Abstract
Graft-versus-host disease (GvHD)–induced apoptosis of the skin targets both epidermal keratinocytes and dermal endothelial cells. We studied the donor-versus-recipient origin of GvHD of these target cells in skin of 18 sex-mismatched hematopoietic stem-cell transplant (HSCT) recipients. Combining XY fluorescence in situ hybridization (FISH) and double immunostaining, and further 3D tissue Z-stack analysis, we found keratinocytes and endothelial cells of donor origin, but only in patients with GvHD. Using terminal dUTP nick-end labeling (TUNEL) assay on sister sections, we found a correlation between the numbers of chimeric and apoptotic epidermal and endothelial cells. Moreover, donor-derived cells were more numerous and preferentially distributed in the areas of severe GvHD damage in biopsies performed early in the course of GvHD, whereas they were less numerous and found in the whole epidermis in late biopsies. Because donor-derived cells were found at the site and at the time of maximum tissue damage, they could contribute to epidermal and microvessel repair.
Introduction
Keratinocyte apoptosis and inflammatory infiltrate are the hallmarks of acute cutaneous graft-versus-host disease (GvHD), but endothelial-cell damage is also found in human1 and experimental studies. In an animal model we demonstrated that endothelial cells in all organs are targets of GvHD.2 In humans with acute GvHD we have found disruption of microvessel walls in gut biopsies.3 Endothelial damage has also been demonstrated at the chronic phase of GVHD in the skin.4
Studies in animal models,5 and in human hematopoietic stem-cell transplant (HSCT) recipients, have shown that marrow stem cells could differentiate into cell types other than blood cells, particularly in oral mucosa,6 digestive tract,7 and liver.8 A study demonstrated that human bone marrow contributes to vascular endothelium in the skin.9 However, the donor-versus-recipient origin of endothelium as target in GvHD lesions has not been studied in humans, so far.
Patients, materials, and methods
Eighteen female patients received an allogeneic, non–T-cell–depleted, marrow transplant from a male sibling donor. Three patients were transplanted for aplastic anemia and 15 for malignant disease, after a myeloablative conditioning regimen (irradiation-based therapy in 10 patients; chemotherapy alone in 8). All patients were full hematopoietic donor chimera, without relapse, at the time of biopsy. A skin rash led to biopsy, and, among the 18 patients, 13 had biopsy-confirmed pathologic acute GvHD. Pathologic grade was grade 3 in 6 patients, grade 2 in 5 patients, and grade 1 in 2 patients. All patients were given steroids which were rapidly tapered in 5 patients in whom pathologic diagnosis was not acute GvHD but suspected drug-induced rash.
Biopsies from female patients grafted with a female donor were considered as negative controls (n = 10), and biopsies from male recipients grafted with a male donor were considered as positive controls (n = 8). No significant differences in terms of pathologic-confirmed acute GvHD, diagnosis, or conditioning regimen emerged when comparing these control to case patients under analysis. All patients gave their informed consent to this study according to the Institution's procedures, and in accordance with the Declaration of Helsinki. This study was approved by the Institutional Review Board of the Hospital Saint Louis, Paris, France.
Combined methods of immunohistochemistry (CD45), fluorescence in situ hybridization (FISH; X/Y) and immunofluorescent labeling (CD31 or AE1/AE3) were performed on each section, as previously described.10 The tissue sections were blindly analyzed on a motorized Z-axis Olympus BX61 microscope. For chromosomal analysis, 10 sequential Z-stack images of the same field, at 0.5-μm intervals, were captured with the motorized z-axis system controlled by SIS software analySIS (Soft Imaging System, Münster, Germany). To detect in situ fragmented DNA, a terminal dUTP nick-end labeling (TUNEL) assay was performed on the following paraffin sections.2 Quantitative results were expressed as the number of apoptotic cells per field through a UPlan FI 40 ×/0.75 NA objective in epidermis and vessels of upper and lower dermis.
Results and discussion
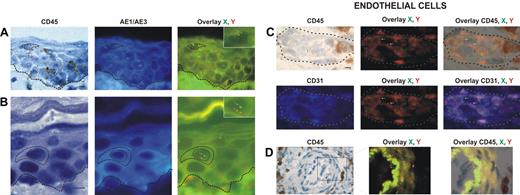
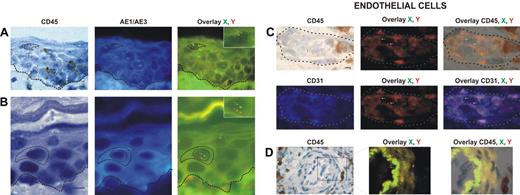
Donor-derived keratinocytes, CD45−, AE1/AE3+, and XY genotype (Figure 1A-B), were found in 12 of our 18 patients. The percentage of donor-derived keratinocytes averaged 5.5% (range, 1.6%-7.8%).
Identification and distribution of chimeric cells in epidermal and dermal compartments of the skin in patients with GvHD. Presence of chimeric keratinocytes, is characterized by CD45 negativity, AE1/AE3 positivity, and X and Y markers (arrows), in the superficial layers of epidermis in a patient in whom a biopsy was taken late in the course of GvHD (day 60) (A), and in the suprabasal layer of epidermis in a patient in whom a biopsy was taken earlier in the course of GvHD (day 35) (B). Presence of chimeric endothelial cells is characterized by CD31 positivity, CD45 negativity, and X and Y markers (arrows) in microvessels of the superficial dermis in all patients with GvHD (C), and in larger vessels of the deep dermis in only one patient with GvHD (D). All scale bars represent 10 μm. Formalin-fixed, paraffin-embedded skin biopsy specimens were cut into 5-μm thick sections. Sister following sections were used for combined immunohistochemistry, FISH, immunofluorescent study and for TUNEL assay. An indirect immunoperoxydase procedure was performed with monoclonal mouse anti–human CD45 antibody (clone PD7/26 and 2B11; Dako, Carpinteria, CA) and secondary biotinylated antibody (Nexes; Ventana, Tucson, AZ). For FISH, the CEP X (SpectrumGreen)/Y (SpectrumOrange) DNA probe (Vysis, Downers Grove, IL) kit was used. Immunofluorescent labeling was carried out with monoclonal mouse anti–human cytokeratin (clone AE1/AE3; Boehringer Mannheim, Indianapolis, IN) and anti–human CD31 (clone JC70A; Dako) as primary antibodies, and AMCA-conjugated horse anti–mouse IgG antibody (Vector Laboratories, Burlingame, CA) as secondary antibody. Bright-field and fluorescent images were obtained by a Provis AX 70 microscope (Olympus, Rungis, France), with wide-field eyepiece number 26.5, providing a field size of 0.34 mm2 , through a UPlan FI 40×/0.75 NA objective, and were captured with a ColorView III digital camera (Olympus) using analySIS software version 3.2 (Soft Imaging System).
Identification and distribution of chimeric cells in epidermal and dermal compartments of the skin in patients with GvHD. Presence of chimeric keratinocytes, is characterized by CD45 negativity, AE1/AE3 positivity, and X and Y markers (arrows), in the superficial layers of epidermis in a patient in whom a biopsy was taken late in the course of GvHD (day 60) (A), and in the suprabasal layer of epidermis in a patient in whom a biopsy was taken earlier in the course of GvHD (day 35) (B). Presence of chimeric endothelial cells is characterized by CD31 positivity, CD45 negativity, and X and Y markers (arrows) in microvessels of the superficial dermis in all patients with GvHD (C), and in larger vessels of the deep dermis in only one patient with GvHD (D). All scale bars represent 10 μm. Formalin-fixed, paraffin-embedded skin biopsy specimens were cut into 5-μm thick sections. Sister following sections were used for combined immunohistochemistry, FISH, immunofluorescent study and for TUNEL assay. An indirect immunoperoxydase procedure was performed with monoclonal mouse anti–human CD45 antibody (clone PD7/26 and 2B11; Dako, Carpinteria, CA) and secondary biotinylated antibody (Nexes; Ventana, Tucson, AZ). For FISH, the CEP X (SpectrumGreen)/Y (SpectrumOrange) DNA probe (Vysis, Downers Grove, IL) kit was used. Immunofluorescent labeling was carried out with monoclonal mouse anti–human cytokeratin (clone AE1/AE3; Boehringer Mannheim, Indianapolis, IN) and anti–human CD31 (clone JC70A; Dako) as primary antibodies, and AMCA-conjugated horse anti–mouse IgG antibody (Vector Laboratories, Burlingame, CA) as secondary antibody. Bright-field and fluorescent images were obtained by a Provis AX 70 microscope (Olympus, Rungis, France), with wide-field eyepiece number 26.5, providing a field size of 0.34 mm2 , through a UPlan FI 40×/0.75 NA objective, and were captured with a ColorView III digital camera (Olympus) using analySIS software version 3.2 (Soft Imaging System).
