Abstract
Mammalian β-globin loci contain multiple genes that are activated at different developmental stages. Studies have suggested that the transcription of one gene in a locus can influence the expression of the other locus genes. The prevalent model to explain this transcriptional interference is that all potentially active genes compete for locus control region (LCR) activity. To investigate the influence of transcription by the murine embryonic genes on transcription of the other β-like genes, we generated mice with deletions of the promoter regions of Ey and βh1 and measured transcription of the remaining genes. Deletion of the Ey and βh1 promoters increased transcription of βmajor and βminor 2-fold to 3-fold during primitive erythropoiesis. Deletion of Ey did not affect βh1 nor did deletion of βh1 affect Ey, but Ey deletion uniquely activated transcription from βh0, a β-like globin gene immediately downstream of Ey. Protein analysis showed that βh0 encodes a translatable β-like globin protein that can pair with alpha globin. The lack of transcriptional interference between Ey and βh1 and the gene-specific repression of βh0 did not support LCR competition among the embryonic genes and suggested that direct transcriptional interference from Ey suppressed βh0.
Introduction
The mammalian β-globin loci consist of multiple genes that are activated at different developmental stages in a tissue-specific manner. In the mouse, 2 “embryonic” β-like globin genes, Ey and βh1, are transcribed at high levels only during primitive erythropoiesis in the embryonic yolk sac. The “adult” expressed β-type globin genes—β-major and β-minor—are expressed at low levels in embryos and at high levels during fetal and adult definitive erythropoiesis. This developmental up-regulation of the adult β-like globin genes is coincident with the silencing of the embryonic β-like globin genes and is hypothesized to be mechanistically related to the silencing of the embryonic genes.
Regulatory elements of each β-like globin gene include a promoter and associated gene proximal cis-regulatory elements bound by multiple-tissue specific or ubiquitous transcription factors. High-level expression of all the genes at the locus requires a gene distal cis-regulatory element, the locus control region (LCR), which is located 5 to 22 kb upstream of the embryonic Ey gene in the mouse locus (for a review, see Stamatoyannopoulos and Grosveld1 ). The role, if any, of the LCR in the developmental regulation of individual genes within the locus is unclear.
Previous studies of β-globin gene regulation in transgenic mice carrying portions of the human β-globin locus have suggested that developmental expression of the embryonic and adult genes is regulated through different mechanisms. For the embryonic genes, the developmental silencing is gene autonomous and is achieved through binding or dissociation of specific transcription factors to or from the gene proximal cis-regulatory elements. When directly linked to the LCR, the human embryonic ϵ is expressed only at embryonic stages.2 When the LCR is deleted from the murine endogenous locus or from the human transgenic locus, all the genes are expressed at a very low level, yet tissue specificity and developmental silencing of the genes are maintained.3–5
The human β-globin gene is not autonomously suppressed in the embryo. When directly linked to the LCR or inserted in place of the endogenous embryonic gene in a transgene containing the entire human locus, the adult β gene is activated at all developmental stages.6–9 Therefore, neither gene proximal cis-elements nor trans-acting factors in primitive erythroid cells directly suppress transcription of the adult β-globin gene during the embryonic stage. However, insertion of a γ gene (which as a transgene is expressed during embryonic erythropoiesis) between the LCR and the adult β-globin gene results in suppression of the adult β gene during embryonic erythropoiesis.6,7 Based on these and other observations, the prevalent model for the developmental silencing of the adult β-globin gene is an LCR competition model10 (for a review, see Stamatoyannopoulos and Grosveld1 ). These and other studies have led to models in which all β-like globin genes compete for a rate-limiting interaction with the LCR. Proximity to the LCR is thought to favor LCR–promoter interaction, and the gene-autonomous silencing of the LCR-proximal embryonic genes is proposed to block interaction of the earlier stage genes with the LCR and thereby stimulate fetal or adult gene transcription.
LCR–gene interaction and gene competition studies have been performed with small LCR–gene constructs or human β-globin YAC constructs in which the general organization of the locus has been altered. This disturbance of the locus structure complicates the interpretation of those studies. Furthermore, because even large, intact, wild-type human b-globin YAC transgenes can occasionally have little or no expression11,12 or experience some degree of variegation at a higher frequency,13 concerns remain that the YACs do not, in fact, harbor the full cis-regulatory requirements of the endogenous human locus. Therefore, it is important to reexamine the conclusions drawn from transgene constructs at the endogenous mouse locus with minimal disturbance of the overall structure.
In addition, none of these experiments explicitly addressed how genes expressed at the same developmental stage influence one another. Given that the mouse genome can be modified through homologous recombination (HR) in embryonic stem (ES) cells to generate mutant mice and that there are multiple genes for each development stage, the murine β-globin locus provides an excellent alternative system to study the interactions among the β-like globin genes. Previous studies in our laboratory and in others have found that when the endogenous βmajorΔ gene was deleted by random mutation from the mouse endogenous locus (Thal-1 mutation), expression of the downstream βminorΔ gene increased14 (J. Roach, S.F., unpublished observations, October 2001).
To investigate the gene interaction mechanisms at the endogenous locus, we previously produced and reported analyses of the individual deletions of the promoters of each of the 2 major murine embryonic genes, Ey (ΔEy) and βh1 (Δβh1), in ES cells and in mice bearing these mutations.15 In the current study, the promoters of both major embryonic genes were simultaneously deleted, and the phenotype was analyzed in mice. Results reveal that the deletion of Ey, βh1, or both increases βmajor and βminor expression in the embryo, consistent with the LCR competition model. However, deletion of Ey did not affect the transcription of βh1 nor did the transcription of βh1 affect the deletion of Ey, which is not consistent with the LCR competition model. Moreover, deletion of Ey greatly increases transcription of βh0 (a weakly expressed embryonic gene), without affecting βh1, whereas deletion of βh1 had no effect on either Ey or βh0. These results are consistent with direct transcriptional interference of Ey on βh0.
Materials and methods
Generation of Ey and βh1 promoter replacement and deletion mice
Targeting constructs used for Ey promoter deletion (pEyprd-Hygro) and for βh1 promoter deletion (pβh1prd-Neo) have been described.15 To generate mice with Ey and βh1 promoters deleted in cis, embryonic stem cell (ES) clones with the βh1 promoter targeted by pβh1prd-Neo were electroporated with the pEyprd-Hygro construct. ES clones correctly targeted in cis with both promoter replacements were used to generate promoter replacement chimeric mice.
Southern blot assay for ES cell and mouse genotyping
Mouse tail DNA was prepared with the PureGene DNA Isolation kit (catalog no. D-70KB; Gentra Systems, Minneapolis, MN). The probe used for Southern blotting is a 0.7-kb BamHI/XbaI fragment located upstream of βh1 (BamH1 site is 7633 bp and XbaI site is 8307 bp from the transcription start site of Ey).
RT-PCR assay of expression of β-like globin genes
The assay system uses polymorphisms in the gene-coding regions between mice with the diffuse Hbb(d), (D) haplotype, on which the mutations are made, and mice with the single Hbb(s), (S) haplotype of β-like globin. The assay compares expression from the S allele to expression from the D allele in D/S heterozygous mice that are either wild-type or mutant at the D allele. The PCR primers and the system for the assay of Ey, βh1, β-major, and β-minor have been described.16 In short, the primers are exact matches for genes from the D and S haplotypes, but within the amplified region of the cDNA is a restriction site found in one haplotype but not the other. Amplified products were labeled with radioactive nucleotides during the last cycle of the PCR, digested with the appropriate restriction enzyme, and separated by size on an acrylamide gel, and the product of each allele was quantitated. Each product was amplified and assayed by itself with no multiplexing, and each gene-specific PCR had its own optimized number of cycles, as reported.16 The βh0 expression was achieved with RT-PCR to amplify the βh0 and the βh1 cDNA and with RFLP differences between the amplified regions to compare levels of βh0 with levels of βh1. Primers for βh0 expression were 5′CTCTGGGAAGGCTCCTGATTG3′ and 5′CCCAGGAGCTTGAAGTTCTC3′. PCR products (242 bp) were digested with BslI to differentiate amplicons from βh1 (242 bp) and βh0 (131 bp and 111 bp) cDNA. For this assay in cells that are D/S, the total βh1 signal from the S allele (the S allele lacks βh0 because of a natural deletion) and the D allele was divided by 2 to represent the βh1 transcripts from one allele. Primers for βh2 expression were 5′GTGCTGCCACTGAAGGTA3′ and 5′CTCAAAAGCAGCTTGCAGTG3′. Primers for βh3 expression were 5′ACTTTGGCAAGGAATTCAA3′ and 5′GGCCTCTGTGGTACTTGTG3′. Primers are perfect matches for βh3 and imperfect matches for Ey, and the products can be differentiated by restriction digest.
Protein assays of β-like globin components in primitive and definitive erythroid cells
Circulating primitive blood was collected from embryonic day 10.5 (e10.5) wild-type or mutant embryos. As described previously, HPLC analyses were performed with a 3-step acetonitrile gradient ranging from 35% to approximately 55% after treatment of the samples with cystamine.17
Protein identification by LC-FTMS
Soluble protein samples were digested with trypsin (Promega, Madison, WI) in ammonium bicarbonate buffer (50 mM) overnight at 37°C, followed by acidification with 5% formic acid and desalting on STAGE tips.18 Liquid chromatography–tandem mass spectrometry (LC-MS/MS) was performed using an LTQFT hybrid linear (2-D) ion trap-Fourier transform ion cyclotron resonance (FTICR) mass spectrometer (ThermoElectron, San Jose, CA) as described previously.19,20
Results
Production of the double embryonic gene promoter deletion mice
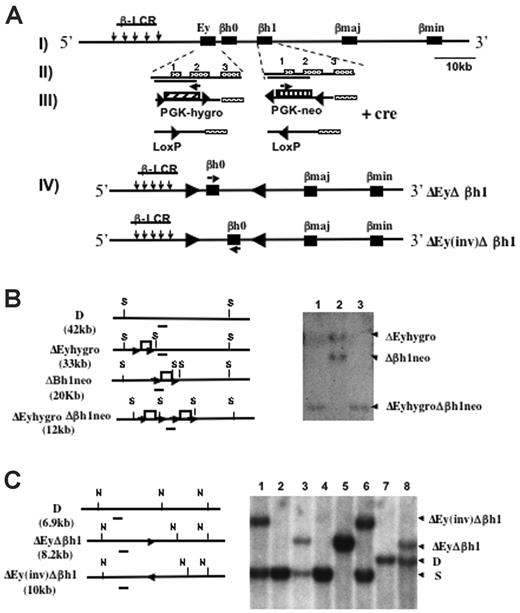
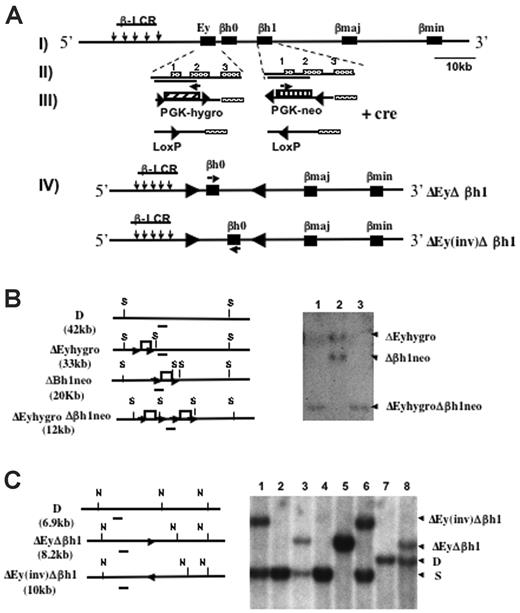
Mice were generated with the promoter regions of Ey and βh1 deleted in cis through sequential homologous recombination (Figure 1A). Each deleted region roughly measured 1.1 kbp, spanning from 700 bp 5′ to the transcription start site to near the 3′ end of exon 2 of each gene.15 One of the ES clones previously targeted to replace the βh1 promoter with neo (Δβh1neo) was retargeted with the construct that replaced the Ey promoter with hygro (ΔEyhygro). ES clones were screened for targeting of Ey, and Southern blots were used to identify clones that were targeted on both alleles in cis rather than in trans (Figure 1B).
Deletion and Southern blot strategies to generate ΔEyΔβh1 promoter deletion mice. (A, I) Schematic map of the Hbb(d) allele. (arrows) LCR hypersensitive sites. (▪) β-like globin genes. (II) Ey and βh1 genes with exons marked. Deleted regions from each gene are indicated by solid lines under the gene names. (III) Ey promoter was replaced by PGK-hygro, and βh1 promoter was replaced by PGK-neo. (▴) Selectable markers were each flanked by 2 convergent lox P sites and were removed by Cre recombinase-mediated deletion. (arrows) Selectable marker transcriptional start and orientation (IV). Cre recombinase also mediated an additional inversion of DNA between the 2 LoxP sites left from the deletion of the selectable markers and resulted in 2 double-promoter deletion alleles, ΔEyΔβh1 and ΔEy(inv)Δβh1. (arrows) Transcriptional orientation of βh0 on each allele. (B) Southern blot strategies and representative Southern blot used to screen for ΔEyhygroΔβh1neo ES clones modified in cis after homologous recombination. Restriction sites for SpeI are marked as S. The probe is denoted as a short line under each map. Note that the expected band for wild-type Hbb(d) measures 42.2 kb, which is not shown on the gel. Each lane represents a single ES clone. (C) Southern blot strategies and representative Southern blot to screen for ΔEyΔβh1 and ΔEy(inv)Δβh1 promoter deletion alleles in mice after Cre-mediated selectable marker deletion in vivo. Restriction sites for NsiI are marked as N, and the probe used is a line underneath each allele. Expected band sizes for each genotype are marked accordingly.
Deletion and Southern blot strategies to generate ΔEyΔβh1 promoter deletion mice. (A, I) Schematic map of the Hbb(d) allele. (arrows) LCR hypersensitive sites. (▪) β-like globin genes. (II) Ey and βh1 genes with exons marked. Deleted regions from each gene are indicated by solid lines under the gene names. (III) Ey promoter was replaced by PGK-hygro, and βh1 promoter was replaced by PGK-neo. (▴) Selectable markers were each flanked by 2 convergent lox P sites and were removed by Cre recombinase-mediated deletion. (arrows) Selectable marker transcriptional start and orientation (IV). Cre recombinase also mediated an additional inversion of DNA between the 2 LoxP sites left from the deletion of the selectable markers and resulted in 2 double-promoter deletion alleles, ΔEyΔβh1 and ΔEy(inv)Δβh1. (arrows) Transcriptional orientation of βh0 on each allele. (B) Southern blot strategies and representative Southern blot used to screen for ΔEyhygroΔβh1neo ES clones modified in cis after homologous recombination. Restriction sites for SpeI are marked as S. The probe is denoted as a short line under each map. Note that the expected band for wild-type Hbb(d) measures 42.2 kb, which is not shown on the gel. Each lane represents a single ES clone. (C) Southern blot strategies and representative Southern blot to screen for ΔEyΔβh1 and ΔEy(inv)Δβh1 promoter deletion alleles in mice after Cre-mediated selectable marker deletion in vivo. Restriction sites for NsiI are marked as N, and the probe used is a line underneath each allele. Expected band sizes for each genotype are marked accordingly.
Mice derived from double-promoter replacement ES cells (ΔEyhygroΔβh1neo) were generated by standard techniques and bred with CMV-Cre transgenic mice to delete the selectable marker. The deletion was efficient and generated 2 new strains, denoted ΔEyΔβh1 and ΔEy(inv)Δβh1 (Figure 1C). Because 2 Lox P sites in opposing orientations were retained in the locus, the Cre recombinase induced an additional inversion of the intergenic sequence between Ey and βh1, which is flanked by the 2 Lox P sites to produce ΔEy(inv)Δ βh1. In the ΔEy(inv)Δ βh1 strain, the βh0 gene was relocated 5 kbp further from the LCR and was transcribed in the direction opposite that of the endogenous gene (Figure 1A). To maintain genotype stability, ΔEyΔβh1 and ΔEy(inv)Δ βh1 mice were bred with 129 SvJ mice, and offspring were screened for the Cre gene by PCR. Only Cre-negative mice were used to generate mice for subsequent studies
Because the major embryonic β-like globins are not expressed from the ΔEyΔβh1 allele, it was possible that thalassemia would develop in homozygous embryos in utero. Sibling matings of each of the promoter deletion strains were set up to test the viability of homozygous mutant mice. Both ΔEyΔβh1 and ΔEy(inv)Δβh1 homozygous mice are born at normal Mendelian frequency and are viable through adulthood.
Transcription of other β-type globin genes in the Ey and βh1 promoter-deleted embryos
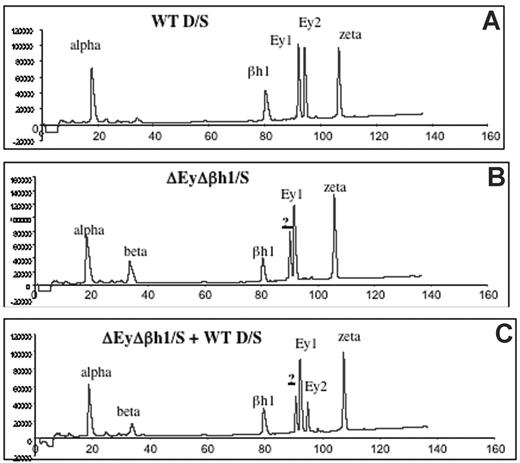
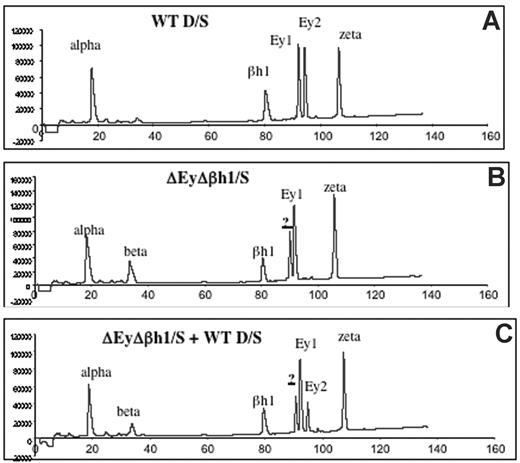
Viability of homozygous ΔEyΔβh1 mutants suggests that other β-like globin genes are up-regulated during primitive erythropoiesis. HPLC analysis of β-like globin protein content in primitive erythroid cells was performed on heterozygous mice to investigate the identity of expressed globin chains. Levels of β-major and β-minor in ΔEyΔβh1/S embryos increased from less than 3% (below detection) to 14% of the total β-like globin output. In addition, a prominent unidentified protein peak composing more than 24% of the total β-like globin output from the mutant allele was observed (Figure 2B-C). HPLC characteristics suggest that this peak is a novel embryonic β-like globin chain.
HPLC assays identify a new peak in peripheral primitive erythroid cells from ΔEyΔβh1 embryos. (A) HPLC of cell lysate of e10.5 circulating blood from wild-type D/S embryo shows identified elution peaks from the wild-type D and S alleles. Ey1 is from the S allele, and Ey2 is from the D allele. The βh1 peak includes protein from D and S, and alpha and zeta are α-like globin chains. (B) HPLC of cell lysate of d10.5 circulating blood from ΔEyΔβh1/S embryo. Identified elution peaks include alpha and zeta and from the S allele, Ey1, and βh1. The Ey2 peak from D is missing. A peak represents β-major and β-minor (beta), and a new peak (?) represents a novel β-like globin chain. (C) HPLC of cell lysate of mixed e10.5 circulating blood from wild-type D/S and ΔEyΔβh1/S embryos shows that the new peak (?) is distinguished from Ey1 and Ey2.
HPLC assays identify a new peak in peripheral primitive erythroid cells from ΔEyΔβh1 embryos. (A) HPLC of cell lysate of e10.5 circulating blood from wild-type D/S embryo shows identified elution peaks from the wild-type D and S alleles. Ey1 is from the S allele, and Ey2 is from the D allele. The βh1 peak includes protein from D and S, and alpha and zeta are α-like globin chains. (B) HPLC of cell lysate of d10.5 circulating blood from ΔEyΔβh1/S embryo. Identified elution peaks include alpha and zeta and from the S allele, Ey1, and βh1. The Ey2 peak from D is missing. A peak represents β-major and β-minor (beta), and a new peak (?) represents a novel β-like globin chain. (C) HPLC of cell lysate of mixed e10.5 circulating blood from wild-type D/S and ΔEyΔβh1/S embryos shows that the new peak (?) is distinguished from Ey1 and Ey2.
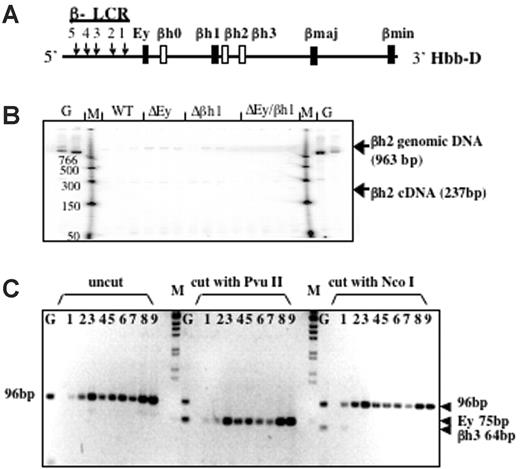
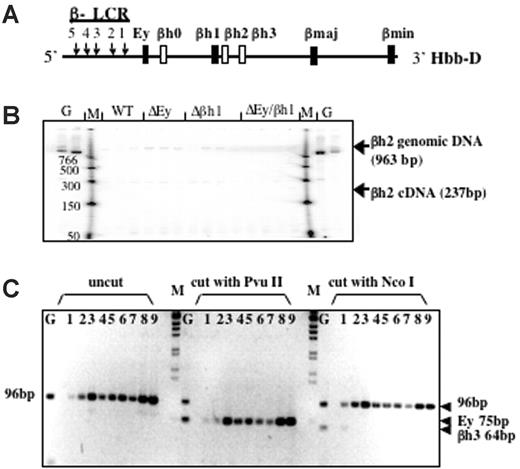
Based on DNA sequence and gene structure, 3 β-like globin genes—βh0, βh2, and βh3—have been predicted in the mouse D allele; however, these putative genes have not been analyzed extensively. βh0 is located 3′ of Ey, whereas βh2 and βh3 are located 3′ of βh1 (Figure 3A). Low-level expression of βh0 has been observed,21 but no expression of βh2 or βh3 has been reported and they have been considered pseudogenes, though their intron/exon structure is that of a normal globin gene. To determine whether the new protein peak is encoded by any of these genes, we designed RT-PCR assays to examine their potential transcription.
βh2 and βh3 are not expressed in any of the promoter-deletion embryos. (A) Schematic map of the D allele with the highly expressed β-like globin genes as dark bars and genes potentially expressed at low levels as white bars. (B) RT-PCR analysis of βh2 transcription in cDNA from e10.5 yolk sac. Lanes with genomic DNA (50 ng) are labeled G, marker lanes are labeled M, and genotypes of yolk sac samples are labeled. Expected sizes of PCR products from the βh2 cDNA and genomic DNA are marked. Genomic bands verify the ability to amplify the βh2 cDNA if it was present. (C) RT-PCR analysis of βh3 transcription in cDNA from e10.5 wild-type D/S (lanes 1-3), Δβh1/S (lanes 4, 5), ΔEy/S (lanes 6, 7), and ΔEyΔβh1/S (lanes 8, 9) yolk sac. G represents genomic DNA. PCR primers preferentially amplified cDNA from βh3 and, less efficiently, from Ey (2 mismatches on both primers). Uncut bands from any gene measured 96 bp. βh3 can be distinguished from Ey because the βh3 amplicon cut with NcoI to 64 bp but not with PvuII and the Ey amplicons cut with PvuII to 75 bp but not with NcoI.
