Abstract
Fc receptor–like proteins (FcRLs) are a growing family of molecules homologous to FcγRI. Whereas all 7 previously reported Fc receptor homologs are expressed by B cells, here we report a new receptor, FcRL6, that is expressed by cytolytic cells including natural killer (NK) cells and effector and effector-memory CD8+ T cells. FcRL6 contains a novel cytoplasmic cysteine-rich motif and recruits SHP-2 through a phosphorylated ITIM, indicating a potential signaling function in effector lymphocytes. In vitro, FcRL6 does not greatly influence NK-cell or CD8+ T-cell–mediated cytotoxicity and has minimal impact on cytokine secretion. However, FcRL6 expression among T lymphocytes is greatly expanded in human immunodeficiency virus type 1 (HIV-1)–infected individuals, and includes not only effector and effector-memory CD8+ T cells but also populations of CD4+ T cells. Expansion of FcRL6-positive lymphocytes is not related to viral load, but is indicative of the dysregulated expansion of terminally differentiated effector lymphocyte populations in response to chronic HIV-1 infection and may serve as an important marker for chronic immune activation and for tracking the generation of effector cells following immune stimulation.
Introduction
Natural killer (NK) cells are large granular lymphocytes capable of providing cellular immunity to some tumors and virally infected cells.1 In order to maintain tolerance to healthy cells, while eliminating infected or otherwise abnormal cells, NK cells display a variety of germ-line–encoded receptors with the potential to activate or inhibit cytolytic responses.2,3 In humans, these receptors include the killer cell immunoglobulin-like receptors (KIRs); the leukocyte immunoglobulin-like receptors (LILRs); the NKG2 family of proteins; “natural cytotoxicity” receptors NKp30, NKp44, and NKp46; and members of the CD2 family, as well as other surface proteins necessary for adhesion and signaling
Activating NK receptors often deliver signals through associated signaling chains such as DAP10, DAP12, FcRγ, and CD3ζ.4,5 These chains contain immunoreceptor tyrosine-based activation motifs (ITAMs), which, upon phosphorylation, provide docking sites for SH2 domain–containing protein kinases, which in-turn recruit and activate downstream signaling partners. Conversely, inhibitory NK receptors commonly have cytoplasmic tails containing one or more consensus immunoreceptor tyrosine-based inhibitory motifs (ITIMs), which, when tyrosine phosphorylated, recruit phosphatases such as SHP-1, SHP-2, or SHIP to attenuate cytolytic responses.
In addition to cytotoxicity, NK cells are also important for regulating immune responses through the release of cytokines such as IFNγ and TNFα. Subsets of human NK cells have been identified with differential abilities to carry out the effector functions of cytotoxicity or cytokine release. In peripheral blood, they are often identified by their expression levels of CD56 and CD16.6 CD56bright CD16− CD25+ cells are weakly cytotoxic but fairly efficient at producing IFNγ. In contrast, CD56dim CD16+ CD25− NK cells show a very limited ability to secrete cytokines in the absence of IL-12, IL-15, or high-dose IL-2, but are capable of cellular cytotoxicity.
Recognition of virally infected cells by specific antibody is followed by antibody-dependent cell-mediated cytotoxicity (ADCC) by NK cells through the low-affinity receptor for IgG, CD16. This bridge between adaptive humoral immune responses and innate effector cells is found repeatedly on leukocyte populations through expression of various activating or inhibitory Fc receptors.7 Closely related to these Fc receptors is a group of immunoglobulin-superfamily receptors known as Fc receptor–like (FCRL) proteins.8–11 Currently, 7 members of this family have been described, and are all expressed by B cells at various stages of differentiation. FcRL1-5 are type I transmembrane receptors that contain ITAMs or ITIMs in their cytoplasmic tails, whereas FCRLA and FCRLB are intracellular proteins without canonical transmembrane domains or signaling motifs. While none of these proteins has been found to bind immune complexes, their expression by B cells indicates a likely role in regulating generation of humoral immune responses.
Here, we have identified a new Fc receptor homolog, FcRL6, primarily expressed by cytotoxic NK cells and effector CD8+ T cells. Its expression pattern among effector lymphocytes, abundance following chronic immune stimulation by human immunodeficiency virus (HIV), regulation by cytokines, and recruitment of an inhibitory phosphatase implicate it in the regulation of cellular immunity during chronic infections.
Materials and methods
Molecular cloning of human and mouse FcRL6
The putative open-reading frame encoding human FcRL6 was identified by comparing the sequence of a known Fc receptor homolog, FCRLA, to the human genome using the tBLASTn algorithm available on the NCBI website. The cDNA encoding FcRL6 was amplified from human peripheral blood mononuclear cell (PBMC) RNA by reverse-transcription–polymerase chain reaction (RT-PCR) using sense primer 5′ATGCTGCTCTGGACGGCTGTGCTGCTCTTTG and antisense primer 5′CTAGCAGAGAACCTCCTCACAGTCGCTAAG. The amplimer was cloned into pCR2.1 TOPO vector (Invitrogen, Frederick, MD), and clones were selected and sequenced. Many smaller splice forms were detected; however, our analyses have focused on the longest mature splice form, which encodes a 434–amino acid protein within 10 exons. Analysis of the sequence indicated that this protein was a type I transmembrane of the immunoglobulin superfamily, closely related to the classical Fc receptors and Fc receptor homologs. To confirm that the protein was expressed on the cell surface, the cDNA encoding FcRL6 was transiently expressed in 293T cells using the vector pFLAG-CMV3 (Sigma, St Louis, MO), which contains an N-terminal FLAG epitope tag. Thirty-six hours after transfection of the 293T cells, surface expression of the FLAG-tagged FcRL6 was verified by flow cytometry.
Molecular cloning of mouse FcRL6 was carried out by comparison of the orthologous rat gene sequence encoding Gp42 with mouse genomic DNA sequence available from NCBI databases. The cDNA encoding FcRL6 was amplified by nested 3′ RACE using the GeneRacer Kit (Invitrogen) and gene-specific primers for the 5′ end. The gene-specific primer sequences used to amplify the 5′ end were 5′TCTGCCTCAAGAAAGGCACCATGCTGCT recognizing the putative 5′ UTR, and 5′ATGCTGCTCTGGATGGTTCTCCT for the translation-initiation site. The source RNA for this transcript was derived from cultured, IL-2–activated, adherent murine splenocytes.
Generation of monoclonal antibodies specific for human FcRL6
Stable clones of the mouse mastocytoma cell line P815 expressing FcRL6 were generated using the FLAG-CMV3 vector. For immunization, a Balb/c mouse was immunized 4 times with FcRL6-expressing cells, and the splenocytes were fused with SP2/0 cells. The resulting hybridoma clones were screened for the ability to react to FcRL6-expressing P815, but not to the parent cell line. The screen resulted in a panel of FcRL6-specific hybridoma clones. Purified antibodies from clones 9H8 (IgG2a) and 2H3 (IgG2b) were generated from ascites fluid and used for further analyses. In this study, clone 2H3 was used for most assays, since the relatively rare IgG2b isotype makes this antibody convenient for multicolor flow cytometry. For redirected lysis assays, the high affinity of IgG2a for Fc receptors was exploited by using the IgG2a clone 9H8. Aside from those differences related to isotype, no qualitative differences between these 2 antibodies have been found, either in the specificity or affinity for FcRL6. For assays to detect cytokine induction by FcRL6, F(ab) fragments specific for FcRL6 were generated by peptic digest of antibody 2H3 using the ImmunoPure F(ab′)2 Preparation Kit (Pierce Biotechnology, Rockford, IL). Purity of the antibody fragment was verified by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE). Reactivity of the antibody fragment with FcRL6 was confirmed by flow cytometry using a PE-conjugated antimouse kappa light chain secondary antibody (Southern Biotechnology, Birmingham, AL). Isotype controls for whole antibody stainings and redirected lysis assays were obtained from Sigma, and purified F(ab′)2 control antibody was obtained from Pierce Biotechnology.
Analysis of FcRL6 expression and regulation by cytokines
Human PBMCs isolated by Ficoll-gradient centrifugation of whole blood or buffy coats were analyzed by flow cytometry for coexpression of FcRL6 with cell lineage markers. Cells were gated by forward- and side-scatter to separate lymphocytes and monocytes, then analyzed for expression of markers CD3, CD4, CD8, CD14, CD16, CD19, CD56, CD66b, and γδTCR (Table S1, available on the Blood website; see the Supplemental Materials link at the top of the online article). For subset analysis of FcRL6 expression on CD8+ T cells, cells were first magnetic-activated cell sorter (MACS) purified using human CD8 microbeads (Miltenyi, Auburn, CA). Subsequently, 4-color flow cytometry was performed using antibodies to CD28, CD45RA, 2B4, and biotinylated anti-FcRL6 clone 2H3. All flow cytometry instrumentation was performed on a BD FACSCalibur (Becton Dickinson, Lincoln Park, NJ).
For analysis of cytokine regulation of FcRL6 expression, PBMCs were cultured for 48 hours in the presence of IL-3 (100 ng/mL), IL-6 (50 ng/mL), IL-7 (50 ng/mL), IL-8 (50 ng/mL), IL-10 (50 ng/mL), IL-12p70 (50 ng/mL), IL-15 (100 ng/mL), IL-18 (50 ng/mL), TGF-β1 (5 ng/mL), TNFα (100 ng/mL), IFNα 1000U/mL, IFNγ (50 ng/mL), VEGF (40 ng/mL), GM-CSF (200 ng/mL), or tissue culture supernatant containing recombinant IL-21, IFNλ, FLT-3L, IL-4, and IL-2. Expression levels of FcRL6 on NK cells were measured by flow cytometry.
Biochemical analysis of human FcRL6
NK cells were purified by CD56+ MACS enrichment (Miltenyi) following Ficoll-gradient centrifugation of buffy coats. Purified NK cells were stimulated with freshly prepared pervanadate for 15 minutes.12 Following pervanadate treatment, cells were lysed and cleared lysates were immunoprecipitated using purified 2H3 antibody, or anti-CD56 antibody as a control. The resulting eluate was separated by reducing SDS-PAGE, and phosphoproteins were detected by Western blotting with antiphosphotyrosine antibody. For mutational analysis of FcRL6 phosphorylation and function, site-directed and truncation mutants of FcRL6 were generated. Site-directed mutants lacking cytoplasmic tyrosines were generated by specifically mutating one or both tyrosines at positions 356 and 371 to phenylalanine. To control for other potential signaling motifs, a truncation mutant lacking the entire C-terminal cytosolic tail was generated. These mutants were retrovirally expressed in NK92 cells using the vector pMX-neo13 and φNX packaging cell line. Stable bulk populations of NK92 cells expressing surface FcRL6 were generated following 2 rounds of fluorescence-activated cell sorting (FACS) on FACSVantage and MoFlow cell sorters. To measure tyrosine phosphorylation, cells were pervanadate treated, and immunoprecipitation/Western blot analysis was performed. Association of the protein tyrosine phosphatase SHP-2 with phosphorylated FcRL6 was detected by immunoprecipitating FcRL6 from pervanadate-treated NK92 transfectants followed by Western blotting with an anti–SHP-2 antibody (Santa Cruz Biotechnology, Santa Cruz, CA). Association of SHP-2 with phosphorylated NKG2A was detected by Western blotting of proteins immunoprecipitated with mouse anti-NKG2A mAb Z199 (Beckman-Coulter, San Diego, CA) from the pervanadate-treated NK92 cells.
