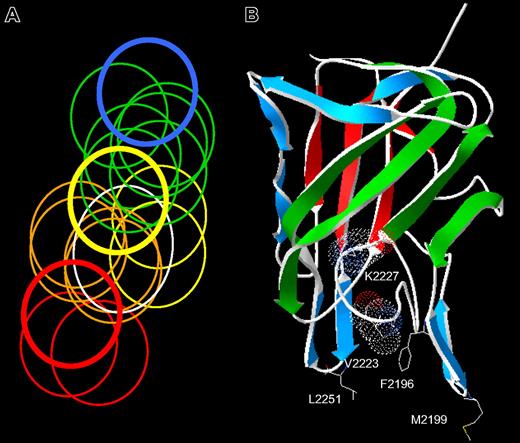
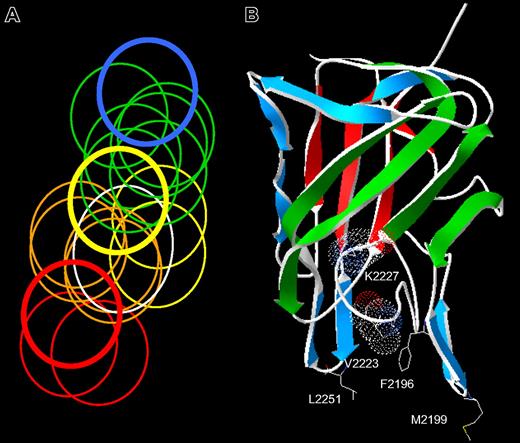
The spectrum of anti-C2 antibody epitopes. (A) Venn diagram representing overlapping anti-C2 MAbs. The overlap pattern of 18 ellipses is derived from the competition matrix of 30 MAbs in Figure 1B. Colors depict the 5 classes of MAbs: red, group A; orange, group AB; yellow, group B; green, group BC; and blue, group C. The thick lines represent the basis set MAbs: red, ESH4 (group A); yellow, 1B5 (group B); and blue, ESH8 (group C). The white ellipse represents the epitope of BO2C11. (B) Amino acid side chains recognized by anti-C2 MAbs. The strands representing the front and back faces of the β-sandwich are shown in green and red, respectively.23 Single-letter nomenclature is used to designate amino acids. Val2223 and Lys2227 are shown as van der Waals surfaces. The group AB MAbs, I109 and BO2C11, bind to the 2 phospholipid-binding β-hairpins containing Met2199 and Leu2251, respectively. The group B MAb, 3D12, binds to Phe2196. The group BC MAbs bind to Lys2227 and possibly Val2223.
The spectrum of anti-C2 antibody epitopes. (A) Venn diagram representing overlapping anti-C2 MAbs. The overlap pattern of 18 ellipses is derived from the competition matrix of 30 MAbs in Figure 1B. Colors depict the 5 classes of MAbs: red, group A; orange, group AB; yellow, group B; green, group BC; and blue, group C. The thick lines represent the basis set MAbs: red, ESH4 (group A); yellow, 1B5 (group B); and blue, ESH8 (group C). The white ellipse represents the epitope of BO2C11. (B) Amino acid side chains recognized by anti-C2 MAbs. The strands representing the front and back faces of the β-sandwich are shown in green and red, respectively.23 Single-letter nomenclature is used to designate amino acids. Val2223 and Lys2227 are shown as van der Waals surfaces. The group AB MAbs, I109 and BO2C11, bind to the 2 phospholipid-binding β-hairpins containing Met2199 and Leu2251, respectively. The group B MAb, 3D12, binds to Phe2196. The group BC MAbs bind to Lys2227 and possibly Val2223.
The spectrum of epitopes depicted in Figure 2A raises the question whether its area would be larger or if a discontinuous island of epitopes would be identified if more MAbs were analyzed. To address this, an additional 26 anti-C2 antibodies were purified from hybridoma supernatants, biotinylated, and used as secondary MAbs in the competition ELISA. All of these MAbs could be classified within groups A, AB, B, BC, or C, and the area covered by the epitopes was not increased. These results show that anti-C2 MAbs bind to a continuum of epitopes. However, the entire surface of the C2 domain is not covered because the epitopes do not circularize into a ring.
Orientation of epitopes on the surface of the C2 domain
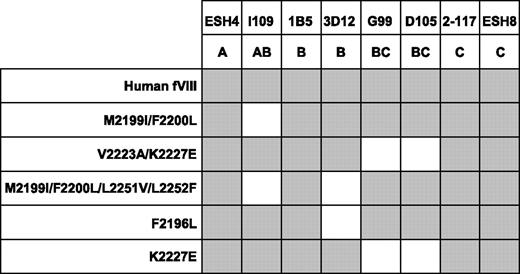
MAbs from groups A, AB, B, BC, and C were tested for binding to a panel of recombinant fVIII molecules containing mutations in the C2 domain (Figure 3). The fVIII mutants used were Met2199I/Phe2200L and Val2223A/Lys2227E double mutants, a Met2199I/Phe2200L/Leu2251V/Leu2252F quadruple mutant, and F2196L and Lys2227E single mutants. All of the mutants retained procoagulant activity, indicating that the native fold of the protein was preserved. The MAbs used were ESH4 (group A), I109 (group AB), 1B5 and 3D12 (group B), G99 and D105 (group BC), and ESH8 and 2–117 (group C). The C2 mutants were captured in a sandwich ELISA using an anti-A2 MAb, followed by addition of the anti-C2 MAbs and detection by visual inspection using streptavidin–alkaline phosphatase conjugate. In this assay, the absence of binding of a MAb (loss of antigenicity) indicates the mutated region contains the structural epitope for the MAb.
Binding of anti-C2 MAbs to fVIII C2 mutants. The binding of anti-C2 antibodies to fVIII captured in microtiter wells using an anti-A2 MAb was detected using streptavidin–alkaline phosphatase conjugate. Gray boxes represent binding of the MAb detected by color development.
Binding of anti-C2 MAbs to fVIII C2 mutants. The binding of anti-C2 antibodies to fVIII captured in microtiter wells using an anti-A2 MAb was detected using streptavidin–alkaline phosphatase conjugate. Gray boxes represent binding of the MAb detected by color development.
The group AB MAb, I109, did not bind the Met2199I/Phe2200L mutant, indicating that its epitope includes the Met2199/Phe2200 β-hairpin (Figure 3). This result is consistent with the behavior of BO2C11, which also is a group AB MAb that does not recognize the Met2199I/Phe2200L mutant.25 In contrast, the 2 group BC MAbs recognize a region containing Val2223 and Lys2227. This important finding fixes the direction of the map of overlapping epitopes (Figure 2A) and orients the epitopes on the “front” face of the C2 domain β-sandwich (Figure 2B). Antibodies representing groups A and C did not appear to recognize the Met2199/Phe2200 or Leu2251/Leu2252 β-hairpins or the Val2223/Lys2227 region, which is consistent with their polarization away from the AB, B, and BC groups (Figure 2A). MAb 3D12 recognizes Leu2251/Leu2252 and Phe2196, whereas the other group B MAb, 1B5, apparently does not. The 2 group B MAbs, 1B5 and 3D12, recognize different epitopes. This result is consistent with the pattern in Figure 2A, which predicts substantial spatial variation among epitopes within a given group. The surface buried by anti-C2 MAbs can be imagined by folding the epitope plane in Figure 2A so that group AB MAbs bind to Met2199/Phe2200 and Leu2251/Leu2252 β-hairpins at the bottom of the surface. Amino acid chains recognized by ESH4 and ESH8 at the polar ends of the epitope map have not been identified. The epitope map predicts that the ESH4 epitope is on the rear face of the β-sandwich and ESH8 recognizes an epitope that is superior to Lys2227. The Venn diagram in Figure 2A was constructed using only the pattern of overlapping epitopes shown in Figure 1B. Thus, the ellipses do not represent the size of predicted physical footprints on the C2 surface shown in Figure 2B.
In the competition ELISA, it is conceivable that the binding of one MAb could induce a conformational change in the C2 domain that alters the epitope recognized by another MAb. If so, competition between 2 MAbs would not necessarily mean overlapping epitopes. However, this possibility does not seem likely to account for most of the competitive binding behavior for several reasons. First, it is difficult to envision 18 distinct MAb-induced conformational changes implied by the matrix shown in Figure 1B. Second, MAb group-specific loss of antigenicity was associated with different site-directed mutants (Figure 3), which is consistent with spatially separate epitopes. Finally, crystallographic studies indicate that most antibodies possess a combining site structure that is complementary to that of their cognate protein antigens and do not commonly induce conformational changes.35,–37 Consistent with this, only minor conformational changes were observed in the X-ray structure of the C2 domain bound to the Fab fragment of BO2C11.24
Anticoagulant properties and inhibition of binding of fVIII to phospholipid and VWF by anti-C2 MAbs
The anti-fVIII inhibitor titer for each anti-C2 MAb was determined using the Bethesda assay (Table 1). Some MAbs showed progressive inhibition of fVIII activity with increasing concentration, whereas the majority had inhibitory activity that leveled off at saturation (Figure 4A)—patterns that are consistent with type I and type II inhibition, respectively.38 Most of the MAbs (23/30, 77%) had titers greater than 900 BU/mg IgG. The remaining MAbs had titers of less than 100 BU/mg IgG. Several MAbs had titers greater than 10 000 BU/mg IgG. However, within this group of high-titer MAbs, only BO2C11 displayed type I behavior. Ten of 12 MAbs with titers of greater than 10 000 BU/mg IgG were in group BC. The remaining 2 MAbs were in group C (ESH8) and group AB (BO2C11).
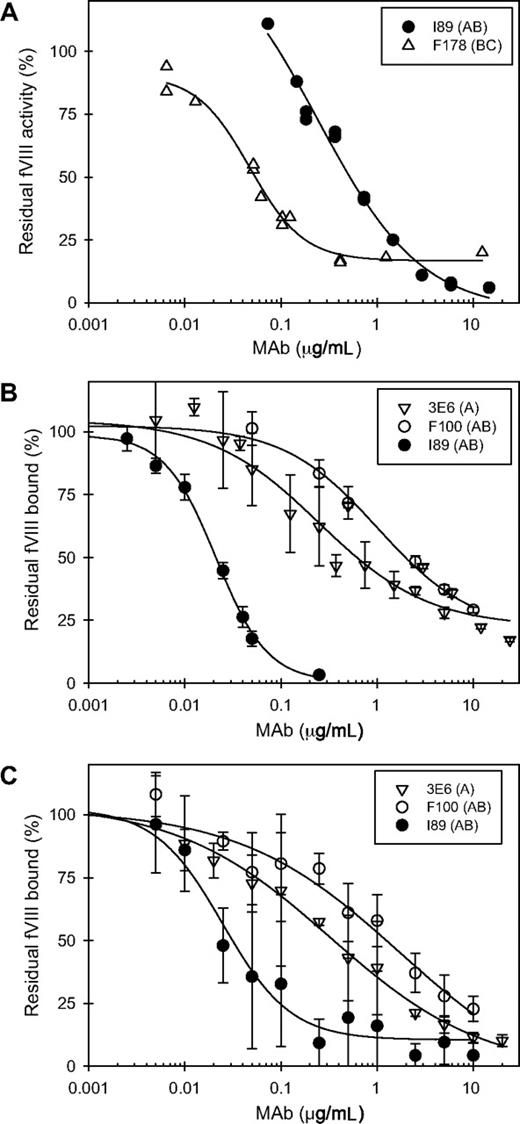
Functional assays of anti-C2 MAbs. (A) Representative Bethesda assays for a type I MAb (I89, ●) and a type II MAb (F178, ▵). (B) Inhibition of binding of fVIII to VWF by 3 representative anti-C2 MAbs. The MAbs shown are 3E6 (▿), F100 (○), and I89 (●). (C) Inhibition of binding of fVIII to phospholipid by the 3 anti-C2 MAbs shown in panel B. Error bars represent sample standard deviations of 3 to 12 replicates.