βh2 and βh3 are not expressed in any of the promoter-deletion embryos. (A) Schematic map of the D allele with the highly expressed β-like globin genes as dark bars and genes potentially expressed at low levels as white bars. (B) RT-PCR analysis of βh2 transcription in cDNA from e10.5 yolk sac. Lanes with genomic DNA (50 ng) are labeled G, marker lanes are labeled M, and genotypes of yolk sac samples are labeled. Expected sizes of PCR products from the βh2 cDNA and genomic DNA are marked. Genomic bands verify the ability to amplify the βh2 cDNA if it was present. (C) RT-PCR analysis of βh3 transcription in cDNA from e10.5 wild-type D/S (lanes 1-3), Δβh1/S (lanes 4, 5), ΔEy/S (lanes 6, 7), and ΔEyΔβh1/S (lanes 8, 9) yolk sac. G represents genomic DNA. PCR primers preferentially amplified cDNA from βh3 and, less efficiently, from Ey (2 mismatches on both primers). Uncut bands from any gene measured 96 bp. βh3 can be distinguished from Ey because the βh3 amplicon cut with NcoI to 64 bp but not with PvuII and the Ey amplicons cut with PvuII to 75 bp but not with NcoI.
Gene-specific PCR primers designed to assay βh2 are in different putative exons; thus, amplified cDNA is distinguishable from genomic DNA by its smaller size. As shown in Figure 3B, the βh2 gene-specific primers did not detect spliced βh2 mRNA from e10.5 yolk sac and only detected contaminating genomic DNA or background bands that were not the expected size and did not show different levels with different mouse genotypes. Thus, βh2 was not expressed in any of the mutant mice. To detect βh3 cDNA, a pair of primers was designed to perfectly match and amplify βh3. When these primers were used under normal annealing conditions, no products were amplified from the cDNA of wild-type or mutant mouse e10.5 yolk sacs. Primers contained mismatches for Ey (2 mismatches on each primer), and, by lowering the annealing temperature, cDNA was amplified from all samples. Any βh3 amplicon should be digested with NcoI but not with PvuII, whereas amplified Ey cDNA should be digested with PvuII but not NcoI. As shown in Figure 3C, the amplification product was not digestible with NcoI and was completely digestible with PvuII, indicating that amplified cDNA was from Ey and that no transcription from βh3 was detected.
Activated transcription of βh0 correlates with deletion of the Ey promoter
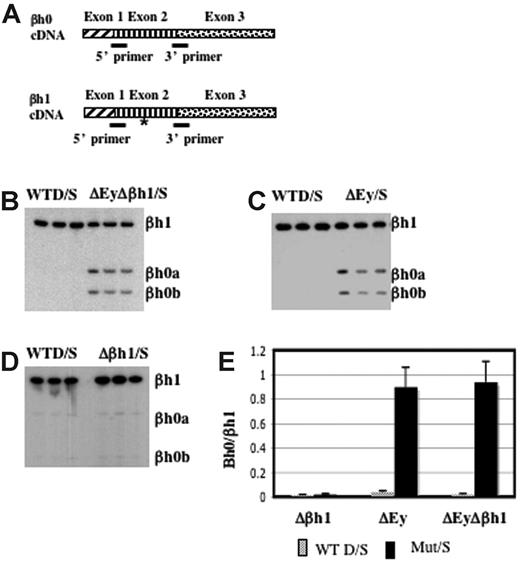
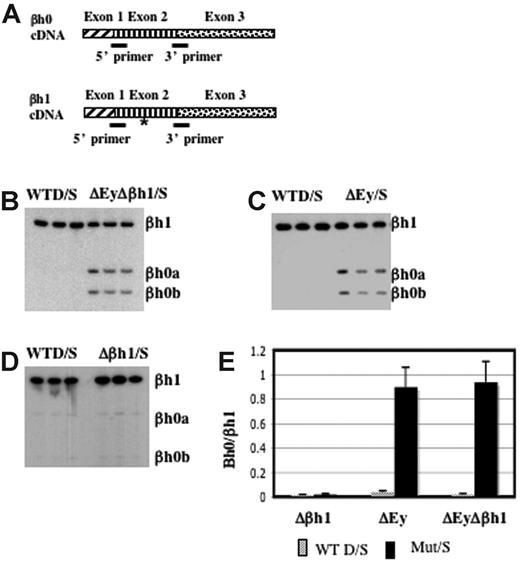
βh0 is highly homologous to βh1 in the coding and the promoter regions. To distinguish the transcription of βh0 from that of βh1, we designed primers to perfectly match βh0 and βh1 cDNA. Primers recognized βh1 from either the D or the S haplotype, and the S haplotype had a deletion of the promoter and the first exon of βh0. The amplified region contained an RFLP at which BslI uniquely digested the PCR product from βh0. This RFLP allowed us to quantify PCR products from βh0 relative to βh1 after BslI digestion (Figure 4A). RT-PCR analysis of e10.5 yolk sac cDNA generated from wild-type D/S and ΔEyΔβh1/S embryos showed that βh0 was activated in the ΔEyΔβh1 mutant primitive erythroid cells (Figure 4B). The distinct increase in βh0 transcription, along with a lack of detectable transcript from any of the other potentially expressed genes, suggested that the novel HPLC peak in ΔEyΔβh1 embryos was βh0 protein and that βh0 mRNA could be translated into a stable globin protein.
βh0 is activated exclusively during primitive erythropoiesis in embryos with the promoter of Ey deleted. (A) Steady state RT-PCR strategy to detect and quantitate transcription from βh0. PCR primers were perfect matches for βh0 and βh1 cDNA. (asterisk) The amplified region contained one restriction enzyme site in βh0 (BslI) that was not present in βh1 cDNA. Amplified cDNA from e10.5 yolk sacs of wild-type D/S and (B) ΔEyΔβh1/S, (C) ΔEy/S, and (D) Δβh1/S. All samples were digested with BslI. (E) Quantitation of βh0 expression in each mutant genotype comparing the ratio of βh1 (uncut) and βh0 (cut), adjusted for the number of active βh1 alleles. Error bars represent SD of at least 3 embryos of that genotype in a litter.
βh0 is activated exclusively during primitive erythropoiesis in embryos with the promoter of Ey deleted. (A) Steady state RT-PCR strategy to detect and quantitate transcription from βh0. PCR primers were perfect matches for βh0 and βh1 cDNA. (asterisk) The amplified region contained one restriction enzyme site in βh0 (BslI) that was not present in βh1 cDNA. Amplified cDNA from e10.5 yolk sacs of wild-type D/S and (B) ΔEyΔβh1/S, (C) ΔEy/S, and (D) Δβh1/S. All samples were digested with BslI. (E) Quantitation of βh0 expression in each mutant genotype comparing the ratio of βh1 (uncut) and βh0 (cut), adjusted for the number of active βh1 alleles. Error bars represent SD of at least 3 embryos of that genotype in a litter.
To further dissect the mechanism underlying βh0 regulation, we assayed βh0 expression in our single-promoter deletion mutants, ΔEy and Δβh1. As reported previously,15 the transcription of βh1 was not changed by the deletion of the Ey promoter. Hence, the transcription level of βh0 could be compared with that of βh1 directly, even on the ΔEy allele. Assays of ΔEy/S and Δβh1/S animals showed that βh0 was activated in ΔEy/S animals (Figure 4C) but not in Δβh1/S animals (Figure 4D). The activation of βh0 correlated with deletion of the Ey promoter, and, when activated, its transcription level was comparable to that of βh1 because the βh0/βh1 ratio was close to 1 (Figure 4E). The βh0 expression level in ΔEy and ΔEyΔβh1 was more than 20-fold higher than in wild-type mice. In the ΔEy/S mutants, βh0 expression was similar to the level in ΔEyΔβh1/S mutants. These results demonstrated that the activation of βh0 was caused by the deletion of the Ey promoter and that the deletion of the βh1 promoter did not have a detectable influence on βh0 transcription.
Transcription of βh0 is inversely proportional to transcription levels of Ey
It has been reported, by us and by others,22–24 that the transcription of one gene can suppress the transcription of other genes linked in cis through direct transcriptional interference (TI), which could involve multiple mechanisms. The physical relationship of the genes involved in TI influences the degree of suppression of each gene.22 It is possible that transcription from Ey suppresses βh0 through direct transcriptional interference rather than competition for the LCR.
If direct transcriptional interference were involved, any change in cis or trans that resulted in reduced Ey transcription would increase the level of βh0. Recently, Tanabe et al (O.T., Yannan Shen, Shoko Kobayashi, David McPhee, William Brandt, Xia Jang, Andrew D. Campbell, Yei-Tsung Chen, Chawnshang Chang, Masayuki Yamamoto, Keiji Tanimoto, and J.D.E., manuscript submitted) generated mice overexpressing the orphan receptors TR2 and TR4. These orphan receptors form a complex that suppresses Ey but does not suppress the βh1 or adult β-globin genes in primitive erythrocytes (O. Tanabe et al, manuscript submitted). Consistent with this, 2 independent transgenic TR2/TR4 lines expressing elevated levels of TR2 and TR4 displayed correspondingly lowered accumulation of Ey mRNA. We found that βh0 is also activated in the yolk sac of those transgenic mice (Figure 5) and that the transcript level of βh0 is inversely proportional to the level of Ey expression. Line 1, which expresses less of the TR2/TR4 transgene than line 2, has 2-fold higher levels of Ey and half the amount of βh0 than line 2. Therefore, a trans alteration that reduced the transcription of Ey increased the transcription of βh0 inversely to the reduction of Ey.
Transcription level of Ey directly regulates βh0. (A) Representative gel of βh0 expression in e10.5 wild-type and TR2/TR4 transgenic yolk sacs. Genotype of each sample is marked above the gel, and 2 TR2/TR4 transgenic lines are noted as line 1s and 2. All samples were digested with BslI. (B) Quantitation of βh0 expression in e10.5 wild-type and 2 different TR2/TR4 transgenic lines. Error bars represent SD of at least 3 embryos of that genotype.
Transcription level of Ey directly regulates βh0. (A) Representative gel of βh0 expression in e10.5 wild-type and TR2/TR4 transgenic yolk sacs. Genotype of each sample is marked above the gel, and 2 TR2/TR4 transgenic lines are noted as line 1s and 2. All samples were digested with BslI. (B) Quantitation of βh0 expression in e10.5 wild-type and 2 different TR2/TR4 transgenic lines. Error bars represent SD of at least 3 embryos of that genotype.
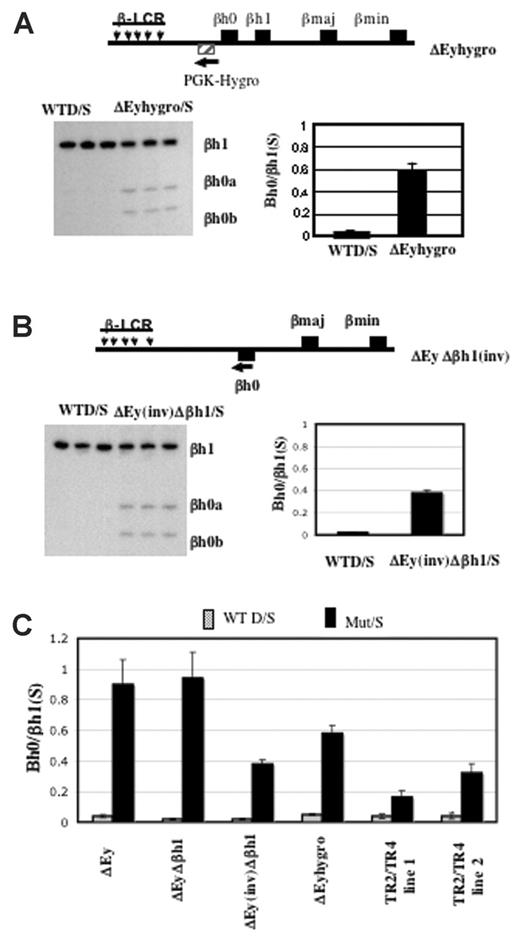
We generated mice in which the Ey promoter was replaced by the PGK-hygro selectable marker transcribed away from βh0. In this strain, the expression of βh0 in yolk sac was well above the amount expressed from a wild-type allele but approximately 60% of the level expressed from an allele with the Ey promoter deleted and in which the selectable marker was removed (Figure 6A).
Activation of βh0 transcription is influenced by multiple parameters in mice with reduced Ey transcription. (A) Schematic map of ΔEyhygro allele. (▨) Selectable marker gene PGK-Hygro. (arrow) Its transcriptional direction. Representative gel and quantitation of βh0 expression in e10.5 wild-type D/S and ΔEyhygro/S yolk sacs. Error bars represent SD of at least 3 littermates with the genotype marked throughout. (B) Schematic map of ΔEy(inv)Δβh1 allele. (▪) βh0 gene that was relocated 5 kb further away from the LCR on the mutant allele compared with the wild-type allele. (arrow) Inversion also changed the transcription direction of the βh0 gene on this mutant allele. Representative gel and quantitation of βh0 expression in e10.5 wild-type D/S and ΔEyΔβh1(inv)/S yolk sacs. (C) Comparative summary of βh0 transcription in various mutants in which it has increased transcription.
Activation of βh0 transcription is influenced by multiple parameters in mice with reduced Ey transcription. (A) Schematic map of ΔEyhygro allele. (▨) Selectable marker gene PGK-Hygro. (arrow) Its transcriptional direction. Representative gel and quantitation of βh0 expression in e10.5 wild-type D/S and ΔEyhygro/S yolk sacs. Error bars represent SD of at least 3 littermates with the genotype marked throughout. (B) Schematic map of ΔEy(inv)Δβh1 allele. (▪) βh0 gene that was relocated 5 kb further away from the LCR on the mutant allele compared with the wild-type allele. (arrow) Inversion also changed the transcription direction of the βh0 gene on this mutant allele. Representative gel and quantitation of βh0 expression in e10.5 wild-type D/S and ΔEyΔβh1(inv)/S yolk sacs. (C) Comparative summary of βh0 transcription in various mutants in which it has increased transcription.
Previous studies with human transgenic loci have shown that changing the distance of a β-like gene from the LCR could affect its developmental expression pattern.8,9,25,26 It is less clear whether distance from the LCR directly affected expression level. During the removal of the selectable markers by Cre, mice were generated with an inversion of the region between Ey and βh1, including the βh0 gene. In the ΔEy(inv)Δβh1 mutants, the βh0 gene is relocated 5 kbp further from the LCR compared with its wild-type location and is transcribed in the reverse direction. To determine how these alterations in location and transcriptional orientation might affect gene expression, we assayed βh0 expression in e10.5 yolk sac from the ΔEy(inv)Δβh1 mutants (Figure 6B). Compared with βh0 transcription in ΔEy and ΔEyΔβh1mutants (Figure 6C), transcription of βh0 in ΔEy(inv)Δβh1 was reduced approximately 2.5-fold. Figure 6C summarizes the levels of βh0 expression in all genotypes of mice assayed.
Bh0 protein is produced and forms a complex with α-globin
The demonstration that βh0 message is increased by at least 20-fold after deletion of the Ey promoter strongly suggests that the unknown protein peak in Figure 2 is βh0. Experiments using mass spectrometry in conjunction with HPLC were undertaken to definitively determine whether βh0 was expressed at the protein level and corresponded to the unknown peak in Figure 2.
Protein extracts of homozygous ΔEyΔβh1 circulating e10.5 primitive cells were run on a nondenaturing isoelectric focusing gel, and the complexes that were visually revealed by staining with o-dianisidine were cut out and eluted from that gel. Three novel bands were analyzed by mass spectrometry. Band 1 contained βh0 as a major component. The protein in this band was then analyzed by HPLC (Figure S2, available on the Blood website; see the Supplemental Figures link at the top of the online article), and results were compared with those from HPLC analysis of protein from circulating primitive erythrocytes of ΔEyΔβh1 mice (Figure S3). Band 1 contained the same peak found in homozygous and heterozygous ΔEyΔβh1 mice; thus, this peak was definitively identified as βh0. In band 1, βh0 and α-globin were found in roughly the same amount as βh0 (Figure S2), demonstrating that βh0 can form a complex with α-globin. No ζ-globin was found in band 1, and bands 2 and 3 did not have significant amounts of βh0. Hence, it appeared that βh0 did not form a complex with ζ-globin.
Deletion of the Ey or βh1 gene promoters induces β-major and β-minor expression in primitive erythroid cells
To test the hypothesis that transcription of the embryonic β-globin genes suppresses transcription of the fetal/adult β-globin genes during embryonic erythropoiesis, we analyzed the transcription and translation of the murine fetal/adult β-major and β-minor genes in the yolk sac primitive erythroid cells from the embryonic gene promoter deletion embryos.
Transcription of β-major and β-minor from the wild-type D allele or the mutant D allele was normalized to the transcription of βs and βt from the wild-type S allele. Results revealed that the deletion of both embryonic gene promoters (ΔEyΔβh1/S) or the deletion of a single embryonic gene promoter (ΔEy/S or Δβh1/S) increased the transcription of β-major and β-minor from the mutant alleles in e10.5 primitive erythrocytes (Figure 7A-B). HPLC quantitation of the β-like globin chains in e10.5 ΔEyΔβh1/S and wild-type D/S primitive erythroid cells showed that the level of adult β-globin chains in ΔEyΔβh1/S primitive erythroid cells increased to 14% of total β-like globin chains (from a possible 50% of total β-like globin chains that can derive from one allele) compared with less than 5% in wild-type D/S primitive erythroid cells (Figure 2). The increase was not apparent in e9.5 day postcoital (dpc) red blood cells. In these cells, very high D/S ratios were identical between wild-type and promoter deletion mice. For clarity, all ratios in wild-type mice except those from e9.5 embryos were normalized to 1.
Deletion of either or both embryonic gene promoter(s) increased transcription of β-major/minor in primitive erythroid cells. (A) Representative RT-PCR/RFLP assay of β-major/minor transcription in e10.5 ΔEy/S, Δβh1/S, and ΔEyΔβh1/S yolk sac primitive erythroid cells. Sample genotypes are marked above the gel. (B) Quantitation of β-major/minor transcription in yolk sac of promoter deletion mutants. Each pair of bars represents data from 2 litters, with a total of at least 12 embryos with error bars denoting SD. Mutations assayed and days of gestation are indicated below the graph. Wild-type ratios from e10.5 and e11.5 were close to 1 and were normalized to 1, and the mutant ratio was similarly adjusted. Wild-type ratios from e9.5 were not normalized and are represented as simple D/S ratios from each genotype. All mutant ratios are statistically different from wild-type ratios (P > .05) for e10.5 and e11.5 but not e9.5. (C) Representative RT-PCR/RFLP assay and (D) quantitation of β-major/minor transcription in ΔEy/S, Δβh1/S, and ΔEyΔβh1/S adult peripheral blood. Genotype of each sample is marked above the gel. Each pair of bars represents data from least 5 adult mice. Wild-type D/S ratios were normalized to 1.
Deletion of either or both embryonic gene promoter(s) increased transcription of β-major/minor in primitive erythroid cells. (A) Representative RT-PCR/RFLP assay of β-major/minor transcription in e10.5 ΔEy/S, Δβh1/S, and ΔEyΔβh1/S yolk sac primitive erythroid cells. Sample genotypes are marked above the gel. (B) Quantitation of β-major/minor transcription in yolk sac of promoter deletion mutants. Each pair of bars represents data from 2 litters, with a total of at least 12 embryos with error bars denoting SD. Mutations assayed and days of gestation are indicated below the graph. Wild-type ratios from e10.5 and e11.5 were close to 1 and were normalized to 1, and the mutant ratio was similarly adjusted. Wild-type ratios from e9.5 were not normalized and are represented as simple D/S ratios from each genotype. All mutant ratios are statistically different from wild-type ratios (P > .05) for e10.5 and e11.5 but not e9.5. (C) Representative RT-PCR/RFLP assay and (D) quantitation of β-major/minor transcription in ΔEy/S, Δβh1/S, and ΔEyΔβh1/S adult peripheral blood. Genotype of each sample is marked above the gel. Each pair of bars represents data from least 5 adult mice. Wild-type D/S ratios were normalized to 1.
Embryonic globin genes were not expressed in definitive cells of the murine fetus or the adult. No effect of embryonic promoter deletion on β-major and β-minor transcription in definitive cells was observed in the ΔEyΔβh1/S mutants (Figure 7C-D). Protein quantification by HPLC analysis confirmed the results of RT-PCR assays (data not shown).
Discussion
Transcriptional interference (TI) is a broad term that describes situations in which the transcription of one gene suppresses the transcription of nearby genes. A variety of mechanisms can be involved in transcriptional interference. It has been reported, primarily from studies of human transgenes in mice, that the transcription of one gene at the β-globin locus can suppress the transcription of other genes. The physical arrangement of genes at the locus is thought to be important for developmental gene regulation through transcriptional interference. The prevalent hypothesis regarding TI among genes at the β-globin locus is that β-like globin genes compete for LCR interaction. It is hypothesized that LCR–promoter interaction is accomplished by a looping mechanism that brings the LCR and the activated promoter in contact in a physical interaction required for transcriptional activation.27 Other possible mechanisms proposed for the TI effects include tracking28 or linking29 of proteins from the LCR and direct transcriptional interference.30 Results reported here suggest that multiple mechanisms are involved in TI among the β-like globin genes and that the mechanisms influencing the embryonic genes differ from those influencing the fetal/adult genes.
It has been shown that high-level transcriptional activation of the embryonic genes at the endogenous locus requires the LCR.35 Therefore, if all the murine embryonic β-like globin genes competed for LCR interaction, deletion of one embryonic gene would increase transcription of the other embryonic β-like globin genes, but this was not the case. As reported previously and reiterated here, deletion of the Ey promoter did not increase βh1 transcription, and deletion of βh1 did not increase Ey transcription. Deletion of the Ey promoter did dramatically increase βh0 transcription, but deletion of βh1 had no effect on βh0. Although these data demonstrated no direct competition between Ey and βh1, Ey transcription interfered with βh0.
The observed βh0 suppression by Ey could have occurred through 2 possible mechanisms, directionally polar LCR competition or direct transcriptional interference. Given that Ey is upstream of βh0, any of the 3 LCR–gene interaction models could potentially explain how Ey suppresses βh0. However, because all the LCR–gene interaction models predicted favored LCR interaction and, therefore, transcription of the LCR proximal gene, they all faced the dilemma of why βh0—which is LCR proximal compared with βh1—was suppressed by Ey transcription whereas the LCR distal βh1 was not affected by Ey transcription. Given that the gene promoters of βh1 and βh0 were almost identical (Figure S3) and that they had similar transcription levels after the removal of the Ey promoter, it was unlikely that the preference resulted from intrinsic properties of different promoters. A plausible alternative explanation is that Ey did not suppress βh0 by competing for the LCR but by direct transcriptional interference. This explanation is consistent with the fact that neither Ey nor βh1 transcriptionally interfered with the other. The finding that the specific reduction in Ey by transgenic overexpression of TR2/TR4 also stimulated βh0 expression supports the model that direct transcriptional interference accounted for the suppression of βh0 by Ey.
Clearly, multiple molecular mechanisms account for transcriptional interference, and they are poorly understood. Because of the proximity and tandem arrangement of the Ey and βh0 genes, we propose that transcription from Ey disrupts the recruitment of transcriptional regulatory factors and transcription machinery to the βh0 promoter (promoter occlusion), as has been demonstrated in other models.23,31 This possibility is related to the fact that mammalian polII proceeds past the polyA site and does not have a specific termination site (for a review, see Rosonina et al32 ). The data showing that PGK-hygro, which replaced the Ey promoter and was transcribed in the opposite direction, only mildly suppressed βh0 supported the hypothesis that direct transcriptional interference by promoter occlusion was the primary mechanism by which Ey suppressed βh0.
Authorship
Contribution: X.H. designed and performed the research and wrote the paper; S.E. designed and performed the research; N.P. performed the research; E.E.B. designed and performed the research and wrote the paper; J.F. performed the research; O.T. contributed new reagents; S.A.G. designed and performed the research; M.B. designed and performed the research and wrote the paper; J.D.E. designed the research, contributed new reagents, and wrote the paper; M.G. designed the research and wrote the paper; and S.F. designed and performed the research and wrote the paper.
Conflict of interest disclosure: The authors declare no competing financial interests.
Correspondence: Steven Fiering, Department of Microbiology/Immunology and Norris Cotton Cancer Center, 622 Rubin, Dartmouth Hitchcock Medical Center, Dartmouth Medical School, Lebanon, NH 03756; e-mail: fiering@dartmouth.edu.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
This work was supported by National Institutes of Health–National Institute of Diabetes and Digestive and Kidney Diseases grant RO1 DK54071 (S.F.) and by a Burroughs-Wellcome Fund Career Award (M.B.).
We thank the staff at the Dartmouth Transgenic Mouse Facility of the Norris Cotton Cancer Center for producing the transgenic mice and Sandra Warner for assisting with ES cell culture.