Analysis of FcRL6 function in NK cells and CD8+ T cells
The influence of FcRL6 signaling on cellular cytotoxicity was measured using a standard redirected lysis assay with NK92 cells as effectors, expressing either wild-type FcRL6 or a mutant form lacking the cytoplasmic domains, and 51Cr-labeled P815 cells as targets. After titration of activating anti-NKp30 antibody to reliably achieve 50% specific lysis, the redirected lysis assay was performed with anti-NKp30 in the presence of anti-FcRL6 antibody (9H8) or an IgG2a isotype control. The effect of FcRL6 signaling alone, or in cooperation with any of CD16, NKp30, 2B4, NKG2D, CD11a, or DNAM-1, was measured using primary NK cells isolated by CD56+ MACS enrichment. Again in this assay, anti-CD16 (B73.1) was titrated to achieve 50% lysis prior to measuring for activation or inhibition by FcRL6. In some assays, primary NK cells were cultured overnight in IL-2 prior to their use as effector cells. For measurement of CD8+ T-cell cytotoxicity, CD8+ T cells were isolated from PBMCs using immunomagnetic enrichment described earlier. Redirected lysis of P815 cells was performed using anti-CD3 antibody (OKT3) in the presence of either anti-FcRL6 antibody or an isotype control.
To measure cytokine output by NK cells following FcRL6 engagement, 96-well plates were coated with either 20 μg/mL F(ab) fragments specific for FcRL6 or control F(ab′)2 fragments. Purified NK cells were plated at 200 000/well and centrifuged to ensure contact of the cells with the antibody-coated plates. After overnight culture in the presence or absence of IL-2, supernatants were harvested and cytokines measured using the human TH1/TH2 Cytometric Bead Array kit (BD Biosciences, San Jose, CA). Assays to measure cytokine responses from CD8+ T cells were similarly performed, with the inclusion of plate-bound anti-CD3 antibody for activation.
Analysis of FcRL6 expression in HIV-1+ individuals
The patient samples reported in this study were from patients chronically infected with HIV-1. Blood samples were obtained via coded access agreement with the Washington University AIDS Clinical Trials Unit, following protocols approved by the institutional review board and human studies committee. Patients provided informed consent in accordance with the Declaration of Helsinki. Blood was stained with antibodies prior to fixation and red cell lysis with BD FacsLyse solution (Becton Dickinson). For analysis, lymphocytes were positively gated by forward and side scatter, and the percentages of FcRL6+CD4+ T cells and FcRL6+CD8+ T cells were quantitated using FlowJo version 4.2 (TreeStar, Ashland, OR). Statistical analyses including Mann-Whitney tests for differences between population medians were performed using Graphpad Prism 4 software (GraphPad Software, San Diego, CA).
Results
FcRL6 is a novel Fc receptor homolog with a unique signaling motif
A search of the human genome sequence on the NCBI database for genes homologous to FCRLA was performed using the tBLASTN algorithm. This generated 3 candidate open-reading frames on human chromosome 1q21-23 for which no current gene designation existed. In an attempt to determine whether these open-reading frames encoded functional proteins, RT-PCR was performed to amplify putative full-length cDNA for each of the genes. Of the 3 candidates, only one was successfully amplified from human PBMC-derived RNA. This cDNA encodes a 434–amino acid protein homologous to FCRLA and FcγRIa. A series of reviews published since the initial cloning of this molecule have described a protein identical to this one and have designated it FcRL6.8
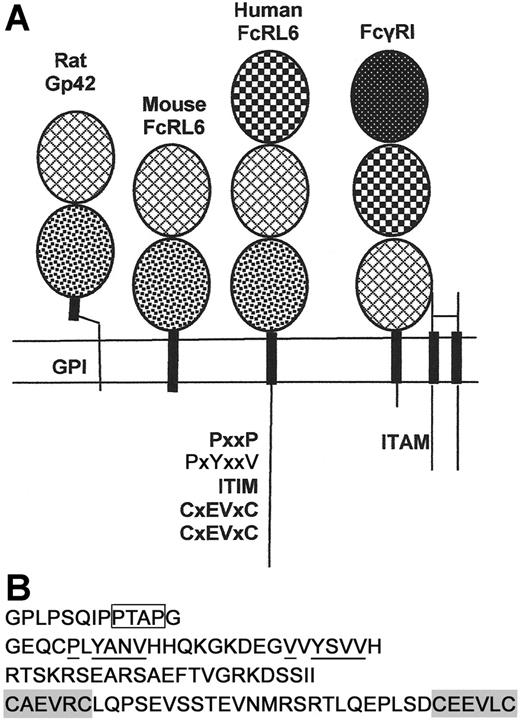
FcRL6 is a type I transmembrane protein of the immunoglobulin superfamily (Figure 1A). The complete coding domain sequence is constructed from 10 exons. As with other Fc receptor homologs, the first 2 exons encode the leader peptide. The ectodomain of the mature protein is composed of 3 immunoglobulin C-type domains. The first and second domains are homologous to the second and third domains of FcγRI, respectively, sharing 42% sequence identity within this region. In addition to the cysteine residues maintaining the structure of the Ig domains, the second and third Ig domains each contain an unpaired cysteine. Since immunoprecipitates of Flag-tagged FcRL6 from P815 transfectants failed to show significant dimerization of the protein by Western blotting under nonreducing conditions, it is unlikely that these cysteines mediate homodimerization of the protein (data not shown). However, it is still possible that they may mediate heterodimerization with an unknown receptor partner.
Structural features of human and mouse FcRL6. (A) Human FcRL6 is a 434–amino acid, type I transmembrane protein of the immunoglobulin superfamily. It contains a leader peptide, encoded by 2 exons, followed by 3 immunoglobulin domains. The protein also contains a cytoplasmic domain with a number of putative signaling motifs. The orthologous gene in mice encodes a type I transmembrane protein highly homologous to the rat protein Gp42. It contains only 2 immunoglobulin domains and lacks a cytoplasmic tail. Patterns within the ovals indicate the sequence homology within the immunoglobulin domains of FcRL6, Gp42, and FcγRI. FcγRI signals through a homodimer of the gamma chain of Fc receptors, which contains a cytoplasmic ITAM. The cDNA and protein sequences for human and mouse FcRL6 are published under GenBank accession numbers AY513661 and EF032497, respectively. (B) The cytoplasmic tail of FcRL6 encodes a series of putative signaling motifs. Most proximal to the membrane is a proline-rich sequence containing a potential SH3-binding PxxP motif (box) followed by 2 YxxV motifs, the second of which is a canonical ITIM (underlined). The last exon encodes a novel cysteine-rich domain containing 2 CxEVxC motifs (shaded) along with several acidic residues that may cooperate for metal binding.
Structural features of human and mouse FcRL6. (A) Human FcRL6 is a 434–amino acid, type I transmembrane protein of the immunoglobulin superfamily. It contains a leader peptide, encoded by 2 exons, followed by 3 immunoglobulin domains. The protein also contains a cytoplasmic domain with a number of putative signaling motifs. The orthologous gene in mice encodes a type I transmembrane protein highly homologous to the rat protein Gp42. It contains only 2 immunoglobulin domains and lacks a cytoplasmic tail. Patterns within the ovals indicate the sequence homology within the immunoglobulin domains of FcRL6, Gp42, and FcγRI. FcγRI signals through a homodimer of the gamma chain of Fc receptors, which contains a cytoplasmic ITAM. The cDNA and protein sequences for human and mouse FcRL6 are published under GenBank accession numbers AY513661 and EF032497, respectively. (B) The cytoplasmic tail of FcRL6 encodes a series of putative signaling motifs. Most proximal to the membrane is a proline-rich sequence containing a potential SH3-binding PxxP motif (box) followed by 2 YxxV motifs, the second of which is a canonical ITIM (underlined). The last exon encodes a novel cysteine-rich domain containing 2 CxEVxC motifs (shaded) along with several acidic residues that may cooperate for metal binding.
Following the uncharged transmembrane domain is a cytoplasmic tail with a series of putative signaling motifs. The most apparent of these are the 2 YxxV motifs, one of which resembles the canonical ITIM sequence V/IxYxxV/I/L.4,5,14 In addition, there is a short proline-rich segment that may bind SH3 domain–containing proteins via a consensus PxxP motif.15 Perhaps the most intriguing feature of the C-terminus is the domain encoded within the last exon of the transcript. This domain contains 2 CxxxxC motifs spaced by 24 amino acids. Further refinement of the sequence illustrates a repeated CxEVxC motif with additional acidic residues found within the domain. This pattern is suggestive of a metal-ion–binding domain, possibly forming a zinc-centered structural motif for signal transduction16,17 (Figure 1B).
The gene encoding FcRL6 in humans is orthologous to one encoding a previously described protein in rat NK cells, namely Gp42.18 This protein varies structurally from human FcRL6 in a few important ways. First, it contains only 2 immunoglobulin domains, homologous to the second and third domains of FcRL6. Second, when transfected into some cell lines, Gp42 is anchored to the membrane through a GPI linkage rather than a transmembrane domain. Lastly, the distinctive cytoplasmic signaling motifs found in human FcRL6 are notably absent in Gp42, indicating divergent functional mechanisms. A BLAST search of mouse genome sequence revealed that a similar mouse homolog of FcRL6 exists. To clone it, murine splenocytes were activated with IL-2 for 5 days. After this activation, RNA was extracted from the adherent lymphocytes and RT-PCR was performed to amplify homologous mouse transcripts. The resulting cloned cDNA sequence encoded a 244–amino acid protein of the immunoglobulin superfamily, highly similar to rat Gp42. Like Gp42, the precursor protein for mouse FcRL6 contains only 2 immunoglobulin domains and a transmembrane domain, with only a few amino acids exposed to the cytosol (Figure 1A). In contrast to Gp42, however, mouse FcRL6 is phospholipase C insensitive when expressed in 293 cells, suggesting the absence of a GPI linkage (not shown). Association of FcRL6 with cell membrane glycolipid-enriched motifs may still be maintained in mice through a cytosolic cysteine residue that is a potential palmitoylation site.
FcRL6 is expressed by NK cells and effector CD8+ T cells in healthy individuals
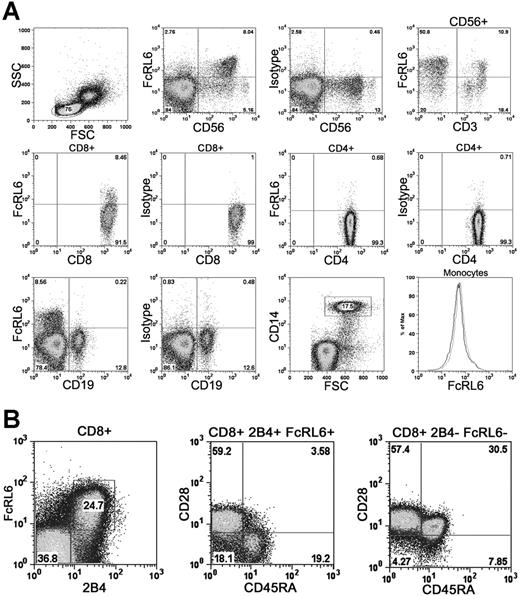
In order to study the expression pattern of FcRL6 within leukocytes, a panel of monoclonal antibodies specific for FcRL6 was developed by immunization of a BALB/c mouse. Staining of human PBMCs with antibody 2H3 (IgG2b) showed that the majority of FcRL6 expression is found within CD56dim CD16+ NK cells. In addition, subsets of CD8+ T cells and CD56+ CD3+ NKT cells were found to express FcRL6. In healthy individuals, FcRL6 is generally not expressed by other major peripheral blood mononuclear cell subsets such as B cells, CD4+ T cells, or monocytes (Figure 2A). This expression pattern is consistent with that found in an earlier study examining the transcript expression of FCRL family members in human peripheral blood leukocytes.19
Expression of FcRL6 on PBMCs. (A) FcRL6 is expressed on CD56dim NK cells, a subset of CD56+ CD3+ NKT cells, and a subset of CD8+ T cells. In healthy individuals, FcRL6 is typically not expressed by CD4+ T cells, B cells, or monocytes. The lack of FcRL6 staining on CD14+ monocytes is indicated on the histogram (black line) overlaying the isotype control (gray line). (B) CD8+ T cells, purified by immunomagnetic isolation, were examined by 4-color flow cytometry to determine FcRL6 expression among CD8+ T-cell subsets. Expression of FcRL6 is found among CD28+ CD45RA− 2B4+ effector-memory cells and CD28− CD45RA+ 2B4+ effector cells. FcRL6 is not expressed by CD28+ CD45RA+ 2B4− naive cells, or by CD28+ CD45RA− 2B4− central memory cells.
Expression of FcRL6 on PBMCs. (A) FcRL6 is expressed on CD56dim NK cells, a subset of CD56+ CD3+ NKT cells, and a subset of CD8+ T cells. In healthy individuals, FcRL6 is typically not expressed by CD4+ T cells, B cells, or monocytes. The lack of FcRL6 staining on CD14+ monocytes is indicated on the histogram (black line) overlaying the isotype control (gray line). (B) CD8+ T cells, purified by immunomagnetic isolation, were examined by 4-color flow cytometry to determine FcRL6 expression among CD8+ T-cell subsets. Expression of FcRL6 is found among CD28+ CD45RA− 2B4+ effector-memory cells and CD28− CD45RA+ 2B4+ effector cells. FcRL6 is not expressed by CD28+ CD45RA+ 2B4− naive cells, or by CD28+ CD45RA− 2B4− central memory cells.
At least 4 functional subsets of circulating CD8+ T cells can be distinguished based on their expression of various cell surface markers including CD28, CD45RA, and 2B4. These include naive cells and antigen-experienced effector, central memory, and effector-memory populations.20,21 Examination of CD8+ T-cell subsets using these cell surface markers found abundant FcRL6 expression within CD28− CD45RA+ 2B4+ effector and CD28+ CD45RA− 2B4+ effector-memory cells. Populations that were 2B4−, including CD28+ CD45RA+ naive and CD28+ CD45RA− central memory cells, do not express FcRL6 with a high frequency (Figure 2B).
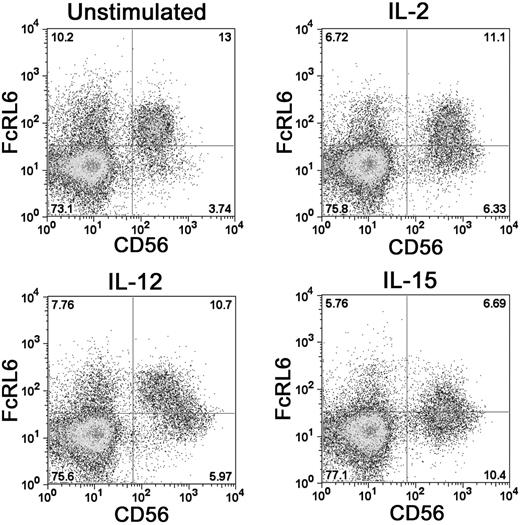
Stimulation of PBMCs with a panel of cytokines found that FcRL6 expression can be regulated by IL-2, IL-12, and IL-15. Activation of NK cells by either IL-2 or IL-15 was found to down-regulate FcRL6 expression on NK cells. Similarly, activation of CD8+ T cells with anti-CD3 antibody reduced the proportion of FcRL6+ cells (not shown). This suggests that FcRL6 expression can be governed by the current activation state of the cell. In addition, IL-12 stimulation led to a clear transition of CD56dim FcRL6+ NK cells to a population of CD56bright FcRL6− cells (Figure 3). This change was remarkably different from the up-regulation of CD56 and down-regulation of FcRL6 elicited by IL-2 and IL-15 stimulation, and may reflect an IL-12–induced differentiation of CD56dim NK cells to a CD56bright phenotype.22
FcRL6 can be down-regulated on NK cells by activating cytokines. Activation of NK cells with IL-2 or IL-15 leads to up-regulation of the surface marker CD56 and down-regulation of FcRL6. The differentiation of resting NK cells from CD56dim “cytotoxic” to CD56bright “regulatory” NK-cell phenotypes with IL-12 stimulation also leads to down-regulation of FcRL6.
FcRL6 can be down-regulated on NK cells by activating cytokines. Activation of NK cells with IL-2 or IL-15 leads to up-regulation of the surface marker CD56 and down-regulation of FcRL6. The differentiation of resting NK cells from CD56dim “cytotoxic” to CD56bright “regulatory” NK-cell phenotypes with IL-12 stimulation also leads to down-regulation of FcRL6.
FcRL6 does not significantly influence NK-cell or CD8+ T-cell cytotoxicity in vitro
In order to test the potential of FcRL6 signaling to influence cellular cytotoxicity, NK92 cells, which have no constitutive expression of FcRL6, were transfected with wild-type FcRL6 or mutant FcRL6 lacking the cytoplasmic tail. Killing of 51Cr-labeled P815 target cells by NK92 cells was measured in a redirected lysis assay. In this assay, NK-cell receptor–specific antibodies cross-link the Fc receptor on P815 with FcRL6 or another activating receptor. Lysis of P815 was not increased in the presence of anti-FcRL6 antibody alone (data not shown). When used together with anti-NKp30 antibody, anti-FcRL6 increased NKp30-mediated cytotoxicity in a dose-dependent manner. However, the magnitude of change in cytotoxicity was identical between the cells expressing wild-type FcRL6 and those expressing the truncated mutant form that lacks any signaling motifs. This suggests that the observed cytotoxicity was not a result of FcRL6 signaling, but rather was due to increased adhesion between effector and target cells, mediated by the anti-FcRL6 antibody (Figure S1).
Since NK92 cells are IL-2 dependent and have no constitutive expression of FcRL6, it is possible that they also lack the necessary signaling partners required for FcRL6 to regulate cytotoxicity. To address this, freshly isolated NK cells were used in redirected lysis assays. It was found that the presence of antibody specific for FcRL6 had no significant effect on cytotoxicity alone, or in the presence of activating antibodies for CD16, either before or after overnight stimulation of the effector cells with IL-2 (Figure S1). No significant effects on cytotoxicity of anti-FcRL6 antibody were observed in freshly isolated NK cells stimulated with antibodies to NKp30, 2B4, DNAM-1, NKG2D, or CD11a (data not shown). Additionally, the use of anti-FcRL6 clone 2H3 rather than 9H8 did not change the results of any of these assays. These data indicate it is unlikely that FcRL6 plays a role in regulating NK-cell–mediated cytotoxicity in vitro. Influence of FcRL6 on CD8+ T-cell cytotoxicity was similarly absent. Cytotoxicity by CD8+ T cells isolated by immunomagnetic isolation was either minimally inhibited or unaffected by the presence of anti-FcRL6 antibody in redirected lysis assays (Figure S1).
Cross-linking of FcRL6 has minimal effects on cytokine production by NK cells and CD8+ T cells in vitro
Another important function of NK cells is the release of cytokines to regulate inflammatory processes or influence T-cell polarization. Measurement of cytokine release from NK cells was performed by assaying the concentration of various cytokines in the supernatants of cultured primary NK cells. CD56+ NK cells enriched by immunomagnetic isolation were stimulated by culturing the cells for 24 hours in plates coated with FcRL6-specific F(ab) fragments, either in the presence or absence of IL-2. In the absence of IL-2 stimulation, IFNγ secretion was not detected in control F(ab)2-stimulated NK-cell supernatants, and only sporadically detectable after anti-FcRL6 F(ab) fragment stimulation. With the addition of IL-2 to the culture, IFNγ production was readily detectable in both FcRL6-stimulated and control cultures, with a consistent increase in IFNγ production witnessed in those cells stimulated through FcRL6 in 4 separate experiments (Figure S2). In addition to increased IFNγ production, some increase in the production of TNFα was seen following engagement of FcRL6. Again, increased cytokine production was most evident with IL-2 costimulation, and was generally low in magnitude, with TNFα production never exceeding 200 pg/mL (Figure S2A). IL-4, IL-5, and IL-10 were generally at or below the limit of detection (< 10 pg/mL). When compared with the effects of IL-2 addition, the induction of cytokines by FcRL6 engagement is relatively weak in these in vitro experiments and may be a secondary effect of some as-yet-undetected function of FcRL6. In similar assays, no significant difference was seen in the levels of cytokine production by CD8+ T cells with FcRL6 cross-linking, suggesting that FcRL6 does not influence cytokine production in vitro. However, we cannot exclude the possibility that FcRL6 may have significant effects on cytokine production by cytotoxic cells in vivo during pathogen challenge.
Recruitment of SHP-2 by FcRL6 is dependent on phosphorylation of tyrosine 371
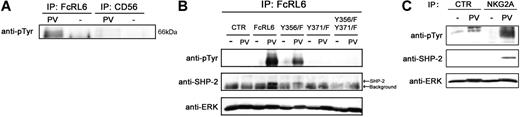
Understanding the functions triggered by FcRL6 engagement depend on specific knowledge of motifs for protein-protein interactions within the cytoplasmic tail. Based on sequence analysis, it appeared likely that FcRL6 would recruit some SH2 domain–containing protein in the event that one or both of the tyrosines, found within ITIM-like motifs, are phosphorylated. Verification that FcRL6 is tyrosine phosphorylated was obtained by immunoprecipitation of FcRL6 from pervanadate-treated human NK cells. Western blot analysis found abundant phosphotyrosine residues within the expected 66-kDa band in samples immunoprecipitated with anti-FcRL6 antibody, but not in the anti-CD56 control. In addition, no such band was found when cells were not treated with pervanadate, demonstrating the specificity of the phosphotyrosine antibody (Figure 4A). Specific importance of tyrosine 371 was obtained by expression of wild-type and site-directed mutants of FcRL6 in NK92 cells. Analysis of protein samples immunoprecipitated from pervanadate-treated cells found extremely high levels of phosphorylation in cells expressing wild-type FcRL6. Tyrosine phosphorylation of FcRL6 was abrogated in cells expressing FcRL6 that had been mutated to replace tyrosine 371 with phenylalanine (Y371F), but not in cells with mutated tyrosine 356 (Y356F), defining tyrosine 371 as the target residue for phosphate modification (Figure 4B). Of importance, specific recruitment of the protein tyrosine phosphatase SHP-2, but not SHP-1 or the inositol phosphatase SHIP, was detected in cells expressing wild-type FcRL6 or Y356F mutants, but not in mutants carrying the Y371F mutation (Figure 4B). This result implicates a role for phosphotyrosine-based signaling by FcRL6 through SHP-2. In addition, the proline-rich sequences and CxEVxC motif described earlier may assist in the recruitment of proteins that contribute to this function.
Recruitment of SHP-2 by FcRL6 is dependent on tyrosine 371. (A) IP/Western analysis of pervanadate-treated primary NK cells shows phosphotyrosines in samples immunoprecipitated with anti-FcRL6 antibody, but not anti-CD56 control. (B) Phosphotyrosine-specific antibodies detected a strong 66-kDa band in immunoprecipitates from pervanadate-treated (PV), but not untreated (UT), NK92 cells stably expressing wild-type FcRL6. In addition, SHP-2 is recruited by FcRL6 in pervanadate-treated cells. Mutation of tyrosine 371, but not tyrosine 356, to phenylalanine leads to an abrogation of FcRL6 phosphorylation and a failure to recruit SHP-2. ERK immunoblot is shown as loading control. This result has been repeated twice. (C) Recruitment of SHP-2 by phosphorylated NKG2A in pervanadate-treated NK92 cells is shown as positive control. NKG2A recruits more SHP-2 than FcRL6, suggesting low stoichiometry of interaction between FcRL6 and SHP-2. Samples immunoprecipitated from untreated or pervanadate-treated NK92 cells with anti-NKG2A or a control antibody were immunoblotted with phosphotyrosine- and SHP-2–specific antibodies. ERK immunoblot is shown as loading control.
Recruitment of SHP-2 by FcRL6 is dependent on tyrosine 371. (A) IP/Western analysis of pervanadate-treated primary NK cells shows phosphotyrosines in samples immunoprecipitated with anti-FcRL6 antibody, but not anti-CD56 control. (B) Phosphotyrosine-specific antibodies detected a strong 66-kDa band in immunoprecipitates from pervanadate-treated (PV), but not untreated (UT), NK92 cells stably expressing wild-type FcRL6. In addition, SHP-2 is recruited by FcRL6 in pervanadate-treated cells. Mutation of tyrosine 371, but not tyrosine 356, to phenylalanine leads to an abrogation of FcRL6 phosphorylation and a failure to recruit SHP-2. ERK immunoblot is shown as loading control. This result has been repeated twice. (C) Recruitment of SHP-2 by phosphorylated NKG2A in pervanadate-treated NK92 cells is shown as positive control. NKG2A recruits more SHP-2 than FcRL6, suggesting low stoichiometry of interaction between FcRL6 and SHP-2. Samples immunoprecipitated from untreated or pervanadate-treated NK92 cells with anti-NKG2A or a control antibody were immunoblotted with phosphotyrosine- and SHP-2–specific antibodies. ERK immunoblot is shown as loading control.
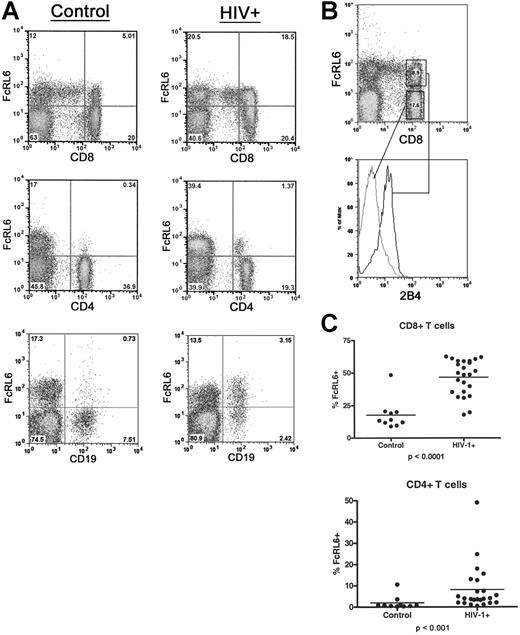
FcRL6-expressing cells are greatly expanded in HIV-1–infected individuals
A screening of immunoreceptor expression in a blood sample from an HIV-1–infected individual found an abnormally high level of T lymphocytes expressing FcRL6. This expression level could not be explained simply by a depletion of CD4+ T cells, since FcRL6 expression by CD8+ lymphocytes is generally not frequent, and does not exceed 50% in healthy individuals. Further analysis of lymphocyte populations from HIV-1–infected individuals confirmed this observation and indicated that the increase in FcRL6+ cells was largely due to an expansion of effector and effector-memory CD8+ T-cell populations (Figure 5). We also separately analyzed patients with detectable viral titers who were untreated or who had been on antiretroviral therapy for 3 months or fewer, and individuals with undetectable viral loads who had been on antiretroviral therapy for 6 months or longer. However, the median frequency of FcRL6+ CD8+ T cells increased nearly 4-fold in both groups compared with healthy controls regardless of the viral titer and/or CD4 counts (data not shown).
FcRL6 expression is dramatically up-regulated on lymphocytes in HIV-1–infected patients. (A) Two-color FACS analysis of blood from HIV-1+ patients and healthy controls shows relative increases in FcRL6+ lymphocytes in HIV-1+ individuals. FACS plots showing increases in CD8+ FcRL6+ cells and CD4+ FcRL6+ cells are representative of the median expression levels for healthy and infected individuals. Up-regulation of FcRL6 on B cells in HIV-1+ is also seen, though less frequently than for T cells. Cells are gated by FSC/SSC to include lymphocytes. (B) Three-color FACS analysis of CD8+ T cells in HIV-1+ individuals. CD8+ FcRL6+ T cells (upper plot) express 2B4 (lower histogram) and therefore correspond to the effector/effector-memory population. (C) The distribution of FcRL6+ cells among CD4+ and CD8+ T cells is shown. Dramatic and statistically significant increases in FcRL6+ can be seen in both major populations of αβ-T cells. Elevation in the number of FcRL6+ CD8+ or CD4+ T cells does not correlate significantly with viral titer or CD4 count (not shown). Horizontal lines indicate mean values.
FcRL6 expression is dramatically up-regulated on lymphocytes in HIV-1–infected patients. (A) Two-color FACS analysis of blood from HIV-1+ patients and healthy controls shows relative increases in FcRL6+ lymphocytes in HIV-1+ individuals. FACS plots showing increases in CD8+ FcRL6+ cells and CD4+ FcRL6+ cells are representative of the median expression levels for healthy and infected individuals. Up-regulation of FcRL6 on B cells in HIV-1+ is also seen, though less frequently than for T cells. Cells are gated by FSC/SSC to include lymphocytes. (B) Three-color FACS analysis of CD8+ T cells in HIV-1+ individuals. CD8+ FcRL6+ T cells (upper plot) express 2B4 (lower histogram) and therefore correspond to the effector/effector-memory population. (C) The distribution of FcRL6+ cells among CD4+ and CD8+ T cells is shown. Dramatic and statistically significant increases in FcRL6+ can be seen in both major populations of αβ-T cells. Elevation in the number of FcRL6+ CD8+ or CD4+ T cells does not correlate significantly with viral titer or CD4 count (not shown). Horizontal lines indicate mean values.
Along with increased FcRL6 expression among CD8+ T cells, additional lymphocyte subsets in HIV-1–infected patients were found to express FcRL6. Most prominent of these was a subset of CD4+ T cells generally absent in uninfected controls. FcRL6+ CD4+ T cells comprise less than 1% of the circulating CD4 T cells in healthy individuals. However, in HIV-1–infected patients, the median frequency of FcRL6+ CD4+ T cells rose to 4% (Figure 5). Lastly, in addition to increased expression on T-cell subsets, FcRL6 was found to be expressed by a subset of circulating B cells in a few individuals (Figure 5). Altogether, these data expand the expression of FcRL6, during HIV infection, to populations of cells within all major classes of lymphocytes.
Discussion
We have reported a new human Fc receptor homolog that may play a role in regulating lymphocyte function during chronic viral infection. FcRL6 was identified by its homology to other Fc receptor homologs and FcγRI, and although it conserves some residues important for binding to IgG, no binding of FcRL6-expressing cells was detected to soluble or heat-aggregated human IgM or IgG (data not shown). This indicates that this molecule is not an Fc receptor, and leaves the ligand for its ectodomain undefined. The cytoplasmic tail of FcRL6 recruits SHP-2, suggesting a function for the protein in modulating some aspect of cellular activation by ligand-induced cross-linking. SHP-2 has been shown to act as negative regulator in the signaling pathway of several inhibitory receptors.23–28 However, SHP-2 also acts as positive regulator in many growth factor receptor and cytokine receptor signaling pathways, such as promoting Ras-ERK activation, resulting in proliferation and survival.29 The consequences of FcRL6 recruitment of SHP-2 on NK-cell and CD8+ T-cell functions await further study. In addition to the canonical signaling motifs identified, FcRL6 contains a repeated CxEVxC motif in the C-terminus of the protein. While we have found no precedent for this motif within other proteins, the repeated cysteine and acidic residues are reminiscent of Zn2+-binding sites found in zinc-finger– or ring-finger–containing proteins.17
We have also identified the mouse homolog of FcRL6 and found that it closely resembles rat Gp42. It is interesting that between human FcRL6 and mouse or rat FcRL6/Gp42, neither the first immunoglobulin domain nor any of the cytoplasmic signaling motifs have been conserved. Such changes underscore the rapidly evolving nature of the Fc receptor homologs, and likely reflect the divergent evolutionary pressures facing rodents and humans. However, in mice, 2 of the immunoglobulin domains have been conserved, leaving the potential to mediate ligand binding and/or cellular adhesion. In addition, the presence of a putative palmitoylation site on the C-terminus of the protein maintains the potential for mouse FcRL6 to signal through association with glycolipid-enriched motifs.
Expression of FcRL6 in the peripheral blood of healthy individuals is mostly limited to CD56dim CD16+ NK cells, and antigen-experienced CD8+ effector T-cell subsets. Expression of FcRL6 by these cytolytic subsets led us to examine whether this protein had a role in regulating cell-mediated cytotoxicity. However, we found no role for FcRL6 in either the attenuation or induction of cytolytic activity in vitro despite recruitment of the protein tyrosine phosphatase SHP-2. Certain subsets of NK cells and CD8+ T cells are also important in cytokine production. While a trend exists indicating increased IFNγ and TNFα production by NK cells following FcRL6 cross-linking, the magnitude of increase in production was relatively mild when compared with the effect of IL-2 induction or intrinsic variation among the donor cells. In addition, no effect on IFNγ and TNFα production by CD8+ T cells was found upon FcRL6 cross-linking, indicating that FcRL6 does not influence cytokine production in these cells.
Besides cytotoxicity and cytokine release, many important potential functions remain to be tested. Our preliminary studies, using CFSE-labeled primary NK cells or CD8+ T cells in vitro, have found no indication that FcRL6 influences cell proliferation or survival. However, we cannot exclude the possibility that this molecule may influence these processes in vivo in such a way that is not represented in our experiments. Additionally, participation by FcRL6 in other processes, such as cellular adhesion and motility, or in metabolic regulation remains to be tested.
Although effects of FcRL6 signaling were not evident in vitro, the presence of a phosphorylated ITIM motif, the recruitment of the protein tyrosine phosphatase SHP-2, and the expression of this NK receptor on effector populations of CD8+ T cells suggests a potential role for this protein in regulating pathogen clearance in vivo. Determining the function of FcRL6 in immune effector cells will become especially important in light of the observation that the frequency of FcRL6 expression by lymphocyte populations is dramatically up-regulated in HIV-1–infected patients. In uninfected, healthy individuals, FcRL6 expression on T cells is limited to a fraction of CD8+ T cells composed of the effector-memory and effector subsets. Previous studies have demonstrated an expansion of these subsets in HIV-1–infected individuals.30–33 Accordingly, in our study, the expansion of FcRL6+ CD8+ T cells was evident in all HIV-1+ samples, even in those without detectable virus and essentially normal CD4 counts. In fact, the frequency of expression of FcRL6 by any cell type was statistically unrelated to both viral titer and CD4 count. Thus, FcRL6, unlike CD38,34,35 is not a prognostic indicator of HIV-1 disease progression, but rather indicates the expansion of terminally differentiated effector lymphocyte populations in response to chronic viral infection.30,31
It is also possible that the high frequency of FcRL6-expressing cells reflects the perturbation of the cytokine network during HIV-1 infection. For instance, we have demonstrated that the activation of cells through IL-15 strongly down-regulates expression of FcRL6. It has been reported that in many cases, IL-15 is depleted from the serum of HIV-1+ patients.36 Moreover, the ability of myeloid dendritic cells (DCs) to secrete IL-12 is also impaired.37 Therefore it is possible that the increase in FcRL6+ cells is due to the reduced inhibitory effect of IL-15 and IL-12.
In conclusion, we have examined the structural characteristics and expression pattern of FcRL6 in lymphocytes. While the exact function of the protein has remained elusive thus far, its ability to recruit SHP-2 and limited expression of FcRL6 in healthy individuals—but widespread expression of FcRL6 by lymphocytes in HIV-1–infected patients—are an indication that this molecule reflects the immune dysregulation occurring during chronic viral infection and may modulate the function of terminally differentiated effector lymphocyte populations.
Authorship
Contribution: T.J.W. identified receptor, generated vital reagents, performed research, and wrote the paper; R.M.P. performed research and contributed to the paper; I.T. generated vital reagents and performed research; E.T.O. provided clinical samples and corresponding data; M. Cella provided vital reagents, performed research, and directed research; M. Colonna contributed to the paper and directed research.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Marco Colonna, Department of Pathology and Immunology, Department of Medicine, Washington University School of Medicine, St Louis, MO; e-mail. mcolonna@pathology.wustl.edu.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
This work was supported in part by the Center for HIV/AIDS Vaccine Immunology, CHAVI AI067854-02. Additional support was provided by the Adult AIDS Clinical Trials Group funded by the National Institute of Allergy and Infections Diseases, NIH AI-25903-18. T.J.W. is supported by a predoctoral training grant in Tumor Immunology by the Cancer Research Institute.
We gratefully acknowledge the assistance of Dr Max Arens and his staff at the Children's Retrovirology Lab for their assistance in providing and processing the HIV-1–positive blood samples. We would also like to thank Dr Persephone Borrow for critical reading of the paper.