Donor-derived endothelial cells, CD31+, CD45−, and XY genotype (Figure 1C-D), were found in skin biopsies of 13 of the 18 patients. The percentage of chimeric endothelial cells obtained with Z-stacking analysis was rather low (mean, 4.3%; range, 2.2%-9.4%). This is in accordance with a study demonstrating 2% donor-derived endothelial cells in the skin of human transplant recipients as a mean.9 In this latter study,9 3 of 12 sex-mismatched transplant recipients had GvHD, and all 3 patients had donor-derived endothelial cells.
Donor-derived inflammatory cells, CD45+, AE1/AE3−, and XY genotype, were found in the dermis. The percentage of chimeric inflammatory cells was high (mean, 95.2%; range, 90.3%-98.1%). In the 18 skin biopsies studied using 3D Z-stack analysis, no endothelial or epidermal cells disclosed a tetraploid genotype.
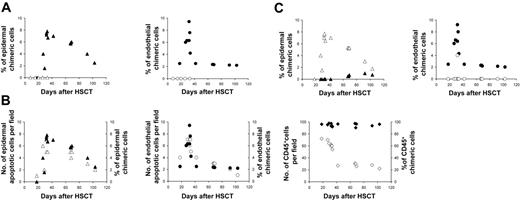
There was a close association between chimeric cells and pathologically proven acute GvHD. None of the 5 patients without GvHD had evidence of endothelial or epidermal chimeric cells, whereas in all 13 patients with GvHD donor-derived endothelial cells were detected (P < .001) (Figure 2A). Epithelial chimeric cells were also detected in 12 of 13 patients with GvHD and in 0 of 5 patients without GvHD (P < .001). There was a strong correlation between the numbers of donor-derived epidermal cells and of apoptotic epidermal cells (r = 0.885, P < .001) and between the numbers of donor-derived endothelial cells and of apoptotic endothelial cells (r = 0.903, P < .001) in these patients.
Chimerism studies in skin biopsy. (A) Percentage of epidermal and endothelial chimeric cells in patients with or without GvHD; (▴) epidermal chimeric cells in patients with GVHD, (▵) epidermal chimeric cells in patients without GVHD, (•) endothelial chimeric cells in patients with GVHD, (○) endothelial chimeric cells in patients without GVHD. (B) Relation between apoptotic and chimeric cell numbers in epidermis, endothelium, and inflammatory infiltrate in early and late biopsies of patients with GvHD; (▵) epidermal apoptotic cells, (▴) epidermal chimeric cells,(○) endothelial apoptotic cells, (•) endothelial chimeric cells, (⋄) CD45+ cell, (♦) CD45+ chimeric cells. (C) Distribution patterns of epidermal and endothelial chimeric cells in skin biopsies of patients with GvHD; (▴) epidermal chimeric cells in superficial layers, (▵) epidermal chimeric cells in basal layers, (○) endothelial chimeric cells in upper dermis, (•) endothelial chimeric cells in deep dermis.
Chimerism studies in skin biopsy. (A) Percentage of epidermal and endothelial chimeric cells in patients with or without GvHD; (▴) epidermal chimeric cells in patients with GVHD, (▵) epidermal chimeric cells in patients without GVHD, (•) endothelial chimeric cells in patients with GVHD, (○) endothelial chimeric cells in patients without GVHD. (B) Relation between apoptotic and chimeric cell numbers in epidermis, endothelium, and inflammatory infiltrate in early and late biopsies of patients with GvHD; (▵) epidermal apoptotic cells, (▴) epidermal chimeric cells,(○) endothelial apoptotic cells, (•) endothelial chimeric cells, (⋄) CD45+ cell, (♦) CD45+ chimeric cells. (C) Distribution patterns of epidermal and endothelial chimeric cells in skin biopsies of patients with GvHD; (▴) epidermal chimeric cells in superficial layers, (▵) epidermal chimeric cells in basal layers, (○) endothelial chimeric cells in upper dermis, (•) endothelial chimeric cells in deep dermis.
We then studied the relative importance of epidermal and endothelial apoptotic counts, and of CD45+ XY cells, according to the delay between HSCT and biopsy (Figure 2B). The highest density of CD45+ XY cells was observed in biopsies performed 18 to 35 days after the graft, whereas the highest density of apoptotic target cells was only found in biopsies performed after day 30. Apoptotic endothelial-cell counts sharply decreased after day 45, whereas apoptotic epidermal-cell counts were still high at day 60 and progressively decreased later. This discrepancy in decline of apoptotic counts between the 2 cell types might be linked to a more rapid clearing process of apoptotic cells in the endothelium than in the epidermis. When donor-derived cell counts were also studied according to the delay between HSCT and biopsy, a striking correspondence could be observed between levels of apoptotic and donor-derived cells, both for epidermal and endothelial cells, in early and late biopsies. In all but one patient, donor-derived endothelial cells were only found in the upper dermis (Figure 2C), when donor-derived keratinocytes were found in the epidermal basal layers. It has been shown that lymphocyte migration into skin in GvHD is promoted through a recognition system in the papillary dermal endothelium.11 The preferential distribution of donor-derived cells in our cases suggests that these cells are present, not only when GvHD damage occurs but also where this damage occurs. Sale et al12 showed that keratinocyte stem cells in the tips of rete ridges are preferred targets in acute skin GvHD. This has been confirmed both in humans and in experimental models.13–15 The epidermal rete ridge microdomain is strongly associated with vascular capillary loops in the upper dermis where we found donor-derived endothelial cells. This suggests that endothelial cells of donor origin are mainly found at the site of the first immune injury during the effector phase of acute GvHD. We have previously shown, in sequential skin biopsies of 7 patients with chronic GvHD, that lichenoid and sclerotic skin lesions of chronic GvHD have morphologic biochemical features of tissue repair.16 Experimentally, it has been demonstrated that bone marrow–derived cells contribute to epithelial engraftment during wound healing.17 In this mouse model, the proliferation index of epidermal cells correlated with the number of donor-derived cells within the wound. An interesting point in our study was the distribution of donor-derived keratinocytes in the basal layers, and also in the upper epidermis (Figure 1A), in late biopsies (after day 60) (Figure 2C). This suggests that donor-derived keratinocytes, when engrafted in areas of GvHD lesions, follow the kinetic of normal keratinocytes within the epidermis.
Our results suggest the participation of donor-derived cells in endothelial repair of GvHD-induced lesions. Recent experimental studies18 demonstrated a similar mechanism in vessel repair 3 to 5 days after brain and skin injuries. We did not characterize the cell at the origin of chimeric cells. Circulating endothelial cells have been found in HSCT recipients,19,20 but the chimeric cells could also derive from a bone marrow multipotent mesenchymal stem cell,21,22 from a trans-differentiated adult hemangioblast,23 or from myeloid precursors.24
Authorship
Contribution: G.S. and A.J. designed the study and wrote the manuscript; H.M. performed the experiments; H.M., A.J., and C.L. analyzed the results, captured the pictures, and performed figures design; J.S. and V.M. participated in performing the experiments. E.G. and J.S. gave expert advice.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
G.S. and A.J. contributed equally to this study and should be considered both as senior author.
Correspondence: Gérard Socié, Service d'Hématologie Greffe de Moelle & U728 INSERM, Université Paris VII, Hôpital Saint Louis, 1 Avenue Claude Vellefaux, 75475, Paris Cedex 10, France; e-mail: gsocie@paris7.jussieu.fr.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgment
This work was supported by Université Paris VII, INSERM, Association pour la Recherche sur le Cancer (ARC) 1999, Programme Hospitalier de Recherche Clinique (PHRC).