Functional assays of anti-C2 MAbs. (A) Representative Bethesda assays for a type I MAb (I89, ●) and a type II MAb (F178, ▵). (B) Inhibition of binding of fVIII to VWF by 3 representative anti-C2 MAbs. The MAbs shown are 3E6 (▿), F100 (○), and I89 (●). (C) Inhibition of binding of fVIII to phospholipid by the 3 anti-C2 MAbs shown in panel B. Error bars represent sample standard deviations of 3 to 12 replicates.
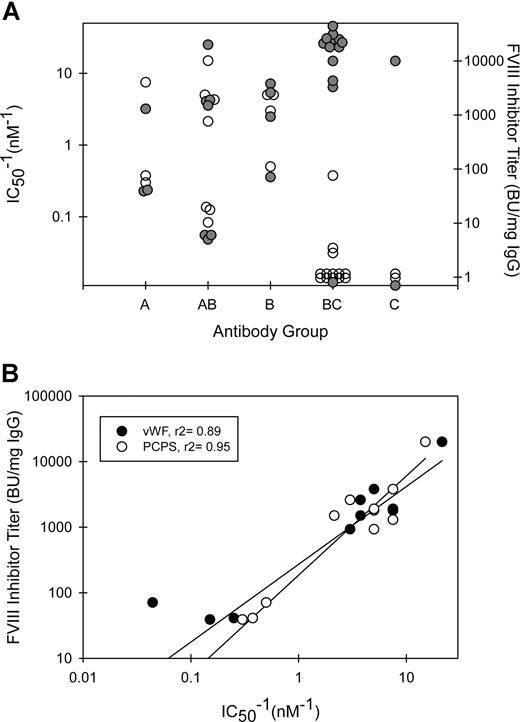
The MAbs also were tested for their ability to inhibit the binding of fVIII to phospholipid (Figure 4B) or to VWF (Figure 4C). MAb concentrations producing 50% inhibition (IC50) for both phospholipid and VWF binding at less than 2 μg/mL were found only in groups A, B, and AB (Figure 5A; Table 1). Inhibition of binding of fVIII to phospholipid paralleled inhibition of binding to VWF with some exceptions. 3C6 (group B) displayed a 10-fold lower IC50 for phospholipid than for VWF. G86 and B9 (group BC) inhibited phospholipid binding at IC50's of 4.1 and 0.4 μg/mL, respectively, but had undetectable inhibition of VWF binding. In contrast, MAb I55 (group BC) did not inhibit phospholipid binding but inhibited VWF binding with an IC50 of 0.02 μg/mL. These results demonstrate that the C2-binding sites for VWF and phospholipid are very similar but are not identical. In the set of MAbs that inhibited VWF and phospholipid binding to fVIII at IC50's less than 2 μg/mL, there was an inverse correlation between inhibitor titer and the IC50 (Figure 5B). Low IC50's for inhibition of fVIII binding to phospholipid presumably reflect high-affinity binding of anti-C2 MAbs to fVIII. Inhibition of binding of fVIII or fVIIIa to phospholipid is detected in the Bethesda assay. Inhibition of binding of fVIII to VWF does not reduce procoagulant activity in the in vitro Bethesda assay. Thus, the inverse correlation between inhibitor titer and the IC50 for VWF binding occurs because inhibition of the binding of fVIII to phospholipid or VWF usually goes hand-in-hand.
Functional properties of anti-C2 MAb groups. (A) Relationship between MAb group, fVIII inhibitor titer (●), and IC50−1 values for inhibition of binding of fVIII to phospholipid (○). (B) The inhibitor titers of MAbs with demonstrable inhibition of binding of fVIII to phospholipid (○) or VWF (●) (Table 1) are plotted versus IC50−1 for inhibition of binding.
Functional properties of anti-C2 MAb groups. (A) Relationship between MAb group, fVIII inhibitor titer (●), and IC50−1 values for inhibition of binding of fVIII to phospholipid (○). (B) The inhibitor titers of MAbs with demonstrable inhibition of binding of fVIII to phospholipid (○) or VWF (●) (Table 1) are plotted versus IC50−1 for inhibition of binding.
Inhibitory properties of anti-C2 MAbs in intrinsic fXase assay systems
Anti-C2 MAbs were tested for their ability to inhibit fVIII or fVIIIa function in 2 configurations of an intrinsic Xase assay. In the first configuration, fVIII was activated at a very high concentration of thrombin (100 nM) to attempt to overwhelm any effects of the MAb on the activation of fVIII itself. FVIII was incubated with MAbs for 2 hours, activated by thrombin in the presence and absence of VWF, and the resulting fVIIIa was assayed by dilution into factor IXa/PCPS vesicles followed by addition of factor X. Under the conditions of the assay, the initial velocity of factor X activation is proportional to fVIIIa activity.
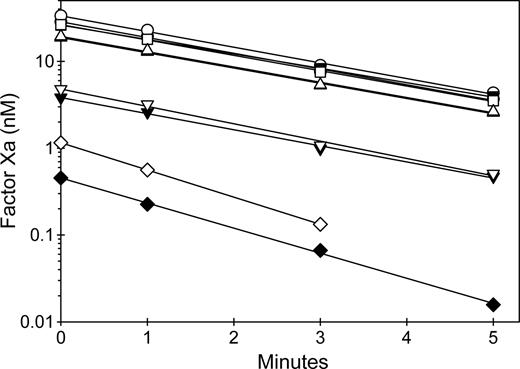
Results using 4 representative MAbs are shown in Figure 6. The zero time point represents peak activation of fVIII, which is followed by first-order decay of activity due to A2 subunit dissociation.39 MAbs from groups A and B, but not groups BC and C, were potent inhibitors of fVIIIa activity, consistent with their ability to block binding to phospholipid. The decay of fVIIIa activity was not affected by any of the MAbs, with the possible exception of 1B5. However, the marked inhibition of fVIIIa at zero time by 1B5 resulted in very low rates of factor X activation, which made fVIIIa decay rates difficult to measure. The presence of VWF did not significantly affect the inhibition of peak activity or the decay rates. These results indicate that anti-C2 MAbs that inhibit phospholipid binding to fVIII act by blocking assembly of fVIIIa into the intrinsic fXase complex.
Inhibition of intrinsic Xase activity by anti-C2 MAbs. FVIII (1 nM) was incubated for 2 hours at 37°C in the absence (closed symbols) or presence (open symbols) of 10 μg/mL VWF and in the absence (○) or presence of 20 nM anti-C2 MAbs: 3E6 (group A) (▿), 1B5 (group B) (◇), 2-77 (group BC) (▵), or 2-117 (group C) (□). Then fVIII was activated rapidly by thrombin (100 nM) for 30 seconds, followed by addition of desulfatohirudin (150 nM) to inhibit thrombin. At this concentration of thrombin, fVIII is activated completely within 10 seconds.27 The fVIIIa sample was added to 2 nM factor IXa/20 μM PCPS, followed immediately by addition of 300 nM factor X and measurement of the initial velocity of factor X activation as described in “Intrinsic fXase assays for measurement of fVIIIa activity.”
Inhibition of intrinsic Xase activity by anti-C2 MAbs. FVIII (1 nM) was incubated for 2 hours at 37°C in the absence (closed symbols) or presence (open symbols) of 10 μg/mL VWF and in the absence (○) or presence of 20 nM anti-C2 MAbs: 3E6 (group A) (▿), 1B5 (group B) (◇), 2-77 (group BC) (▵), or 2-117 (group C) (□). Then fVIII was activated rapidly by thrombin (100 nM) for 30 seconds, followed by addition of desulfatohirudin (150 nM) to inhibit thrombin. At this concentration of thrombin, fVIII is activated completely within 10 seconds.27 The fVIIIa sample was added to 2 nM factor IXa/20 μM PCPS, followed immediately by addition of 300 nM factor X and measurement of the initial velocity of factor X activation as described in “Intrinsic fXase assays for measurement of fVIIIa activity.”
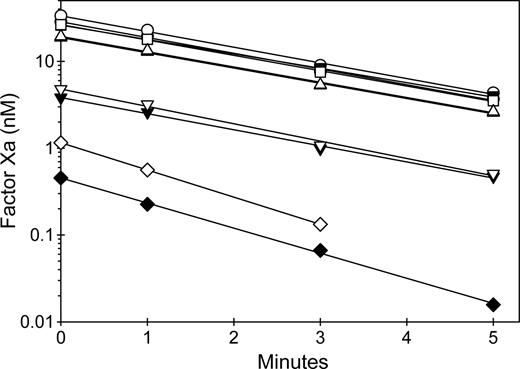
The second assay configuration evaluated the ability of anti-C2 MAbs to inhibit the rate of fVIII activation by thrombin or factor Xa. FVIII was preincubated in the presence and absence of MAbs and in the presence or absence of VWF and activated at low (nanomolar) concentrations of thrombin or factor Xa. Figure 7 shows representative results of MAbs from groups BC or C. In the absence of MAbs, VWF did not affect the activation of fVIII by thrombin. VWF is a cofactor for cleavage of the fVIII light chain by thrombin.40 This leads to rapid dissociation of cleaved fVIII from VWF, which therefore does not inhibit the rate of development of fVIIIa activity. In contrast, in the absence of MAbs, VWF decreased the rate of fVIII activation by factor Xa. FVIII that is bound to VWF cannot bind to phospholipid membranes and is recognized poorly by factor Xa.41 ESH8 partially inhibited the activation of fVIII by thrombin in the absence of VWF and markedly inhibited fVIIIa activity in the presence of VWF. In contrast, 2-77 partially inhibited the activation of fVIII by thrombin in the presence and absence of VWF. ESH8 markedly inhibited fVIII activation by factor Xa in the presence and absence of VWF, while 2–77 partially inhibited the activation of fVIII by factor Xa in the absence of VWF and markedly inhibited fVIIIa activity in the presence of VWF. Overall, the results of Figures 6 and 7 indicate that group A, AB, and B antibodies inhibit fVIIIa activity by interfering with assembly of intrinsic fXase complex on phospholipid membranes, whereas group BC and C MAbs inhibit the activation of fVIII by thrombin or factor Xa.
Inhibition by anti-C2 MAbs of the activation of fVIII by thrombin or factor Xa. (A) FVIII (1 nM) was incubated for 2 hours at 37°C in the absence (closed symbols) or presence (open symbols) of 10 μg/mL VWF and in the absence (○) or presence of 130 nM 2–77 (group BC) (▵) or ESH8 (group C) (□). The fVIII sample was diluted 0.5 nM in 1.5 nM factor IXa/20 μM PCPS and thrombin (0.55 nM) was added for the indicated times, followed by measurement of the initial velocity of factor X activation as described in “Intrinsic fXase assays for measurement of fVIIIa activity.” (B) FVIII (4 nM) was incubated for 2 hours at 37°C in the absence (closed symbols) or presence (open symbols) of 40 μg/mL VWF and in the absence (○) or presence of 520 nM 2–77 (group BC) (▵) or ESH8 (group C) (□). The fVIII sample was diluted to 2 nM and activated with 2.5 nM factor Xa in the presence of 1.5 nM factor IXa/20 μM PCPS for the indicated times, followed by measurement of the initial velocity of factor X activation as described in “Intrinsic fXase assays for measurement of fVIIIa activity.”
Inhibition by anti-C2 MAbs of the activation of fVIII by thrombin or factor Xa. (A) FVIII (1 nM) was incubated for 2 hours at 37°C in the absence (closed symbols) or presence (open symbols) of 10 μg/mL VWF and in the absence (○) or presence of 130 nM 2–77 (group BC) (▵) or ESH8 (group C) (□). The fVIII sample was diluted 0.5 nM in 1.5 nM factor IXa/20 μM PCPS and thrombin (0.55 nM) was added for the indicated times, followed by measurement of the initial velocity of factor X activation as described in “Intrinsic fXase assays for measurement of fVIIIa activity.” (B) FVIII (4 nM) was incubated for 2 hours at 37°C in the absence (closed symbols) or presence (open symbols) of 40 μg/mL VWF and in the absence (○) or presence of 520 nM 2–77 (group BC) (▵) or ESH8 (group C) (□). The fVIII sample was diluted to 2 nM and activated with 2.5 nM factor Xa in the presence of 1.5 nM factor IXa/20 μM PCPS for the indicated times, followed by measurement of the initial velocity of factor X activation as described in “Intrinsic fXase assays for measurement of fVIIIa activity.”
Discussion
In this study, a large panel of antibodies was used to identify C2 epitopes that are recognized by hemophilia A mice in response to clinically relevant doses of human fVIII. To characterize these epitopes, a basis set of 3 anti-C2 MAbs was defined that did not compete with each other for binding to human fVIII, but as a group competed with all other antibodies. The overlap patterns of the basis set MAbs produced 5 groups of MAbs, designated A, AB, B, BC, and C, and yielded an overlapping set of 18 distinct epitopes (Figure 2A). No MAb overlapped both group A and C MAbs. This, along with the large number of distinct epitopes, allowed us to develop a Venn diagram representing a map in which the groups A and C are located at polar ends of a continuous band of epitopes. Loss of antigenicity was associated with mutations at the Met2199/Phe2200 β-hairpin for group AB and Lys2227 for group BC (Figure 3). This allowed orientation of the epitope map and indicates that epitope structure is a continuum that covers most or all of the front face of the C2 β-sandwich (Figure 2).
MAbs from groups A, AB, and B represent classical anti-C2 antibodies that inhibit the binding of fVIII or fVIIIa to phospholipid membranes. The specific anti-fVIII inhibitory activity of these MAbs correlates with their ability to inhibit the binding of fVIII to phospholipid membranes (Table 1; Figure 5B). The pathogenicity of MAbs from groups A, AB, and B could involve inhibition of assembly of fVIIIa into the intrinsic fXase complex (Figure 6). Alternatively, they potentially could inhibit the activation of fVIII by factor Xa, which also is phospholipid dependent.41 Most of these MAbs also inhibit the binding of fVIII to VWF. Thus, they could possibly increase the clearance of fVIII by displacing it from VWF.
Interestingly, BO2C11, which is the most thoroughly characterized anti-fVIII MAb,16,24 was atypical in that it was the only MAb in groups A, AB, or B with a fVIII inhibitor titer exceeding 20 000 BU/mg IgG. The other MAbs in these groups had inhibitor titers less than 3800 BU/mg IgG. The inhibitory activity of BO2C11 can be explained by its high affinity binding to fVIII,16 which is manifested by its low IC50's for inhibition of fVIII binding to phospholipid or VWF (Table 1). The Venn diagram in Figure 2A could be constructed using ellipses of identical sizes except for BO2C11, which required a larger ellipse. Consistent with this, BO2C11 buries 1200 Å2 of the surface of the C2 domain,24 whereas murine MAbs typically bury only 700 to 850 Å2 .42 The CDR3 regions of the BO2C11 are responsible for most of the contacts with the C2 domain,24 which generally is the case in antibody-antigen interactions. The combined heavy and light chain CDR3 regions of BO2C11, 17 amino acid residues, are larger than the average length of the corresponding murine CDR3 regions (14 amino acid residues).43
Surprisingly, group BC represented the most common MAb group and displayed the highest fVIII inhibitor activities (Table 1). We have observed by competition ELISA that 7 of 9 human polyclonal fVIII inhibitor plasmas recognize group BC and C epitopes (S.L.M, J.F.H., and P.L., unpublished observations, July 2007), indicating that the murine hemophilia A model may accurately mimic the human anti-C2 antibody response. An important result of the present study is that group BC MAbs have the previously unknown property of inhibiting the activation of fVIII by either thrombin or factor Xa (Figure 7). A possible mechanism is the inhibition of fVIII light chain cleavage at Arg1689, which has been reported for ESH8 (a group C MAb) and an inhibitory human anti-C2 polyclonal IgG, A-FF.9,10 Most group BC MAbs are characterized by poor ability to inhibit the binding of fVIII to phospholipid membranes or VWF. High-resolution epitope mapping and characterization of the mechanism of action of group BC MAbs should be useful in structure-function studies of the role of the C2 domain in the activation of fVIII.
Group BC MAbs incompletely inactivated fVIII and were classified as type II inhibitors (Figure 4A; Table 1). Human type II inhibitors are pathogenic because they are associated with a poor clinical response to fVIII in congenital hemophilia A patients and frequently occur in patients with acquired hemophilia.44 The pathogenicity of type II inhibitors is puzzling because substantial fVIII activity remains at saturating concentrations of antibody. It has been proposed that type II inhibitors compete with the binding of fVIII to VWF, resulting in the dissociation of the fVIII-VWF complex and the rapid clearance of fVIII from the circulation.38 However, with the exception of I55, group BC MAbs compete poorly with VWF for binding to fVIII (Table 1). The behavior of group BC MAbs raises the hypothesis that although inhibition of the activation of fVIII by type II inhibitors is incomplete when measured by in vitro assays, it is sufficient to inhibit hemostasis in vivo. Alternatively, because group BC MAbs can bind fVIII in the presence of VWF, they could form immune complexes that lead to Fc receptor–mediated clearance of fVIII. Further studies are required to understand the pathogenicity of this important group of antibodies.
Another extensively characterized anti-fVIII MAb, ESH8,9,19,20 appears to be an atypical anti-C2 antibody. Unlike group A, AB, and B MAbs, it does not inhibit the binding of fVIII to VWF or phospholipid (Table 1). In addition, in contrast to the group BC MAb 2–77, the presence of VWF increases the inhibition by ESH8 of the activation of fVIII by thrombin (Figure 7A). This latter behavior is consistent with inhibition of the dissociation of thrombin-cleaved fVIII from VWF by ESH8.20 Analysis of ESH8 by competition ELISA (Figure 1B) and site-directed mutagenesis (Figure 3) shows that it binds to an epitope that is distinct from group BC epitopes. The only other group C MAb, 2–117, was the only MAb in the set that had no detectable fVIII inhibitor activity and is functionally distinct from ESH8.
The immunogenicity of human fVIII can be reduced in a murine hemophilia A model by mutagenesis of the Arg484-Ile508 A2 domain inhibitory epitope, which apparently is confined to a single continuous loop.45 Combined mutagenesis of epitopes in the A2 and C2 domains could produce a recombinant fVIII product with reduced immunogenicity. In addition to mutagenesis of phospholipid-binding residues, the results of the present study indicate that high-resolution mapping of group BC epitopes may yield additional candidate sites for mutagenesis.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by grants from the National Institutes of Health (HL082609 and HL40921) and Hemophilia of Georgia, (P.L.), and National Hemophilia Foundation Clinical Fellowship (S.L.M.).
We thank Dr Marc Jacquemin for providing the cell line expressing BO2C11.
National Institutes of Health
Authorship
Contribution: S.L.M. and J.F.H. designed and performed research, analyzed data, and cowrote the paper; E.T.P. and R.T.B designed and performed research; P.L. designed research, analyzed data, and cowrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Pete Lollar, Emory Children's Center, Rm 426D, 2015 Uppergate Dr, Atlanta, GA 30322; e-mail:jlollar@emory.edu.