Abstract
Natural killer (NK) cells directly lyse tumor or viral-infected cells but also an important role for NK cell cytotoxicity in regulating the extent of immune responses is emerging. Here, we show that autologous human macrophages activated NK cell proliferation and cytokine secretion, increased expression of activating receptors, and primed NK cell cytotoxicity against susceptible target cells. Ligation of NK cell 2B4, and not NKp30 (known to be important for DC-mediated NK cell activation), is critical for this macrophage-mediated NK cell activation. Reciprocally, however, NK cells regulated macrophage activity by directly killing macrophages stimulated by high doses of LPS. Cytolysis was triggered by NKG2D recognition of stress-inducible class I major histocompatibility complex (MHC)–like ligands on macrophages: high doses of LPS induced transcription and surface expression of ULBP1, ULBP2, and ULBP3 and surface expression of constitutively transcribed MICA. Thus, these data suggest a new function for NK cell cytotoxicity in eliminating overstimulated macrophages. Additionally, these interactions define, for the first time, 2 distinct activating NK cell synapses: lytic and nonlytic. Triggering NK cell proliferation and cytokine secretion, but not cytolysis, specifically associated with synaptic accumulation of macrophage F-actin and NK cell 2B4, while macrophages were killed when NK cell F-actin and macrophage ICAM-1 accumulated around a central cluster of NK cell NKG2D/DAP10.
Introduction
Natural killer (NK) cells are important effector cells of the innate immune response through their production of cytokines and lysis of transformed or infected cells without prior sensitization. NK cells exert killing by sensing “missing self” and/or by triggering of activating receptors upon interaction with specific ligands.1 One of the best-characterized activating receptors is NKG2D, expressed on NK, NKT, and T cells, which recognizes stress-inducible class I major histocompatibility complex (MHC)–like proteins.2 Other activating receptors, natural cytotoxicity receptors (NCRs), are expressed almost exclusively on NK cells.3 Ligands for activating NK cell receptors are found on many cancer cell lines4 and cells infected with bacteria5 or viruses.6–8
Immunoregulatory crosstalk between NK cells and dendritic cells (DCs) has emerged as important in both innate and adaptive immune responses.9,10 However, the extent of crosstalk between human NK cells and macrophages has been less studied. Macrophages are also important effector cells of the innate immune response, exerting their function by using a range of receptors that recognize pathogen molecules such as bacterial lipopolysaccharide (LPS).11 LPS is a powerful endotoxin that activates macrophages, although at high doses macrophages become refractory to further stimulation (ie, endotolerant).12 Here, we set out to examine the potential for immunoregulatory crosstalk between human NK cells and macrophages or macrophages activated with LPS and probe the molecular basis for this.
At the intercellular contact between immune cells, proteins are commonly seen to segregate into central and peripheral supramolecular activating clusters (c- and p-SMACs) at the immune synapse (IS). Functions of the NK cell IS could be to provide a framework for establishing checkpoints for cellular activation and/or directing secretion of lytic granules or cytokines in some circumstances.13–15 Here we define, for the first time, 2 distinct NK cell–activating synapses. The macrophage–NK cell IS associated with priming, but not triggering, NK cytolysis and the IS between NK cells and macrophages treated with a high dose of LPS that triggers NK cytolysis.
Materials and methods
Generation of macrophages and DCs
Peripheral blood mononuclear cells (PBMCs) were isolated by density gradient centrifugation (Ficoll-Paque Plus; Amersham Pharmacia Biotech, Piscataway, NJ). Serum was collected, heat inactivated for 30 minutes at 56°C, and filtered. PBMCs were incubated for 2 hours in plastic plates previously coated overnight with 2% gelatin (Sigma-Aldrich, St Louis, MO). After 2 hours, the flask was washed intensively to remove the nonadherent cells. After 24 hours of incubation in serum-free media (X-vivo 10; Bio-Whittaker, Walkersville, MD) with 1% autologous serum, monocytes were washed with cold PBS and 98% of cells were positive for CD14 as assessed by flow cytometry (CELLQuest; Becton Dickinson, San Jose, CA). CD14-expressing cells were then cultured in X-vivo media with 1% autologous serum for 10 to 12 days to generate macrophages. For activation when appropriate, macrophages were incubated with LPS from Salmonella minnesota (Sigma-Aldrich) for 48 hours.
DCs were generated by incubating 2 × 106 CD14-expressing cells per milliliter in RPMI containing 5% human serum (Type AB; Sigma-Aldrich), 150 ng/mL rhIL-4, and 150 ng/mL rhGM-CSF (R&D Systems, Minneapolis, MN). The medium was replenished after 3, 5, and 7 days, and after 10 days, floating cells were harvested and confirmed to be DCs. The phenotype of monocytes, macrophages, and DCs after preparation is shown in Figure S1 (available on the Blood website; see the Supplemental Materials link at the top of the online article).
Isolation and culture of NK cells
NK cells were isolated from PBMCs by negative selection using magnetic-activated cell sorting (Human NK Cell Enrichment Cocktail; Stem Cell Technologies, Vancouver, BC, Canada) and monitored to be CD3− and CD56+. NK cells were grown in DMEM (GIBCO-BRL, Gaithersburg, MD) supplemented with 10% human serum, 1 mM l-glutamine, 1 mM sodium pyruvate, 1 mM penicillin-streptomycin, 1 mM MEM nonessential amino acids, and 20 μM β-mercaptoethanol. Freshly isolated NK cells were used unless stated that cultured NK cells were used, in which case 100 U/mL human recombinant IL-2 (Roche, Basel, Switzerland) was added to the media and NK cells were used 8 days after restimulation with human recombinant IL-2 (ie, when resting). Where it is stated that IL-2–activated NK cells were used, NK cells were used one day after restimulation with rhIL-2.
Cytotoxicity assays
The cytolytic activity of NK cells was assessed in standard 5-hour 35S-Met release assays. For blocking experiments, NK cells were incubated with 20 μg/mL anti-NKG2D monoclonal antibody (mAb) or an isotype-matched control mAb for 30 minutes on ice and washed prior to assaying their cytolytic activity. To assess NK cell cytotoxicity after coincubation with antigen-presenting cells (APCs), NK cells were first dissociated from APCs by incubation in 5 mM EDTA/0.2% BSA/PBS for 30 minutes at 4°C, and free-floating NK cells were then harvested and washed with cold PBS before use. The purity of isolated NK cells was 98% as determined by being CD56+ and CD3− by flow cytometry, and viability was confirmed by cells being unstained by trypan blue.
Flow cytometric analysis
For analysis of macrophages after activation with LPS, cells were scraped gently in cold PBS. For analysis of NK cells after coculture with APCs, cells were first dissociated from APCs as described for cytotoxicity assays. Isolated NK cells or macrophages were then incubated with 10% human serum/1% BSA/0.01% azide/PBS for 30 minutes on ice and washed 3 times. Then, cells were incubated with the appropriate mAb in 1% BSA/0.01% azide/PBS for 45 minutes on ice, washed 3 times, and incubated for 45 minutes on ice with FITC- or PE-conjugated secondary antibody. Finally, cells were washed 3 times and analyzed by flow cytometry (FACSCalibur; Becton Dickinson).
Antibodies
The following mAbs were used: anti-CD14 (61D3, IgG1; eBioscience, San Diego, CA), anti–class I MHC (W6/32, IgG2a; American Type Culture Collection, Manassas, VA), anti-CD154 (TRAP-1, IgG1; Santa Cruz Biotechnology, Santa Cruz, CA), anti-2B4 (C1.7, IgG1; Immunotech, Marseille, France); anti-ULBP1 (170818), anti-ULBP2 (165903), anti-ULBP3 (166510), anti-MICA/B (159207) (IgG2a; R&D Systems), anti-ULBP1 (M295), anti-ULBP2 (M311), anti-ULBP3 (M551, for flow cytometry and Western blotting), and anti-MICA (M673, for Western blot) (all IgG1; Amgen, Thousand Oaks, CA); and anti-MICA (for flow cytometry, BAM195, IgG1; as described previously4 ), anti-NKG2D (for blocking and flow cytometry, 149810, IgG1, R&D Systems; for immunofluorescence staining, BAT221, IgG1; as described previously4 ), and anti-NKp30 (A76, IgG1, for flow cytometry; or F252, IgM, for blocking).16 Anti-NKp44 and anti-NKp46 were kind gifts from A. Moretta, University of Genova, Italy. Other antibodies used were anti–HLA-DR (L243, IgG2a), -CD54 (HA58, IgG1), -CD44 (515, IgG1), -CD3 (UCHT1, IgG1), -CD56 (B159, IgG1), -CD3ζ (8D3, IgG1), -CD11c (B-ly6, IgG1), -CD1a (HI149, IgG1), -CD48 (Tü145, IgM), -actin (C4, IgG1), -perforin (δG9, IgG2b), -CCR7 (3D12, IgG2a), -CD86 (FUN1, IgG1), -CD69 (FN50, IgG1), -CD25 (M-A251, IgG1), IgG2a control (G155-178), IgG2b control (MPC-11), IgG1 control (MOPC-21), and IgM control (R6-60.2) (all from BD PharMingen, Franklin Lakes, NJ). FITC- or PE-conjugated secondary antibodies were used (Jackson ImmunoResearch, West Grove, PA). Finally, we generated a rat anti-DAP10 mAb (4E1, IgG2a) using a synthetic peptide corresponding to DAP10 intracytoplasmic domain. Cytokine secretion by macrophages was assayed by flow cytometry (Cytometric Bead Array - Human Inflammation kit; BD PharMingen). For Western blotting, macrophages were incubated with different doses of LPS for 48 hours, lysed, and blotted with the indicated antibodies.
Assays for NK cell responses after coculture with macrophages, DCs, or monocytes
Freshly isolated NK cells (105 per well) were incubated with macrophages or DCs, derived from 10 to 12 days of culture, or monocytes, freshly isolated from the same donor, at the indicated ratio in media used to culture NK cells (without IL-2) in 96-well flat-bottom plates. To test for IFN-γ secretion after 48 hours of coculture, supernatants were assayed by enzyme-linked immunosorbent assay (ELISA) using anti-IFNγ capture mAb (NIB42), biotinylated anti-IFNγ detection mAb (4S.B3), and streptavidin-HRP (all from BD PharMingen). To test for proliferation after 24 hours of coculture, 1 μCi (0.037 MBq ) [3H] thymidine (ICN Biomedicals, Irvine, CA) was added to each well, and NK cells were harvested the following day (Harvester Mach IIIM; Tomtec, Hamden, CT) and tested for incorporated [3H] thymidine (1540 MicroBeta TriLux; PerkinElmer, Boston, MA). For blocking experiments, APCs were incubated with 10% human serum for 30 minutes, washed, and cocultured for 48 hours with NK cells previously incubated with 4 μg/mL anti-2B4, anti-LFA1, anti-CD40L, anti-NKp30, or isotype-matched control IgG1 or IgM mAbs for 30 minutes on ice. Alternatively, 4 μg/mL anti-CD48 or an isotype-matched control mAb was added to each APC for 30 minutes on ice and washed prior to coculture with NK cells for 48 hours.
Reverse transcriptase–polymerase chain reaction (RT-PCR)
Macrophages were incubated with 50, 100, or 200 ng/mL LPS for 24 or 48 hours. Total RNA was extracted from macrophages (RNeasy Kit, Qiagen, Crawley, United Kingdom), and samples were treated with DNase I (Ambion, Warrington, United Kingdom) before 4 μg RNA was used to synthesise first-strand cDNA (SUPERSCRIPT, Invitrogen, Paisley, United Kingdom). One microliter of the resulting cDNA was used in a 20 μL PCR reaction using AmpliTaq Gold (Applied Biosystems, Foster City, CA). Primers and conditions used for PCR of each MIC/ULBP gene are given in Table S1 (available at the Blood website; see the Supplemental Materials link at the top of the online article). PCR for GAPDH was used as a control for cDNA synthesis. PCR reactions were run in parallel with negative (water) and positive control reactions. HT1080 cell cDNA was used as a positive control for ULBP3, RAET1E, RAET1G, MICA, and MICB expression, and Jurkat cDNA was used as a positive control for containing ULBP1 and ULBP2 transcripts. For RAET1L, a pooled Universal cDNA library was used (Stratagene, La Jolla, CA). PCR products were cloned using a pGEM-T Easy kit (Promega, Madison, WI) and sequenced to confirm their identity.
Confocal microscopy
For imaging, macrophages were derived from monocytes on coverslips over 10 to 12 days of culture and then incubated with autologous IL-2–cultured NK cells (resting NK cells) for 5, 10, and 20 minutes in a ratio of 1:5 (macrophage/NK cells). Cells were fixed (Cytofix/Cytoperm; BD PharMingen) at 4°C, washed 3 times with 0.1% Tween 20/PBS, and incubated with the indicated antibodies, as described previously.17 For imaging F-actin, cells were stained with 5 U/mL Alexa Fluor 488–conjugated phalloidin (Molecular Probes, Eugene, OR) for 1 hour at 4°C and washed 3 times with 0.1% Tween 20/PBS. When appropriate, macrophages were first incubated with LPS for 48 hours. For ATP depletion or cytoskeletal inhibition, cells were incubated respectively with 50 mM sodium azide (Sigma-Aldrich) for 2 hours or 10 μM cytochalasin D (Sigma-Aldrich) for 1 hour. More than 85% depletion of ATP was confirmed by a luciferase-based assay (ATPLite-M; Packard Bioscience, Groningen, Netherlands). Cell conjugates were imaged under a 63×/1.32 oil immersion objective using a laser scanning confocal microscope (TCS SP2; Leica, Wetzlar, Germany). Conjugates were scanned in the xy direction every 0.3 μm throughout the z plane. The face of the IS was then reconstructed using a maximum intensity projection (Volocity; Improvision, Coventry, United Kingdom). Protein was considered clustered at the IS if the fluorescence intensity of labeled proteins at the intercellular contact reached at least greater than twice that of the intensity at unconjugated regions of the same cell membrane. The percentage of fluorescence and the ratio of fluorescence at the IS compared with another region of the same area were quantified from 3-dimensional reconstructions of conjugate fluorescence (Volocity; Improvision).
Statistical analysis
Data were analyzed by analysis of variance (ANOVA) and the Student t test for unpaired values unless stated otherwise. P values less than .05 were considered significant. The results are expressed as the mean ± SE.
Results
Macrophages can stimulate autologous NK cells to proliferate, secrete IFN-γ, and augment NK cytotoxicity against susceptible target cells
Pure populations of macrophages and DCs were prepared (Figure S1), and blood from the same donor was then taken 10 to 12 days later to prepare autologous NK cells and monocytes. In this manner, autologous NK cells could be coincubated with macrophages or DCs derived from monocytes over 10 to 12 days of culture or monocytes that were freshly isolated from the same donor. In addition, macrophages were stimulated for 48 hours with 100 or 200 ng/mL LPS, hereafter denoted macrophageslow LPS or macrophageshigh LPS, respectively, during the coincubation with NK cells.
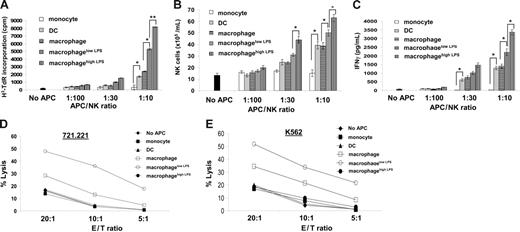
After coincubation for 48 hours, the rate of NK cell proliferation was measured by thymidine incorporation (Figure 1A). Additionally, the total extent of NK cell proliferation was assessed by counting the number of live NK cells, unstained by trypan blue, present after 48 hours of coculture (Figure 1B). Counting adherent APCs after coincubation with NK cells confirmed that they did not proliferate (data not shown). By either assay, it was clear that NK cells had been activated to proliferate when coincubated with either macrophages or DCs but not monocytes. Macrophages activated by LPS caused even greater NK cell proliferation. There was no increase in NK cell proliferation when NK cells were incubated with LPS in the absence of macrophages (Figure S2). Additionally, IFN-γ was detected by ELISA in the supernatant of NK cells cocultured with macrophages, LPS-activated macrophages or DCs, but not monocytes (Figure 1C). Thus, both macrophages and DCs can induce NK cell proliferation and cytokine secretion.
Human macrophages trigger NK cell proliferation, IFN-γ secretion, and prime NK cell cytotoxicity to susceptible target cells. Autologous NK cells were coincubated for 48 hours with monocytes, DCs, macrophages, or macrophages in the presence of 100 ng/mL (macrophagelow LPS) or 200 ng/mL (macrophagehigh LPS) LPS at the ratios shown. (A) 3H-TdR incorporation by NK cells. The first data point shows the extent of 3H-TdR incorporation in NK cells cultured in media alone. (B) Viable NK cells, unstained by trypan blue, after 48 hours of coincubation with APCs at the ratios indicated. Cells were counted using a hemocytometer. The first bar indicates the number of viable NK cells after culture in media alone. (C) IFN-γ secretion from NK cells cocultured with the indicated APCs, at the ratios shown, measured by ELISA. NK cells were isolated after coincubation with indicated APCs and tested for cytolytic activity against (D) the EBV-transformed B-cell line 721.221 and (E) the myeloid leukemia cell line K562. *P < .05, **P < .01 by Student t test for unpaired values. The results are expressed as the mean ± SE of at least 3 independent experiments using cells from different donors.
Human macrophages trigger NK cell proliferation, IFN-γ secretion, and prime NK cell cytotoxicity to susceptible target cells. Autologous NK cells were coincubated for 48 hours with monocytes, DCs, macrophages, or macrophages in the presence of 100 ng/mL (macrophagelow LPS) or 200 ng/mL (macrophagehigh LPS) LPS at the ratios shown. (A) 3H-TdR incorporation by NK cells. The first data point shows the extent of 3H-TdR incorporation in NK cells cultured in media alone. (B) Viable NK cells, unstained by trypan blue, after 48 hours of coincubation with APCs at the ratios indicated. Cells were counted using a hemocytometer. The first bar indicates the number of viable NK cells after culture in media alone. (C) IFN-γ secretion from NK cells cocultured with the indicated APCs, at the ratios shown, measured by ELISA. NK cells were isolated after coincubation with indicated APCs and tested for cytolytic activity against (D) the EBV-transformed B-cell line 721.221 and (E) the myeloid leukemia cell line K562. *P < .05, **P < .01 by Student t test for unpaired values. The results are expressed as the mean ± SE of at least 3 independent experiments using cells from different donors.
We next set out to determine how NK cell cytotoxicity against susceptible target cells would be influenced by prior coculture with APCs. After coculture, NK cells were dissociated from APCs and harvested to 98% purity. NK cell cytotoxicity was tested against the MHC class I–negative Epstein-Barr virus (EBV)–transformed B-cell line 721.221 (Figure 1D) or the erythroleukemic cell line K562 (Figure 1E). The extent of lysis of either target cell was dramatically increased by prior incubation of NK cells with macrophages. Unexpectedly, however, NK cells coincubated with macrophageslow LPS were subsequently able to kill target cells to a far greater extent than cells that were incubated with macrophageshigh LPS.
NK cell 2B4 recognition of macrophage CD48 is critical for triggering NK cell proliferation and IFN-γ secretion
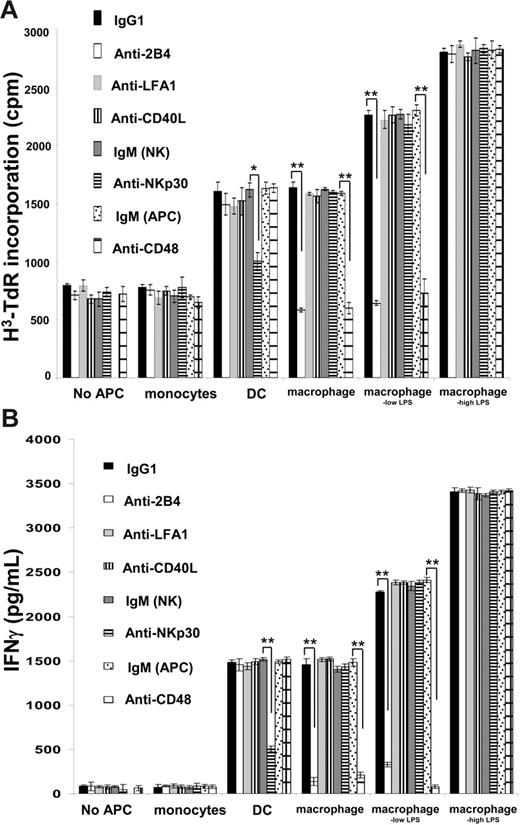
To probe the molecular basis of NK cell activation by macrophages, we tested if various mAbs would block the NK cell response after coculture with monocytes, DCs, macrophages, or macrophages activated with LPS for 48 hours at a 10:1 ratio (NK/APC). We found that proliferation of NK cells (Figure 2A) and secretion of IFN-γ (Figure 2B) triggered by coincubation with macrophages or macrophageslow LPS could be efficiently blocked with antibodies against NK cell 2B4 or its ligand CD48 on macrophages. However, while anti-2B4 or anti-CD48 mAbs were efficient at blocking NK cell proliferation and IFN-γ secretion in response to macrophages or macrophageslow LPS, they had no effect on the response of NK cells cocultured with macrophageshigh LPS (Figure 2). This further suggested that a distinct NK cell response occurs to macrophages stimulated with high doses of LPS.
2B4 and CD48 are critical for macrophage-mediated activation of NK cell proliferation and IFN-γ secretion. (A) Proliferation and (B) IFN-γ secretion of NK cells during 48 hours of coincubation alone or with monocytes, DCs, macrophages, and macrophages activated with 100 ng/mL (macrophageslow LPS) or 200 ng/mL (macrophageshigh LPS) LPS at a 10:1 ratio. NK cells were pulsed with anti-2B4, anti-LFA1, anti-CD40L, anti-NKp30 (F252, IgM), or isotype-matched control mAbs prior to coculture with APCs for 48 hours. Alternatively, APCs were pulsed with anti-CD48 or isotype-matched control mAb (IgM; APC) prior to coculture with NK cells for 48 hours. *P < .05, **P < .01 by Student t test for unpaired values. The results are expressed as the mean ± SE of at least 3 independent experiments using cells from different donors.
2B4 and CD48 are critical for macrophage-mediated activation of NK cell proliferation and IFN-γ secretion. (A) Proliferation and (B) IFN-γ secretion of NK cells during 48 hours of coincubation alone or with monocytes, DCs, macrophages, and macrophages activated with 100 ng/mL (macrophageslow LPS) or 200 ng/mL (macrophageshigh LPS) LPS at a 10:1 ratio. NK cells were pulsed with anti-2B4, anti-LFA1, anti-CD40L, anti-NKp30 (F252, IgM), or isotype-matched control mAbs prior to coculture with APCs for 48 hours. Alternatively, APCs were pulsed with anti-CD48 or isotype-matched control mAb (IgM; APC) prior to coculture with NK cells for 48 hours. *P < .05, **P < .01 by Student t test for unpaired values. The results are expressed as the mean ± SE of at least 3 independent experiments using cells from different donors.
We found that neither NK cell proliferation nor IFN-γ secretion was influenced by mAbs against NKp30, whereas blocking this receptor did abrogate NK cell proliferation and cytokine secretion triggered by DCs (Figure 2), consistent with previous studies.18 NK cell proliferation was also unaffected by mAb blocking of CD40L or LFA1. Thus, the interaction of 2B4 and CD48 between NK cells and macrophages is critical in triggering NK cell proliferation and IFN-γ secretion, whereas NKp30 is critical in triggering NK cell activation by DCs.
Altered expression of NK cell surface proteins after incubation with macrophages
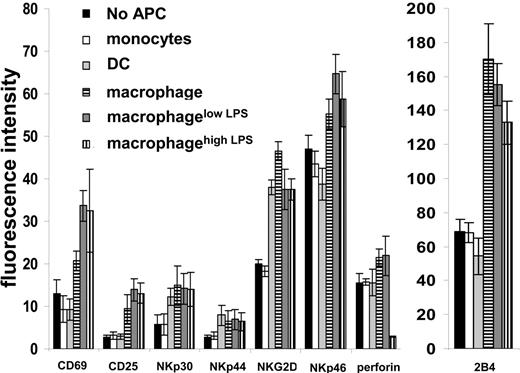
We next examined whether coculture with APCs altered the expression of NK cell surface proteins. After coculture with monocytes, DCs, macrophages, or macrophages activated with LPS for 48 hours at a 10:1 ratio (NK/APC), NK cells were isolated and the cell surface expression of several proteins assessed by flow cytometry. NK cells in coculture with DCs, macrophages, or LPS-activated macrophages expressed increased levels of the activating receptors NKp30, NKp44, and NKG2D. Thus, to some extent, DCs and macrophages show a broadly similar ability to activate NK cells. However, NK cells cocultured with macrophages or LPS-activated macrophages, but not those cultured with DCs, expressed higher levels of the activating receptor NKp46 (Figure 3). Particularly striking was the increased level of 2B4 expression on NK cells coincubated with macrophages or LPS-activated macrophages but not DCs (Figure 3). Surprisingly, intracellular staining revealed dramatically less perforin in NK cells coincubated with macrophageshigh LPS, consistent with the lower cytolytic activity of those NK cells against susceptible targets (Figure 1D-E).
Phenotype of NK cells after coculture with autologous APCs. NK cells were stained for different surface markers after coincubation for 48 hours alone or with monocytes, DC, macrophages, and macrophages activated with 100 ng/mL (macrophagelow LPS) or 200 ng/mL (macrophagehigh LPS) LPS at 10:1 ratio and analyzed by flow cytometry. Fluorescence intensities were calculated as the geometric mean fluorescence intensity of the sample − geometric mean fluorescence intensity after isotype-matched control mAb staining. The mean ± SD from 3 independent experiments using cells derived from different donors is shown. Instrumental settings were adjusted so that the fluorescence intensity of all samples stained with an isotype-matched control mAb was similar.
Phenotype of NK cells after coculture with autologous APCs. NK cells were stained for different surface markers after coincubation for 48 hours alone or with monocytes, DC, macrophages, and macrophages activated with 100 ng/mL (macrophagelow LPS) or 200 ng/mL (macrophagehigh LPS) LPS at 10:1 ratio and analyzed by flow cytometry. Fluorescence intensities were calculated as the geometric mean fluorescence intensity of the sample − geometric mean fluorescence intensity after isotype-matched control mAb staining. The mean ± SD from 3 independent experiments using cells derived from different donors is shown. Instrumental settings were adjusted so that the fluorescence intensity of all samples stained with an isotype-matched control mAb was similar.
Macrophages treated with high doses of LPS are directly lysed by NK cells
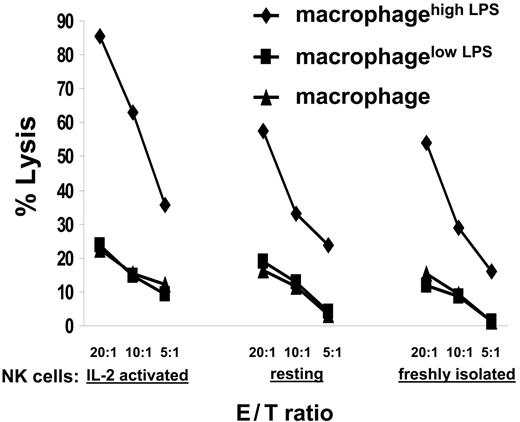
We next set out to assay for NK cell cytolytic activity against macrophages or macrophages activated with different doses of LPS. We found that (1) freshly isolated NK cells, (2) resting IL-2–cultured NK cells last stimulated 8 days earlier with IL-2, or (3) IL-2–activated NK cells stimulated 1 day earlier with IL-2 all could directly lyse autologous macrophageshigh LPS (Figure 4). Macrophages incubated with 200 ng/mL LPS for a shorter time (ie, 12 or 24 hours) or macrophages incubated with a lower dose of LPS (ie, 100 ng/mL) (data not shown) were not lysed by autologous NK cells. Thus, NK cells specifically killed macrophages stimulated with a high dose of LPS. Exhaustion in NK cell cytotoxicity explains the loss of perforin (Figure 3) and the decreased lysis of 721.221 or K562 by NK cells previously coincubated with macrophageshigh LPS (Figure 1).
Macrophages stimulated with a high dose of LPS were lysed by NK cells. IL-2–activated NK cells (ie, NK cells 1 day after restimulation with human recombinant IL-2), resting NK cells (ie, NK cells expanded with IL-2 but used 8 days after restimulation), or freshly isolated NK cells were tested for their cytolytic activity against autologous unstimulated macrophages or autologous macrophages incubated with 100 ng/mL (macrophagelow LPS) or 200 ng/mL (macrophagehigh LPS) LPS for 48 hours. NK cells and target cells were incubated at the ratios indicated, and specific lysis was measured by a standard 35S release assay. In each experiment the spontaneous release of 35S was less than 20% of the total. The mean of at least 3 independent experiments using cells from different donors is shown.
Macrophages stimulated with a high dose of LPS were lysed by NK cells. IL-2–activated NK cells (ie, NK cells 1 day after restimulation with human recombinant IL-2), resting NK cells (ie, NK cells expanded with IL-2 but used 8 days after restimulation), or freshly isolated NK cells were tested for their cytolytic activity against autologous unstimulated macrophages or autologous macrophages incubated with 100 ng/mL (macrophagelow LPS) or 200 ng/mL (macrophagehigh LPS) LPS for 48 hours. NK cells and target cells were incubated at the ratios indicated, and specific lysis was measured by a standard 35S release assay. In each experiment the spontaneous release of 35S was less than 20% of the total. The mean of at least 3 independent experiments using cells from different donors is shown.
High doses of LPS trigger a distinct macrophage phenotype, including expression of stress-inducible ligands for NKG2D
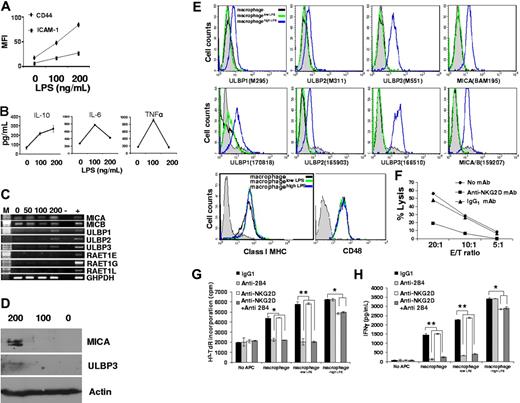
We next set out to compare the phenotypes of macrophageslow LPS and macrophageshigh LPS. ICAM-1 and CD44 expression (Figure 5A) and IL-10 secretion (Figure 5B) increased upon stimulation by LPS in a dose-dependent manner. However, by contrast, 200 ng/mL LPS triggered less secretion of TNF-α or IL-6 (Figure 5B). The high dose of LPS used did not trigger less cytokine secretion due to apoptosis of macrophages, because macrophageshigh LPS remained unstained with propidium iodide (PI) or annexin V (data not shown). Thus, a distinct macrophage phenotype is induced by high doses of LPS.
High doses of LPS caused a distinct macrophage phenotype including protein expression of NKG2D ligands that trigger NK cell–mediated lysis. (A) Macrophages or macrophages incubated with 100 or 200 ng/mL LPS for 48 hours (denoted 0, 100, 200 respectively) were stained with anti-CD54 mAb or anti-CD44 mAb and analyzed by flow cytometry. (B) Cytokines secreted into the supernatant by macrophages stimulated with different doses of LPS for 48 hours. (C) Macrophages that were incubated with different amounts of LPS for 48 hours were probed for transcripts of different NKG2D ligand genes by RT-PCR. M indicates marker; 0, 50, 100, and 200, the amount of LPS in nanograms per milliliter; -, water; +, positive control. (D) Macrophages incubated with different amounts of LPS for 48 hours were tested for expression of ULBP3 and MICA by Western blotting. (E) Macrophages after incubation with 0 (green line), 100 (macrophagelow LPS; black), and 200 (macrophagehigh LPS; blue) ng/mL LPS were stained for NKG2D ligands, class I MHC protein, or CD48 and analyzed by flow cytometry. Expression of each NKG2D ligand was confirmed by using alternative mAb as shown (clone names given in parentheses). Isotype-matched control mAbs were used for comparison (shown in each panel for macrophagehigh LPS; gray filled histograms). (F) NK cells were preincubated with 20 μg/mL anti-NKG2D mAb or an IgG1 isotype-matched control for 45 minutes before testing their cytotoxicity against macrophages incubated with 200 ng/mL LPS for 48 hours. NK cells and target cells were incubated at the ratios indicated, and specific lysis was measured by standard 35S release assays. In each experiment the spontaneous release of 35S was less than 20% of the total. Proliferation (G) and IFN-γ secretion (H) of NK cells after 48 hours incubated alone (no APC) or coincubated with macrophages or macrophages activated with 100 ng/mL (macrophagelow LPS) or 200 ng/mL (macrophagehigh LPS) LPS at a 10:1 ratio. NK cells were pulsed with anti-2B4 (4 μg/mL), anti-NKG2D (20 μg/mL), or isotype-matched control mAbs prior to coculture with APC for 48 hours. *P < .05, **P < .01 by Student t test for unpaired values. The results are expressed as the mean ± SE of at least 3 independent experiments using cells from different donors.
High doses of LPS caused a distinct macrophage phenotype including protein expression of NKG2D ligands that trigger NK cell–mediated lysis. (A) Macrophages or macrophages incubated with 100 or 200 ng/mL LPS for 48 hours (denoted 0, 100, 200 respectively) were stained with anti-CD54 mAb or anti-CD44 mAb and analyzed by flow cytometry. (B) Cytokines secreted into the supernatant by macrophages stimulated with different doses of LPS for 48 hours. (C) Macrophages that were incubated with different amounts of LPS for 48 hours were probed for transcripts of different NKG2D ligand genes by RT-PCR. M indicates marker; 0, 50, 100, and 200, the amount of LPS in nanograms per milliliter; -, water; +, positive control. (D) Macrophages incubated with different amounts of LPS for 48 hours were tested for expression of ULBP3 and MICA by Western blotting. (E) Macrophages after incubation with 0 (green line), 100 (macrophagelow LPS; black), and 200 (macrophagehigh LPS; blue) ng/mL LPS were stained for NKG2D ligands, class I MHC protein, or CD48 and analyzed by flow cytometry. Expression of each NKG2D ligand was confirmed by using alternative mAb as shown (clone names given in parentheses). Isotype-matched control mAbs were used for comparison (shown in each panel for macrophagehigh LPS; gray filled histograms). (F) NK cells were preincubated with 20 μg/mL anti-NKG2D mAb or an IgG1 isotype-matched control for 45 minutes before testing their cytotoxicity against macrophages incubated with 200 ng/mL LPS for 48 hours. NK cells and target cells were incubated at the ratios indicated, and specific lysis was measured by standard 35S release assays. In each experiment the spontaneous release of 35S was less than 20% of the total. Proliferation (G) and IFN-γ secretion (H) of NK cells after 48 hours incubated alone (no APC) or coincubated with macrophages or macrophages activated with 100 ng/mL (macrophagelow LPS) or 200 ng/mL (macrophagehigh LPS) LPS at a 10:1 ratio. NK cells were pulsed with anti-2B4 (4 μg/mL), anti-NKG2D (20 μg/mL), or isotype-matched control mAbs prior to coculture with APC for 48 hours. *P < .05, **P < .01 by Student t test for unpaired values. The results are expressed as the mean ± SE of at least 3 independent experiments using cells from different donors.
Most importantly, transcription of NKG2D ligands ULBP1, ULBP2, and ULBP3 but not RAET1E, RAET1G, and RAET1L was triggered in macrophages only upon stimulation with 200 ng/mL LPS for 48 hours and not with 100 ng/mL LPS (Figure 5C) or with any dose of LPS for 24 hours (data not shown). Transcripts of NKG2D ligands MICA and MICB were detected constitutively in macrophages, but expression of MICA protein was only found in macrophages incubated with 200 ng/mL LPS (Figure 5D). Importantly, ULBP1, ULBP2, ULBP3, and MICA were detected at the surface of macrophageshigh LPS by flow cytometry (Figure 5E). Expression of each NKG2D ligand was confirmed with alternative mAbs of different isotypes, and macrophage expression of class I MHC protein or CD48 (Figure 5E) did not change after LPS activation. Thus, de novo transcription of ULBPs and cell surface expression of both ULBPs and MIC is specifically triggered in macrophages by a high dose of LPS.
NK cell–mediated lysis of macrophageshigh LPS could be abrogated by the addition of 20 μg/mL anti-NKG2D mAb and not an isotype-matched control mAb (Figure 5F). The low NK cytotoxicity against macrophages or macrophageslow LPS was unaffected by anti-NKG2D mAb (data not shown). The addition of anti-NKG2D mAb only slightly reduced NK cell proliferation (Figure 5G) and IFN-γ secretion (Figure 5H) after coincubation with macrophageshigh LPS. Thus, it is possible that other receptor-ligand interactions may also be important in macrophageshigh LPS triggering NK cell proliferation and IFN-γ secretion, such as the very recently reported interaction between NKp80 and AICL.19 Nevertheless, blocking of lysis by anti-NKG2D mAb was potent and, thus, macrophages stimulated with high doses of LPS are lysed by NK cells via NKG2D recognition. NK cell cytotoxicity can therefore serve to eliminate overstimulated macrophages that would perhaps otherwise contribute to an immunopathology or endotoxic shock.
Accumulation of F-actin at the macrophage–NK cell IS
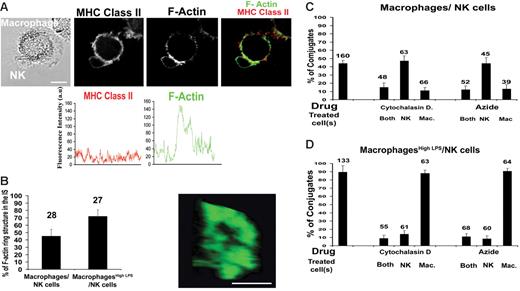
Interactions with macrophages or macrophageshigh LPS define 2 distinct NK cell–activating synapses, one triggering only proliferation and cytokine secretion with the other triggering lysis, and thus we set out to compare their structures. Conjugates between NK cells and autologous macrophages or macrophageshigh LPS were fixed and labeled with phalloidin marking F-actin and imaged by confocal microscopy. The fluorescence intensity around one cell in the conjugate showed, for example, a 3-fold increase of F-actin at the synapse compared with elsewhere at the cell membrane (Figure 6A). Such accumulation of F-actin at the macrophage–NK cell synapse is not merely a reflection of an increase in cell membrane at the synapse, because there was no increase in the amount of MHC class II protein at the synapse (Figure 6A).
Accumulation of F-actin at the NK cell–macrophage IS. (A) NK cells and autologous macrophages were coincubated, fixed, and then stained with anti–MHC class II mAb (red) and with Alexa Fluor 488–conjugated phalloidin (green) to mark the location of F-actin. Analysis of the fluorescence intensity around the cell membrane demonstrates a 3-fold increase in the amount of F-actin, but not MHC class II protein, at the synapse. (B) The organization of F-actin at the IS was often ring-shaped as shown. Bar chart shows the frequency of a clearly ring-shaped distribution of F-actin at the IS between NK cells and unstimulated macrophages or macrophageshigh LPS. (C) The percent of NK cell–macrophage conjugates where F-actin clearly accumulated at the IS was scored and then compared when each cell type was pretreated with cytochalasin D or sodium azide. (D) The percent of NK cell–macrophageshigh LPS conjugates where F-actin clearly accumulated at the IS was scored and then compared when each cell type was pretreated with cytochalasin D or sodium azide. The means ± SD from 3 independent experiments are shown with the total number of cells indicated above each bar. Scale bars, 10 μm.
Accumulation of F-actin at the NK cell–macrophage IS. (A) NK cells and autologous macrophages were coincubated, fixed, and then stained with anti–MHC class II mAb (red) and with Alexa Fluor 488–conjugated phalloidin (green) to mark the location of F-actin. Analysis of the fluorescence intensity around the cell membrane demonstrates a 3-fold increase in the amount of F-actin, but not MHC class II protein, at the synapse. (B) The organization of F-actin at the IS was often ring-shaped as shown. Bar chart shows the frequency of a clearly ring-shaped distribution of F-actin at the IS between NK cells and unstimulated macrophages or macrophageshigh LPS. (C) The percent of NK cell–macrophage conjugates where F-actin clearly accumulated at the IS was scored and then compared when each cell type was pretreated with cytochalasin D or sodium azide. (D) The percent of NK cell–macrophageshigh LPS conjugates where F-actin clearly accumulated at the IS was scored and then compared when each cell type was pretreated with cytochalasin D or sodium azide. The means ± SD from 3 independent experiments are shown with the total number of cells indicated above each bar. Scale bars, 10 μm.
A more than 2-fold increase in phalloidin staining was seen at 45% of the intercellular contacts between NK cells and unstimulated macrophages compared with unconjugated membrane. The arrangement of F-actin at the contact was ring-shaped in 45% of conjugates where F-actin did accumulate between NK cells and macrophages (Figure 6B). No differences in the frequency of F-actin accumulation or ring-shaped arrangements of F-actin were seen when cells were coincubated together for 5, 10, 20, or 30 minutes before fixation (data not shown). Thus, assembly of a ring-shaped distribution of F-actin at the NK cell–macrophage IS is likely to be an early and/or long-lasting event. Strikingly, however, F-actin was seen to accumulate at 85% of intercellular contacts between macrophageshigh LPS and NK cells, and in 65% of these conjugates F-actin was in a ring-shaped arrangement. Thus, F-actin accumulates more frequently at the IS between macrophageshigh LPS and NK cells.
Optical microscopy is unable to resolve directly from which cell type F-actin accumulated at the IS. To test this, NK cells and/or macrophages were treated before coculture, with either cytochalasin D to inhibit actin polymerization or azide to inhibit ATP-dependent processes. For synapses involving either macrophages or macrophageshigh LPS, treatment of both NK cells and macrophages with either drug reduced the frequency of F-actin accumulation at the IS to about 10% (Figure 6C-D). When only the macrophages were treated with either cytochalasin D or azide, the frequency of F-actin accumulating at the IS between NK cells and macrophages was also reduced to about 10% (Figure 6C). Treatment of only the NK cells had no effect on the frequency of F-actin at this noncytolytic IS. In contrast, only when NK cells were treated with either cytochalasin D or azide was the accumulation of F-actin at the IS between NK cells and macrophageshigh LPS abrogated (Figure 6D). Thus, distinct synapses are assembled to facilitate disparate outcomes of NK cell interactions with macrophages or macrophageshigh LPS. Specifically, NK cell–derived F-actin accumulates at cytolytic synapses with macrophageshigh LPS, while macrophage F-actin accumulates at the noncytolytic IS between macrophages and NK cells.
Trafficking of specific surface proteins and signaling adaptors to the NK cell–macrophage IS
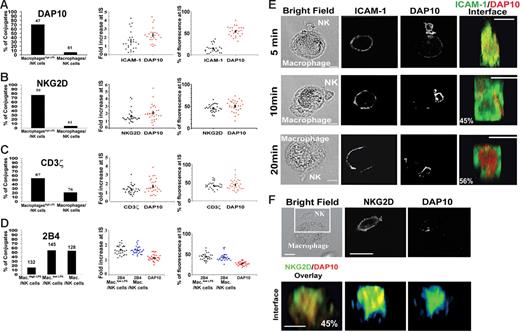
Next, we compared the extent of recruitment and the specific supramolecular organization of proteins key to the outcome of NK cell–macrophage communication. After 20 minutes of coincubation, DAP10 was recruited only to the NK cell IS with macrophageshigh LPS (Figure 7A). Similarly, NKG2D accumulated at 76% of synapses with macrophageshigh LPS while very rarely accumulating at the IS with macrophages (Figure 7B). It is particularly striking that so much NK cell NKG2D and DAP10—on average about 50% of the total amount—is accumulated at the IS. This results in an average approximate 2-fold increase in the amount of these proteins at the IS compared with the unconjugated membrane, implying that the contact between NK cells and macrophages is far larger than other NK synapses (eg, with transformed B cells). Specific accumulation of NKG2D and DAP10 at the NK cell synapse with macrophageshigh LPS further demonstrates the key role of these proteins in triggering cytotoxicity.
Two distinct NK cell–activating immune synapses. (A-D) NK cells were coincubated with macrophages or macrophageshigh LPS for 20 minutes, and conjugates were fixed, stained, and imaged. The left panels show the frequency of conjugates where the indicated protein accumulated at the IS, with the number of conjugates imaged indicated above each bar; middle panels, the fold increase of the fluorescence intensity at the synapse relative to elsewhere at the cell membrane, determined from 3D reconstructions of conjugates; right panels, the percent of fluorescence at the IS as a fraction of the total cell fluorescence in the cell, calculated from 3D reconstructions of conjugates. Figures show data for (A) DAP10 and ICAM-1, (B) DAP 10 and NKG2D, (C) DAP 10 and CD3ζ, and (D) DAP 10 and 2B4. In panels A-C, measurements of fluorescence intensities (middle and right panels) were made on synapses with macrophageshigh LPS, whereas in panel D calculations of 2B4 and DAP10 recruitment (middle and right panels) are made for synapses with (unstimulated) macrophages (Mac.) or macrophageslow LPS (Mac.low LPS). For 3D reconstructions, at least 27 synapses were analyzed in each case. (E) NK cells and macrophageshigh LPS coincubated for different times were fixed and stained for ICAM-1 and DAP10. Reconstructions of the distribution of ICAM-1 (green) and DAP10 (red) at the face of the IS are shown, with the frequency of such distributions marked. Thirty synapses were analyzed for each time point over 3 independent experiments. (F) NK cells were coincubated for 20 minutes with macrophageshigh LPS, and conjugates were fixed, stained, and imaged. The top row shows the bright field image and associated fluorescence within the boxed area marking NKG2D and DAP10. Reconstructions of the distribution at the face of the IS are shown (lower rows), with the frequency of such distribution marked. For the distribution at the face of the IS, about 30 synapses were analyzed over 3 independent experiments. Scale bars, 10 μm.
Two distinct NK cell–activating immune synapses. (A-D) NK cells were coincubated with macrophages or macrophageshigh LPS for 20 minutes, and conjugates were fixed, stained, and imaged. The left panels show the frequency of conjugates where the indicated protein accumulated at the IS, with the number of conjugates imaged indicated above each bar; middle panels, the fold increase of the fluorescence intensity at the synapse relative to elsewhere at the cell membrane, determined from 3D reconstructions of conjugates; right panels, the percent of fluorescence at the IS as a fraction of the total cell fluorescence in the cell, calculated from 3D reconstructions of conjugates. Figures show data for (A) DAP10 and ICAM-1, (B) DAP 10 and NKG2D, (C) DAP 10 and CD3ζ, and (D) DAP 10 and 2B4. In panels A-C, measurements of fluorescence intensities (middle and right panels) were made on synapses with macrophageshigh LPS, whereas in panel D calculations of 2B4 and DAP10 recruitment (middle and right panels) are made for synapses with (unstimulated) macrophages (Mac.) or macrophageslow LPS (Mac.low LPS). For 3D reconstructions, at least 27 synapses were analyzed in each case. (E) NK cells and macrophageshigh LPS coincubated for different times were fixed and stained for ICAM-1 and DAP10. Reconstructions of the distribution of ICAM-1 (green) and DAP10 (red) at the face of the IS are shown, with the frequency of such distributions marked. Thirty synapses were analyzed for each time point over 3 independent experiments. (F) NK cells were coincubated for 20 minutes with macrophageshigh LPS, and conjugates were fixed, stained, and imaged. The top row shows the bright field image and associated fluorescence within the boxed area marking NKG2D and DAP10. Reconstructions of the distribution at the face of the IS are shown (lower rows), with the frequency of such distribution marked. For the distribution at the face of the IS, about 30 synapses were analyzed over 3 independent experiments. Scale bars, 10 μm.
After 20 minutes of coincubation we found that another signaling adaptor protein, CD3ζ, also accumulated at 55% of synapses with macrophageshigh LPS but rarely at contacts with macrophages (Figure 7C). Thus, activating NK cell receptors associated with CD3ζ could also play a role in the recognition of LPS-activated macrophages. Alternatively, CD3ζ may be brought to the cytolytic NK cell–macrophage IS if other activating NK receptors cocluster with NKG2D.
In contrast to the specific accumulation of NKG2D, DAP10, and CD3ζ at synapses with macrophageshigh LPS, 2B4 clustered at 55% or 56% of synapses with macrophages or macrophageslow LPS, respectively, and at only 10% of synapses with macrophageshigh LPS (Figure 7D). Again, a strikingly large fraction, on average close to 50%, of NK cell 2B4 is recruited to the IS. Thus, complementing the effect of blocking mAb against 2B4 and CD48 (Figure 2), these imaging data provide a further line of evidence for the importance of 2B4 in macrophage-mediated NK cell activation and surprisingly not in the lysis of macrophageshigh LPS.
Organization of the IS
Three-dimensional reconstructions of conjugates fixed after different times of coincubation were used to reveal the spatial-temporal organization of proteins at the NK cell IS with macrophageshigh LPS. After 5 minutes of coincubation, there was little or no specific accumulation of ICAM-1 or DAP10 at the IS. By 10 minutes of coincubation, we could readily observe clustering of ICAM-1 at the IS, but only after 20 minutes of coincubation could both ICAM-1 and DAP10 readily be seen to accumulate at the IS (Figure 7E). ICAM-1 was clearly seen in a ring-shaped p-SMAC around a central patch of DAP10 at the c-SMAC. Commonly, NKG2D was colocalized with DAP10 in the c-SMAC (Figure 7F). The observation that the activating receptors accumulate in the c-SMAC is reminiscent of the “prototypical” T-cell IS and could be an important role in augmenting or balancing signaling.13
Discussion
There has been a great deal of interest in crosstalk between DC and NK cells,9,10 while less attention has been given to communication between NK cells and other cells of the innate immune system. Here, we found that macrophages prime NK cell cytotoxicity and trigger NK cell cytokine secretion and proliferation. In different situations, 2B4 can act as an activating or an inhibitory NK cell receptor, though in most situations it acts as a costimulatory receptor augmenting signals from other activating receptors.20,21 Here, we found that the interaction between NK cell 2B4 and macrophage CD48 is critical in triggering NK cell proliferation and IFN-γ secretion. Moreover, macrophages induced increased expression of 2B4 on NK cells, and 2B4 specifically clustered at the NK cell–macrophage IS associated with NK cell priming. It is surprising that, in interactions with macrophages, 2B4 triggers NK cell proliferation and not cytotoxicity, because 2B4 triggers cytotoxicity in other interactions.22,23
High doses of LPS for 48 hours induced a distinct macrophage phenotype characterized by low levels of secretion of TNF-α and IL-6, perhaps related to the phenotype of “tolerant” macrophages24,25 and/or the macrophage response in septic patients.26 Most importantly, we also found that NK cells directly lysed macrophageshigh LPS via NKG2D recognition. NKG2D ligands ULBP1, ULBP2, and ULBP3 but not RAET1E, RAET1G, and RAET1L were transcribed and expressed at the cell surface in macrophages treated with 200 ng/mL LPS, demonstrating that Toll-like receptor signaling can lead to NKG2D ligand expression. Transcription of MICA and MICB occurred constitutively in unactivated and LPS-activated macrophages alike, but protein expression was only detected in macrophageshigh LPS. Up-regulation of NKG2D ligands upon LPS activation has also been reported for mouse macrophages,27 and conservation of this immune cell interaction is perhaps indicative of its importance.
The observation that NK cell F-actin clusters at the IS formed with LPS-activated macrophages, but not unactivated macrophages, is reminiscent of the specific accumulation of F-actin at the cytolytic synapse with EBV-transformed B-cell lines lacking expression of MHC class I protein.17,28 The cytolytic synapse between NK cells and LPS-activated macrophages likely requires a ring-shaped accumulation of F-actin to facilitate directed granule secretion, as recently described for CTL-mediated lysis.29 NKG2D and the signaling adaptor protein associated with NKG2D, DAP10, specifically accumulated at the IS involving LPS-activated macrophages, consistent with the role for NKG2D in triggering NK cell lysis. The activating NK cell adaptor protein CD3ζ was also seen to accumulate in the cytolytic IS. CD3ζ is important in NK cell activation and is known to associate with CD16, NKp46, and NKp30.30 It is possible that an activating receptor that uses CD3ζ to signal may also be important in the cytolytic response of NK cells to macrophageshigh LPS. Alternatively, because it is established that there is crosstalk between activating receptors on NK cells,31 it is possible that they are constitutively clustered together such that CD3ζ is brought to the IS through cis interactions between activating receptors.
At the cytolytic synapse between NK cells and LPS-activated macrophages, ICAM-1 was readily seen in a ring-shaped structure at the IS after 10 minutes of coincubation. Thus, the initial interaction between these cells resembles the antigen-independent cytotoxic T-cell IS.32 Then, after 20 minutes of coincubation of cells, DAP10 accumulates in the c-SMAC while ICAM-1 remains within the p-SMAC. The “bull's-eye” arrangement of activating receptors, signaling adaptors, and integrins is reminiscent of the “prototypical” activating T-cell synapse.33–37 Thus, the function of the specific arrangement of proteins at the NK-macrophage IS would be analogous to the possible functions of the T-cell IS, which include facilitating directed secretion, establishing checkpoints for activation, and/or balancing signaling.13
Together these data beg the question of where macrophage–NK cell interactions would be important in vivo. Interaction between NK cells and macrophages could prime NK effector functions in organs such as the spleen or liver38 as well as promote the cells' cooperation in inflammation sites in rheumatoid arthritis patients39 or in Plasmodium falciparum infection.40 It is also possible that macrophages could play a role in recruiting NK cells to lymph nodes, as previously determined for activated DCs in mice.41 In addition, both NK cells and macrophages are found in abundance in the human deciduas42–44 and could interact to produce appropriate cytokines important for pregnancy.
NK cell–mediated lysis of LPS-activated macrophages may be important during septic shock following infection by Gram-negative bacteria and/or the release of bacterial products. Our data suggest that the presence of high levels of LPS would render macrophages susceptible to NK cell–mediated lysis and, indeed, there is considerable evidence that NK cells are important in the pathogenesis of septic shock. For example, liver NK cells were found to be the principal source of IFN-γ in a murine model of human septic peritonitis.45 In addition, because our data suggest that NK cells participate in elimination of overactivated macrophages, in the absence of cytotoxicity activated macrophages would not be killed, leading to hemaphagocytosis and other inflammatory syndromes. Thus, impaired NK cell–mediated immunoregulation of macrophages likely contributes to the pathology of several diseases associated with a loss of cytolytic activity, such as hemophagocytic lymphohistiocytosis (HLH),46,47 macrophage activation syndrome,48 Hermansky-Pudlak syndrome type 2,49 and Griscelli syndrome.50
Authorship
Contribution: S.N. designed and performed most of the research; S.S. and R.A.E. designed and performed additional research; J.H. helped with macrophage cell culture; F.V. and D.P. provided vital new reagents; J.T., E.V., and S.G. designed research; D.M.D. designed research and supervised the project; and S.N. and D.M.D. wrote the paper with valuable contributions from all other authors.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Daniel M. Davis, Division of Cell and Molecular Biology, Sir Alexander Fleming Bldg, Imperial College London, SW7 2AZ, United Kingdom; e-mail: d.davis@imperial.ac.uk.
The online version of this article contains a data supplement.
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
This work was supported by funds from the Department of Trade and Industry, the Medical Research Council, the Human Frontier Science Program, the Biotechnology and Biological Sciences Research Council, and a Lister Institute Prize Fellowship.
We thank M. Purbhoo, E. N. M. Nolte-'t Hoen, F. Culley, P. Eissmann, C. R. Almeida, and P. Höglund for useful comments on the manuscript.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal