Abstract
Treatment for steroid-resistant acute graft-versus-host disease (GVHD) has had limited success. ABX-CBL is a hybridoma-generated murine IgM monoclonal antibody against the CD147 antigen, weakly expressed on human leukocytes and up-regulated on activated lymphocytes. A prospective, multicenter, open-label, randomized clinical trial comparing ABX-CBL to antithymocyte globulin (ATG) for treatment of steroid-resistant acute GVHD was conducted in 95 patients at 21 centers. Forty-eight patients received ABX-CBL daily for 14 consecutive days followed by up to 6 weeks of ABX-CBL twice weekly. Forty-seven patients received equine ATG, 30 mg/kg every other day for a total of 6 doses with additional courses as needed. By day 180, overall improvement was similar in the patients receiving ABX-CBL and in those receiving ATG (56% versus 57%, P = .91). Patient survival at 18 months was less favorable on ABX-CBL than on ATG (35% versus 45%), with the 95% confidence interval ruling out that ABX-CBL provides at least a 10.4% improvement. Data from this trial suggest that ABX-CBL does not offer an improvement over ATG in the treatment of acute steroid-resistant GVHD. This prospective, multicenter, randomized clinical trial for steroid-resistant acute GVHD serves as a model for future evaluation of new agents.
Introduction
Acute graft-versus-host disease (GVHD) remains a major cause of morbidity and mortality after allogeneic hematopoietic cell transplantation (HCT). Moderate to severe GVHD grades II to IV occurs in up to 30% to 50% of matched related donor recipients1,2 and 50% to 70% of unrelated donor recipients.3,4 Despite many advances in the past decade in the management of complications related to HCT, treatment of acute GVHD remains suboptimal.4,5 Corticosteroids are the standard front-line therapy with overall response (OR) rates of approximately 50%.1–4,6 A myriad of second-line therapies has been used, with none to date showing better or more durable efficacy than ATG.5,7–14
GVHD is triggered by donor-derived T lymphocytes that recognize recipient alloantigens as foreign, resulting in cell activation and cytokine release, leading to destruction of host tissues.15,16 ABX-CBL is a hybridoma-generated purified murine IgM monoclonal antibody, which recognizes the CD147 antigen that is weakly expressed on human leukocytes, granulocytes, red blood cells, and several other cell types. Upon activation, CD147 is markedly up-regulated on activated lymphocytes.17 ABX-CBL is capable of inhibiting the in vitro mixed lymphocyte reaction by depleting monocytes and activated lymphocytes via a complement-dependent cytotoxic mechanism. Both activated T cells (CD4+ and CD8+) and B cells are depleted by ABX-CBL, while resting lymphocytes are unaffected. The half-life of ABX-CBL is 15 to 19 hours.
In a pilot study of CBL-1, a murine ascites-generated precursor of ABX-CBL, 5 complete responses (CRs) and 4 partial responses (PRs) were observed in 10 pediatric patients with steroid-resistant acute GVHD.18 In a subsequent phase 1-2 trial of 59 patients with steroid-resistant acute GVHD, patients received ABX-CBL at 0.01, 0.1, 0.2, or 0.3 mg/kg per day, and an additional 32 patients received ABX-CBL at 0.2 or 0.15 mg/kg per day.19 Among 51 evaluable patients, overall response was observed in 26 (51%), including CRs in 13 patients and PR in 13 patients. Mylagias at doses of 0.2 mg/kg or higher were dose limiting but resolved without sequelae.19
This current report describes the results of a multicenter, open-label, randomized clinical trial comparing ABX-CBL to equine ATG (Atgam) in 95 HCT recipients with steroid-resistant acute GVHD.
Patients and methods
Study design
This was a multicenter, open-label, randomized, controlled study of ABX-CBL versus ATG as therapy for steroid-resistant acute GVHD. The primary end points of the study were survival at day 180 after treatment with ABX-CBL or ATG, and incidence of acute toxicity of ABX-CBL. Secondary end points included time to and duration of acute GVHD improvement, change in organ GVHD, change in ECOG performance status, total steroid dose, incidence and time to onset of chronic GVHD, development of infections, lymphoproliferative disease, and relapse of primary disease. All patients were evaluated through day 180 after randomization.
Patients
From April 2000 to January 2003, 95 patients with steroid-resistant acute GVHD were enrolled in 21 study centers. To be eligible, patients had to have been recipients of a single allogeneic HCT from any donor; had to be 100 or fewer days from transplantation; had to have received no prior treatment for GVHD other than steroids; and had to have understood and signed an IRB/ERC-approved consent form or, if younger than 18 years, had a legal guardian sign. Institutional review board approval was obtained from the participating institutions (University of Minnesota, Minneapolis, MN; M.D. Anderson Cancer Center, Houston, TX; University of Washington, Seattle, WA; Hadassah Hebrew University Hospital, Jerusalem, Israel; the Western Pennsylvania Hospital, Pittsburgh, PA; Washington University School of Medicine, St. Louis, MO; UCLA Medical Center, Los Angeles, CA; University of Nebraska Medical Center, Omaha, NE; UCSF Stanford Healthcare, San Francisco, CA; Baylor College of Medicine, Houston, TX; Fred Hutchinson Cancer Research Center, Seattle, WA; the Cleveland Clinic Foundation, Cleveland, OH; Roswell Park Cancer Institute, Buffalo, NY; University of Chicago, Chicago, IL; Cook Children's Medical Center, Fort Worth, TX; Loyola University Medical Center, Maywood, IL; Emory University, Atlanta, GA; Florida Hospital, Orlando, FL; University of Utah School of Medicine, Salt Lake City, UT; Vanderbilt Clinic, Nashville, TN; Blood and Bone Marrow Transplant Center, Atlanta, GA; Oncology and Hematology Associates of Kansas City, Kansas City, MO), and patients provided informed consent in accordance with the Declaration of Helsinki. Potential patients were excluded if they were recipients of second HCTs or of donor lymphocyte infusions (DLIs) from their donors after HCT; were unwilling to relinquish the option of receiving DLIs through day 100 once randomized to this study; had an IBMTR GVHD Index of A or lower; had received a fully murine antibody product in the past; had changed their prophylactic regimen for acute GVHD within 72 hours of randomization (except change in dose to maintain proper serum levels or due to toxicity, or change to a different medication due to drug toxicity); were the recipients of ATG later than 10 days after HCT; were pregnant or breastfeeding or of childbearing potential not practicing birth control; were human immunodeficiency virus (HIV) positive; had a history of or current substance abuse or any existing condition that may have increased the risks associated with the study; were the recipients of investigational medications (other than antifungals) within 30 days prior to randomization; had chronic GVHD; had serum creatinine 2.5 times the upper limit of normal or required renal dialysis; had respiratory failure; had severe veno-occlusive disease or multisystem organ failure; or had developed posttransplantation lymphoproliferative disease.
Steroid-resistant acute GVHD was defined as either continuing active GVHD despite treatment with methylprednisolone at doses of 2 mg/kg per day or higher or equivalent dose of another steroid for acute GVHD for at least 3 days, or failure during corticosteroid taper following initial treatment, which included 2 mg/kg per day or more of methylprednisolone or equivalent for at least 3 days.
Active acute GVHD was defined as (1) skin rash on more than 25% body surface areas; and (2) total bilirubin level higher than 25.65 μM (1.5 mg/dL), diarrhea output of 500 mL or more per day (children, greater than or equal to 7 mL/kg per day) or nausea/vomiting, and epigastric pain with positive upper gastrointestinal histology for acute GVHD. For patients with isolated liver or upper GI acute GVHD, a confirmatory biopsy of the appropriate organ was required.
Study methods
Patients who met eligibility criteria were randomized in a 1:1 ratio to receive continuing corticosteroids plus either ABX-CBL or ATG. Patients were stratified according to IBMTR Severity Index (D versus B or C)20 and probability of survival based upon type and risk status of the primary disease at the time of randomization. Good risk was assigned to patients in first or second remission of acute myelocytic leukemia (AML) or acute lymphoblastic leukemia (ALL), patients with myelodysplastic syndrome (MDS), and patients with chronic myeloid leukemia (CML) in first chronic phase. All other patients were considered poor risk.
Patients remained on cyclosporine or tacrolimus, and steroids were the only new treatment for acute GVHD treatment permitted before randomization.
ABX-CBL was administered intravenously at a dose of 0.1 mg/kg daily for 14 consecutive days (induction regimen). Patients who qualified were eligible to receive up to 3 maintenance courses, each course consisting of 2 infusions per week for 2 consecutive weeks. To qualify for the first maintenance course, a patient had to have had completed all 14 ABX-CBL infusions during the induction regimen, had no worsening of GVHD in any organ by 1 or more stages, had improvement of 1 or more stages in at least one organ involved with GVHD on the day of randomization, and had no chronic GVHD. In addition, to be eligible for maintenance therapy, patients had to be receiving steroids at a dose on study day 15 that was lower than the dose received when randomized, if they were receiving only ABX-CBL and steroids for GVHD treatment, and if they were able to receive the first dose of maintenance ABX-CBL no later than study day 17.
Patients were eligible for a second or third maintenance course of ABX-CBL if they had received all 4 infusions of the previous maintenance regimen; had no organs involved with GVHD on day 15 (or 29, respectively) worsen by 1 or more stages and had no chronic GVHD; if the dose of steroids on day 29 (or 43, respectively) was lower than the dose of steroids on day 15 (or 29, respectively); if they were receiving only ABX-CBL and steroids for GVHD treatment; and if they were able to receive the first dose of the maintenance course no later than on study day 31 (or 45, respectively).
Equine ATG (Atgam) was administered at doses of 30 mg/kg per day every other day for a total of 6 doses. Patients were eligible for additional courses at the discretion of the investigator.
The study was not blinded with respect to treatment but a blinded reviewer was assigned to assess GVHD and treatment responses in each patient. Patients were assessed weekly until 10 weeks after randomization, then monthly for the remainder of the follow-up period. Weekly assessments for efficacy included survival, 4 organ GVHD scores, development of chronic GVHD, and treatment for GVHD. Safety assessments during this time interval included physical examination, laboratory evaluation, occurrence of adverse events, infections, and concomitant medication administration. Lymphocyte counts were performed during the third and tenth week visits. Blood for human antimurine antibody (HAMA) was drawn from patients assigned to ABX-CBL at one week after the last infusion of the induction regimen and at week 15 of the study. For patients who received the maintenance regimen, samples for HAMA were drawn prior to the start of the first maintenance course, 1 week after the last dose of ABX-CBL, and at week 15. Blood for pharmacokinetic analysis of the ABX-CBL antibody was drawn at the start and completion of the first and 14th ABX-CBL infusion. Plasma Epstein-Barr virus (EBV)–DNA was measured at baseline, and at weeks 3, 7, 11, and 15.21
Statistical analysis
The trial was designed to assess the efficacy of ABX-CBL compared with ATG, where the primary end point was patient survival at 180 days after randomization. The primary analysis of this end point was based on the difference in Kaplan-Meier estimates. Based on a retrospective review of data on second-line therapy for treatment of acute GVHD, it was projected that 40% of patients receiving the ATG regimen would survive through day 180. With 46 patients per arm, the study was designed to provide a screening evaluation that would rule out from further evaluation an intervention failing to improve 180-day survival, and that would identify for further evaluation an intervention providing at least 20% improvement in 180-day survival, when comparing with ATG.22
Safety of ABX-CBL was assessed by comparing the percentage of patients in the 2 groups with grade 3 or 4 adverse events and serious adverse events. Additional safety analyses were based on laboratory data, vital signs, HAMA in patients assigned to ABX-CBL, the incidence of infections, lymphoproliferative disease, and primary disease progression.
A data monitoring committee (DMC) evaluated all grade 4 adverse events, serious adverse events, and grade 3 to 4 myalgias. Toxicity was evaluated on a monthly basis by the chair of the DMC and the entire board as needed. GVHD status of each patient was assessed by a blinded reviewer weekly using the IBMTR Severity Index.20
Results
Patient characteristics
Patient characteristics are shown in Table 1. The groups were balanced for age, sex, primary diagnosis and risk type, donor type, preparative therapy, GVHD prophylaxis, IBMTR Severity Index, and time from HCT to randomization.
Patient characteristics: ITT population
| Characteristic . | Treated with ABX-CBL . | Treated with ATG . |
|---|---|---|
| No. patients | 48 | 47 |
| Mean age, y (range) | 38.1 (2-65) | 39.1 (2-65) |
| Male-female ratio, nos. | 31:17 | 27:20 |
| Primary diagnosis, no. (%) | ||
| AML | 14 (29) | 9 (19) |
| ALL | 3 (6) | 8 (17) |
| CML | 7 (15) | 12 (26) |
| Myelodysplastic syndrome | 6 (12) | 4 (8) |
| Lymphoma | 10 (21) | 8 (17) |
| Other | 8 (17) | 6 (13) |
| Risk type, no. (%) | ||
| Good risk | 25 (52) | 22 (47) |
| Poor risk | 23 (48) | 25 (53) |
| Median days from transplantation to randomization, no. (range) | 35.5 (11-100) | 37.0 (14-99) |
| Donor type, no. (%) | ||
| Matched related | 19 (40) | 22 (47) |
| Mismatched related | 1 (2) | 2 (4) |
| Matched unrelated | 21 (44) | 16 (34) |
| Mismatched unrelated | 7 (14) | 7 (15) |
| Preparative therapy, no. (%) | ||
| Chemotherapy with TBI | 29 (60) | 26 (55) |
| Chemotherapy alone | 19 (40) | 21 (45) |
| GVHD prophylaxis, no. (%) | ||
| CSA alone | 5 (10) | 4 (8) |
| CSA + MTX | 10 (21) | 4 (8) |
| CSA + MTX + other | 5 (10) | 5 (10) |
| CSA + other | 9 (19) | 14 (30) |
| FK506 + MTX | 8 (17) | 10 (21) |
| FK506 + MTX + other | 8 (17) | 3 (6) |
| FK506 + other | 3 (6) | 7 (15) |
| GVHD grade, no. (%) | ||
| B/C | 41 (85) | 43 (91) |
| D | 7 (15) | 4 (9) |
| Characteristic . | Treated with ABX-CBL . | Treated with ATG . |
|---|---|---|
| No. patients | 48 | 47 |
| Mean age, y (range) | 38.1 (2-65) | 39.1 (2-65) |
| Male-female ratio, nos. | 31:17 | 27:20 |
| Primary diagnosis, no. (%) | ||
| AML | 14 (29) | 9 (19) |
| ALL | 3 (6) | 8 (17) |
| CML | 7 (15) | 12 (26) |
| Myelodysplastic syndrome | 6 (12) | 4 (8) |
| Lymphoma | 10 (21) | 8 (17) |
| Other | 8 (17) | 6 (13) |
| Risk type, no. (%) | ||
| Good risk | 25 (52) | 22 (47) |
| Poor risk | 23 (48) | 25 (53) |
| Median days from transplantation to randomization, no. (range) | 35.5 (11-100) | 37.0 (14-99) |
| Donor type, no. (%) | ||
| Matched related | 19 (40) | 22 (47) |
| Mismatched related | 1 (2) | 2 (4) |
| Matched unrelated | 21 (44) | 16 (34) |
| Mismatched unrelated | 7 (14) | 7 (15) |
| Preparative therapy, no. (%) | ||
| Chemotherapy with TBI | 29 (60) | 26 (55) |
| Chemotherapy alone | 19 (40) | 21 (45) |
| GVHD prophylaxis, no. (%) | ||
| CSA alone | 5 (10) | 4 (8) |
| CSA + MTX | 10 (21) | 4 (8) |
| CSA + MTX + other | 5 (10) | 5 (10) |
| CSA + other | 9 (19) | 14 (30) |
| FK506 + MTX | 8 (17) | 10 (21) |
| FK506 + MTX + other | 8 (17) | 3 (6) |
| FK506 + other | 3 (6) | 7 (15) |
| GVHD grade, no. (%) | ||
| B/C | 41 (85) | 43 (91) |
| D | 7 (15) | 4 (9) |
There was no significant difference in any characteristic between treatment groups.
ITT indicates intent-to-treat cohort.
The amount of secondary GVHD treatment was similar between the 2 groups. The induction regimen was completed by 43 patients (90%) in the ABX-CBL group and by 43 patients (92%) in the ATG group. The visits at week 15 and month 12 were completed by all patients.
GVHD responses
The GVHD overall response rates were similar in the 2 treatment groups, as assessed by a blinded review. Twenty-seven (56%) of 48 ABX-CBL–treated patients improved (complete or partial response) their GVHD score at a median of 22 days (range, 7-72 days) after randomization versus 27 (57%) of 47 ATG-treated patients at a median of 28 days (range, 2-50 days) (P = .99). Among patients who showed an improvement, the response lasted a median of 13 days (range, 13-70 days) in the ABX-CBL–treated group and a median of 23 days (range, 13-83 days) in the ATG-treated group.
In the ABX-CBL group, 14 patients (29%) had CR of GVHD at a median of 77 days (range, 14-77 days) versus 15 patients (32%) of the ATG-treated group at a median of 78 days (range, 21-88 days). Of those patients who experienced CR of acute GVHD, the responses lasted a median of 30 days (range, 13-70 days) for the ABX-CBL–treated patients and a median of 21 days (range, 18-62 days) for the ATG-treated patients.
Various patient characteristics and transplantation conditions were analyzed for their association with clinical response to ABX-CBL or ATG. In univariate analysis, donor type was the only factor statistically significantly associated with overall response (CR + PR). CR/PR was achieved in 25 (57%) of 44 HLA-matched related donor recipients, versus 25 (68%) of 37 HLA-matched unrelated donor (URD) recipients, versus 4 (29%) of 14 HLA-mismatched URD recipients (P = .04). There was not a significant association of CR/PR and age, year of transplantation, sex, sex match, underlying diagnosis, cytomegalovirus (CMV) serostatus of the patient and donor, GVHD prophylaxis regimen, conditioning regimen, initial grade of acute GVHD, time to onset of acute GVHD, time to therapy, and type of organ involvement.
Chronic GVHD eventually developed in 44% of the ABX-CBL–treated patients and in 46% of ATG-treated patients.
Survival
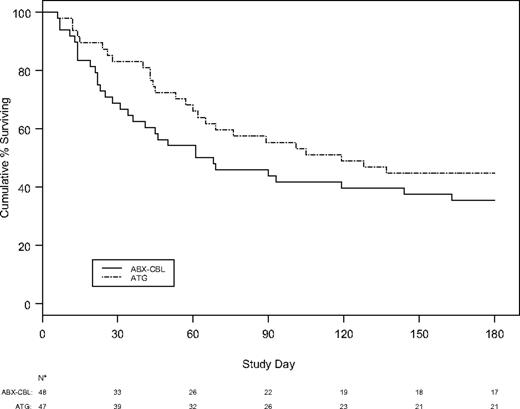
The probability of survival at day 180 after randomization was lower in patients in the ABX-CBL group than in patients in the ATG-treated group (35.4% versus 44.7%, Figure 1). The 95% confidence interval for this difference (−0.289, 0.104) allows one to rule out not only the targeted 20% improvement in 180-day survival, but also that ABX-CBL provides a 10.4% improvement relative to ATG. Causes of death for the entire study duration were similar in the 2 groups (Table 2). There was not a significant association between survival and age, year of transplantation, sex, sex match, underlying diagnosis, CMV serostatus of the patient and donor, donor type, GVHD prophylaxis regimen, conditioning regimen, initial grade of acute GVHD, time to onset of acute GVHD, time to therapy, and type of organ involvement.
Probability of survival after randomization to ABX-CBL or ATG for steroid-resistant acute GVHD.
Probability of survival after randomization to ABX-CBL or ATG for steroid-resistant acute GVHD.
Survival: ITT population, unblinded assessment
| . | Treated with ABX-CBL . | Treated with ATG . |
|---|---|---|
| No. patients | 48 | 47 |
| Survival time | ||
| Median d (min d, max d)* | 64.5 (6, 682) | 119 (6, 768) |
| Censored, no. | 13 | 8 |
| Cause of death, no. (%) | ||
| GVHD related | 19 (40) | 9 (19) |
| Primary disease related | 3 (6) | 2 (4) |
| Other | 13 (27) | 28 (60) |
| Probability of 180-day survival (K-M) | 0.354 | 0.447 |
| 95% confidence interval | (0.219, 0.489) | (0.305, 0.589) |
| 95% confidence interval for difference | (−0.289, 0.104) | |
| . | Treated with ABX-CBL . | Treated with ATG . |
|---|---|---|
| No. patients | 48 | 47 |
| Survival time | ||
| Median d (min d, max d)* | 64.5 (6, 682) | 119 (6, 768) |
| Censored, no. | 13 | 8 |
| Cause of death, no. (%) | ||
| GVHD related | 19 (40) | 9 (19) |
| Primary disease related | 3 (6) | 2 (4) |
| Other | 13 (27) | 28 (60) |
| Probability of 180-day survival (K-M) | 0.354 | 0.447 |
| 95% confidence interval | (0.219, 0.489) | (0.305, 0.589) |
| 95% confidence interval for difference | (−0.289, 0.104) | |
Min indicates minimum uncensored time; max, maximum uncensored time.
Adverse events
The administration of ABX-CBL was generally well tolerated. Adverse events, which were considered possibly, probably, or definitely related to study drug administration, were observed in similar numbers in both treatment groups with the exception of infusion-related reactions and myalgias. Twenty-nine (63%) ABX-CBL–treated patients and 21 (46%) ATG-treated patients had study drug–related adverse events (P = .09). The most common drug-related adverse events were myalgias, which developed in 13 (28%) ABX-CBL–treated patients and in 1 (2%) ATG-treated patient (P ≤ .001); rigors in 2 (4%) ABX-CBL–treated and 5 (11%) ATG-treated patients (P = .44); and back pain in 6 (13%) ABX-CBL–treated and 1 (2.2%) ATG-treated patient (P = .11). Infusion-related adverse events (myalgias, rigors, and back pain) were reported in more ABX-CBL–treated patients (25, 54%) than in ATG-treated patients (16, 35%; P = .06). HAMAs to ABX-CBL were detected in 2 (4%) of 48 ABX-CBL–treated patients.
Patients receiving ABX-CBL or ATG had similar rates of adverse events, including infections. Adverse events (AEs) occurring in more than 20% of patients are shown in Table 3. The most common AEs (infections, positive blood cultures, and hypertension) occurred with similar frequency in the 2 treatment groups, except for pneumonia, which was observed in 15 (33%) patients in the ABX-CBL–treated group versus 30 (65%) patients in the ATG-treated group (P = .002).
Adverse events occurring in less than 20% of patients
| Adverse event . | Treated with ABX-CBL, no. (%) . | Treated with ATG, no. (%) . |
|---|---|---|
| Infections and infestations* | 45 (97.8) | 46 (100) |
| Blood culture positive | 26 (56.5) | 21 (45.7) |
| Hypertension | 14 (30.4) | 13 (28.3) |
| Graft-versus-host disease | 16 (34.8) | 10 (21.7) |
| Hyperglycemia | 11 (23.9) | 12 (26.1) |
| Neutropenia | 10 (21.7) | 13 (28.3) |
| Pyrexia | 9 (19.6) | 14 (30.4) |
| Abdominal pain | 7 (15.2) | 15 (32.6) |
| Edema | 14 (30.4) | 8 (17.4) |
| Depression | 11 (23.9) | 10 (21.7) |
| Hypotension | 10 (21.7) | 11 (23.9) |
| Back pain | 11 (23.9) | 8 (17.4) |
| Cough | 6 (13.0) | 13 (28.3) |
| Dyspnea | 8 (17.4) | 11 (23.9) |
| Rigors | 7 (15.2) | 12 (26.1) |
| Hypocalcemia | 10 (21.7) | 8 (17.4) |
| Myalgia | 14 (30.4) | 3 (6.5) |
| Edema lower limb | 4 (8.7) | 13 (28.3) |
| Thrombocytopenia | 10 (21.7) | 7 (15.2) |
| Headache | 5 (10.9) | 11 (23.9) |
| Renal impairment | 6 (13.0) | 10 (21.7) |
| Arthralgia | 5 (10.9) | 10 (21.7) |
| Gastrointestinal hemorrhage | 4 (8.7) | 10 (21.7) |
| Adverse event . | Treated with ABX-CBL, no. (%) . | Treated with ATG, no. (%) . |
|---|---|---|
| Infections and infestations* | 45 (97.8) | 46 (100) |
| Blood culture positive | 26 (56.5) | 21 (45.7) |
| Hypertension | 14 (30.4) | 13 (28.3) |
| Graft-versus-host disease | 16 (34.8) | 10 (21.7) |
| Hyperglycemia | 11 (23.9) | 12 (26.1) |
| Neutropenia | 10 (21.7) | 13 (28.3) |
| Pyrexia | 9 (19.6) | 14 (30.4) |
| Abdominal pain | 7 (15.2) | 15 (32.6) |
| Edema | 14 (30.4) | 8 (17.4) |
| Depression | 11 (23.9) | 10 (21.7) |
| Hypotension | 10 (21.7) | 11 (23.9) |
| Back pain | 11 (23.9) | 8 (17.4) |
| Cough | 6 (13.0) | 13 (28.3) |
| Dyspnea | 8 (17.4) | 11 (23.9) |
| Rigors | 7 (15.2) | 12 (26.1) |
| Hypocalcemia | 10 (21.7) | 8 (17.4) |
| Myalgia | 14 (30.4) | 3 (6.5) |
| Edema lower limb | 4 (8.7) | 13 (28.3) |
| Thrombocytopenia | 10 (21.7) | 7 (15.2) |
| Headache | 5 (10.9) | 11 (23.9) |
| Renal impairment | 6 (13.0) | 10 (21.7) |
| Arthralgia | 5 (10.9) | 10 (21.7) |
| Gastrointestinal hemorrhage | 4 (8.7) | 10 (21.7) |
For both groups, n = 46.
*This includes all preferred terms in the System Organ Class “Infections and Infestations.”
EBV titers at baseline and after treatment were available for only 14 ABX-CBL–treated patients and 19 ATG-treated patients. Increased EBV titers (any rise above baseline) were observed in 5 ABX-CBL–treated patients and 13 ATG-treated patients. Only one patient developed posttransplantation lymphoproliferative disease (PTLD). He was a 4-year-old white male who was randomized to ABX-CBL for grade B acute GVHD. Two days after randomization, a computed tomography (CT) scan of the neck revealed lymphadenopathy, and a biopsy of cervical lymph nodes performed the subsequent day confirmed an EBV PTLD. The patient required intubation and his respiratory and renal functions worsened over the next few days. Six days after randomization, life support was withdrawn and the patient died.
Lymphocyte counts for the ABX-CBL–treated patients and the ATG-treated patients did not differ significantly. The median lymphocyte count at baseline was 0.3 × 109/L (range, 0-3.2 × 109/L) for 45 ABX-CBL–treated patients and 0.2 × 109/L (range, 0-144.4 × 109/L) for 45 ATG-treated patients. The median lymphocyte count at week 3 was 0.19 × 109/L (range, 0-1.3 × 109/L) for 39 ABX-CBL–treated patients and 0.20 × 109/L (range, 0-232.0 × 109/L) for 42 ATG-treated patients, and the median lymphocyte count at week 10 was 0.40 × 109/L (range, 0-1.56 × 109/L, n = 22) for 22 ABX-CBL–treated patients and 0.32 × 109/L (range, 0-5.3.0 × 109/L, n = 30) for 30 ATG-treated patients.
Discussion
As ABX-CBL had shown promising results in phase 1 and 2 studies for treatment of steroid-resistant acute GVHD,18,19 a phase 3 study was developed to compare outcome between ABX-CBL and standard therapy. As there were no FDA-approved second-line therapies for acute GVHD, when designing this study we had to choose the optimum standard therapy for the comparator arm. After reviewing the literature and transplant center use, we chose ATG as it was the most widely used agent and there were no alternatives that showed higher response rates. Although this ATG treatment regimen has not been proven to be effective therapy in double-blinded, placebo-controlled randomized trials, senior investigators representing large transplantation centers who designed this study agreed that ATG had been studied in the largest numbers of steroid refractory GVHD patients for toxicity, efficacy, and overall survival, leading us to elect to have the ATG control arm used in this study.
The results of this prospective randomized study show that ABX-CBL, like equine ATG, is generally well tolerated with a similar incidence of adverse events when given as therapy for steroid-resistant acute GVHD. However, myalgias occurred more frequently in patients receiving ABX-CBL.
In the subset of patients for whom EBV polymerase chain reaction (PCR) was measured, EBV reactivation was more commonly seen in the ATG arm. This finding may reflect the fact that ABX-CBL will deplete B cells (which may be EBV infected) as well as activated T cells, whereas ATG will deplete T cells while sparing B cells, making outgrowth of EBV-infected B cells more likely. Only one patient developed frank EBV PTLD but as this was diagnosed only 2 days after starting ABX-CBL, it was unlikely due to the antibody.
Although the response rate for ABX-CBL was similar to that observed in previous studies,18,19 there was no clinically or statistically significant advantage in regard to treatment response using ABX-CBL compared with ATG, and survival was sufficiently unfavorable on ABX-CBL that one can rule out even moderate improvement relative to ATG in this randomized risk-stratified trial. The duration of response was also similar between the treatment groups despite the shorter half-life of ABX-CBL (15-19 hours) versus ATG (5.7 ± 3 days).
The clinical responses to ATG observed in this study were similar to previous nonrandomized studies for patients with steroid-resistant GVHD. A report of patients who underwent transplantation at a single institution in the 1990s described an overall improvement in 54% of patients receiving ATG for steroid-resistant acute GVHD, and durable complete responses in 20% of patients.5 The results of the current multicenter trial establish ATG response rates that can be used as a benchmark for future randomized trials.
This study is the first reported prospective, multicenter, randomized clinical trial for steroid-resistant acute GVHD. In designing the study, we considered that survival was the most important end point and that it was important to stratify patients by risk. To reduce bias, we also had an independent observer who was not aware of the randomization arm grade of GVHD at each site. This design may serve as a model for future testing of new agents to treat steroid-resistant acute GVHD.
There are currently few options for therapy of steroid-resistant GVHD. Although ABX-CBL did not show an improvement in outcome compared with ATG and therefore did not meet FDA criteria for approval, it did show activity and it is possible that patients resistant to either ATG or ABX-CBL may respond to the alternate agent. Moreover, because we restricted the study to steroid-resistant GVHD occurring in the first 100 days after a single transplantation, it is also possible that the activities of the study drugs may differ in treating GVHD after DLI. Nevertheless, the activity of each agent was suboptimal and new agents are required. Treatment with combinations of agents may also improve outcomes.
As novel approaches to HCT are developed, studies of the prevention and treatment of acute GVHD are necessary to optimize outcomes. In a recent report from Seattle in a large cohort of patients undergoing nonmyeloablative HCT, a decreased incidence of acute GVHD was observed compared with conventional HCT. Of interest, the onset of acute GVHD was delayed, with some patients presenting with de novo acute GVHD after day 100.23 Whether this form of acute GVHD will be amendable to the same therapy as acute GHVD following conventional HCT remains to be determined.
Authorship
Contribution: M.L.M. wrote the paper; D.C. enrolled patients; D.J.W. enrolled patients and wrote the paper; G.S. designed the study, helped conduct the trial, and helped collect data; N.H. and T.R.F. helped design and conduct the trial; S.H. performed statistical design and analysis; L.R. helped design and conduct the trial; S.S., R.K.S., J.D., M.T., S.P., and C.L. treated patients; H.E.H. helped design and conduct the trial, treated patients, and helped write the paper; H.J.D. helped design the trial, met with investigators, treated patients, and helped write the paper; B.R.B. helped design and conduct the trial, met with investigators, treated patients, and helped write the paper. H.J.D. and B.R.B. contributed equally to this work.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Margaret L. MacMillan, Department of Pediatrics, University of Minnesota, MMC 484, 420 Delaware St, SE, Minneapolis, MN 55455; email: macmi002@umn.edu.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by Abgenix.
The authors wish to acknowledge the following investigators, contributors, and institutions: Other participating investigators: Brian Bolwell, The Cleveland Clinic Foundation, Cleveland, OH; Arif Aram, Roswell Park Cancer Institute, Buffalo, NY; Todd Zimmermann, University of Chicago, Chicago, IL; Gretchen Eames, Cook Children's Medical Center, Fort Worth, TX; Patrick Stiff, Loyola University Medical Center, Maywood, IL; Istvan Redei, Emory University, Atlanta, GA; John Edwards, Florida Hospital, Orlando, FL; Finn Bo Petersen, University of Utah School of Medicine, Salt Lake City, UT; Friedrich Schuening, Vanderbilt Clinic, Nashville, TN; Lawrence Morris, Blood and Bone Marrow Transplant Center, Atlanta, GA; and Sunil Abhyankar, Oncology and Hematology Associates of Kansas City, Kansas City, MO. Data management: Axio Research, Seattle, WA. Study monitoring: ICON PLC, San Bruno, CA, and Farmastudio, Rovigo, Italy. Biostatistics support: Michael White, PhD, Seattle, WA.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal