Abstract
A unique characteristic of the autoimmune liver disease primary biliary cirrhosis (PBC) is the presence of high-titer and extremely specific autoantibodies to the E2 component of the pyruvate dehydrogenase complex (PDC-E2). Autoantibodies to PDC-E2 antigen have only been detected in patients with disease or in those who subsequently develop PBC. One exception has been a subgroup of patients with multiple myeloma (MM) who underwent allogeneic hematopoietic stem cell transplantation (HSCT) and received donor lymphocyte infusions (DLIs) after transplantation. These patients developed high-titer antibodies to a variety of myeloma-associated antigens, including PDC-E2, coincident with rejection of myeloma cells in vivo. To examine the specificity of autoantibodies to PDC in these patients, we screened sera from patients with MM, chronic leukemias, monoclonal gammopathy of unknown significance (MGUS), PBC, and healthy donors. Three of 11 patients with MM (27%) and 2 of 6 patients with chronic leukemias (33%) developed anti–PDC-E2 antibodies in association with DLI response; 2 of 12 (17%) patients in the MGUS pretreatment control population also had detectable anti-PDC responses. Interestingly, the epitope specificity of these PDC-E2 autoantibodies was distinctive, suggesting that the mechanisms leading to loss of tolerance in the transplantation patients are distinct from PBC.
Introduction
In a previous report we demonstrated that patients with multiple myeloma (MM) who exhibited rejection of tumor cells following allogeneic hematopoietic stem cell transplantation (HSCT) and CD4+ donor lymphocyte infusion (DLI) developed high-titer antibody responses against a variety of intracellular or cell surface proteins expressed by myeloma cells.1,2 Importantly, the antibody response against these proteins only developed after DLI and occurred primarily at the time of complete disease response.1 One of the intracellular antigens identified in these studies was dihydrolipoamide acetyltransferase (PDC-E2). PDC-E2 is the E2 component of the multienzyme pyruvate dehydrogenase complex (PDC) localized within the inner mitochondrial membrane that catalyzes several steps in intermediary metabolism.3,4 In addition to its role in cell metabolism, PDC-E2 represents the target of antimitochondrial antibodies (AMAs), the characteristic autoantibodies present in up to 95% of patients with primary biliary cirrhosis (PBC).5–7
PBC is a chronic cholestatic disease characterized by lymphocytic infiltration and progressive destruction of the intrahepatic bile ducts, leading to cirrhosis and liver failure.8 The B-cell epitopes within PDC-E2 are restricted to the inner and outer lipoyl domain of the protein with no reactivity toward other domains.5,9–11 Although in some cases CD4+ T-cell responses have been demonstrated against epitopes external to this region,12,13 in most cases the B, CD4+, and CD8+ T-cell responses recognize highly conserved epitopes in the inner lipoyl domain of PDC-E2.14–16
In the present study, we describe specific anti–PDC-E2 responses in 2 distinct patient populations that are not affected by PBC: patients with MM and chronic leukemias who received allogeneic HSCT and DLI and patients with monoclonal gammopathy of unknown significance (MGUS) and MM before any therapy. To exclude a causative role for HSCT and its frequent complication graft versus host disease (GVHD) alone, we also analyzed 19 additional patients who underwent allogeneic HSCT, including 10 who developed chronic GVHD. Using 85 overlapping peptides representing the entire length of PDC-E2, we further characterized the epitope specificity in patients with hematologic malignancies and PBC, showing how post-DLI anti–PDC-E2 antibodies preferentially target different structural domains of PDC-E2. Importantly, although these antibody responses persisted for more than 2 years, none of the patients showed any evidence of chronic liver disease, again highlighting that autoantibodies that appear in hematopoietic disorders arise via different mechanisms than occur in spontaneous human autoimmune disease.
Materials and methods
Patient samples and treatment
Serum samples were obtained after informed consent from 12 patients with MGUS, 20 patients with MM, 54 patients with PBC (27 known to be positive and 27 negative for anti–PDC-E2), and 36 patients enrolled on clinical trials of allogeneic HSCT. The latter group received myeloablative therapy followed by infusion of marrow stem cells from HLA-matched donors. Seventeen patients received infusions of CD8+-depleted donor lymphocytes as previously described.17 PDC-E2–negative samples from PBC patients were intentionally selected after a Western blot screening to have a larger group of negative PBC as a further control in our enzyme-linked immunosorbent assay (ELISA) screening. Clinical characteristics of patients who developed antibodies against PDC-E2 and PBC patients are summarized in Table 1. Serum or plasma samples from anonymous healthy donors were obtained from the Blood Donor Center at Dana-Farber Cancer Institute. Clinical protocols were approved by the institutional review boards of the Dana-Farber/Harvard Cancer Center or University of California at Davis.
Generation and purification of PDC-E2–GST fusion protein
A cDNA fragment encoding full-length PDC-E2 with BamHI and NotI restriction sites was generated by polymerase chain reaction (PCR) using Pfu-turbo DNA polymerase (Stratagene, La Jolla, CA). The PCR product was inserted into a GST fusion vector pGEX 4T3 (Amershan Pharmacia Biotech, Piscataway, NJ), and the DNA sequence of the constructed plasmid was verified by sequencing. The induction of recombinant protein, synthesis, and subsequent purification of protein by elution from Sepharose GST beads was performed according to manufacturer's protocols (Amershan Pharmacia). Correct size and specificity of the expressed product was confirmed by Western blot using a goat anti–PDC-E2 antibody (N-20) (Santa Cruz Biotechnology, Santa Cruz, CA). Recombinant GST protein (without fusion protein) was also purified by standard techniques and used as a control in all the experiments.
Western blot
PDC-E2–GST fusion protein was subjected to 7% to 14% sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) with tris-glycine buffer and transferred onto a nitrocellulose membrane in 20% methanol in tris-glycine buffer. Membranes were stained with either anti–PDC-E2 antibody (N-20) (Santa Cruz Biotechnology), anti-GST (BD Biosciences Pharmingen, San Jose, CA), or patient serum (1:500 dilution) followed by a secondary horseradish peroxdase–conjugated antibody (rabbit antigoat or goat antihuman) from Jackson ImmunoResearch Labs (West Grove, PA) and visualized using chemiluminescence.
Indirect immunofluorescence for serum autoantibody analysis
AMAs were determined blindly using indirect immunofluorescence analysis. Sera were tested for the presence of AMAs at different dilutions using human Hep-2 cells as substrate. FITC-conjugated anti-IgA/G/M was used as secondary antibody (DAKO, Carpinteria, CA). Immunofluorescence microscopy was performed as described previously,39 using a Zeiss Axioskop microscope, Zeiss AxioCam camera, AxioVision software version 4.0, a pl 10×/20 ocular, and a 40×/0.75 numerical aperture objective (total magnification, 400×) (all from Carl Zeiss Microimaging, Thornwood, NY). The presence of AMAs at serum dilutions greater than 1:40 was considered positive.
Inhibition of PDC enzyme activity
Patient serum (1:100 dilution) was incubated with PDC (Sigma, St Louis, MO) for 10 minutes at room temperature. The solution was then added to a PDC reaction mixture containing 5 mM sodium pyruvate, 2.5 mM NAD+, 0.2 mM magnesium chloride, and 50 mM potassium phosphate buffer, pH 8.0. UV absorbance change per minute at 340 nm was monitored for 5 minutes. Enzyme activity measurements without addition of serum were run in parallel on sera from patients with PBC, MM, other leukemia after DLI, and negative human controls.
ELISA assays
A total of 50 μL purified PDC-E2–GST or GST alone was coated onto ELISA plates (VWR Scientific, South Plainfield, NJ) at the concentration of 5 μg/mL in coating buffer (PBS and 0.05% sodium azide) overnight at 4°C. Plates were washed 5 times with PBS with 0.05% Triton and blocked overnight at 4°C with 200 μL per well of nonfat milk with 0.05% Triton. Patient serum (50 μL per well) at serial dilutions ranging from 1:50 to 1:10 000 was added to each well for 3 hours at room temperature. After 5 washes, the plates were incubated with 50 μL per well of alkaline phosphatase–conjugated goat anti–human IgG antibody (Jackson ImmunoResearch Labs) and diluted 1:1000 for 1 hour at room temperature. Plates were then washed and incubated with 75 μL per well of PNPP substrate (Sigma) for 25 minutes at room temperature and read at optical density (OD) 405 by Spectramax 190 Microplate reader (Molecular Devices, Sunnyvale, CA). For IgG subclass analysis, 5 μg/mL PDC-E2–GST or GST alone was incubated with 1:50 dilution of patient serum followed by a biotin-conjugated IgG1, IgG2, IgG3, and IgG4 at 1:500 dilution (Sigma) for 1 hour at room temperature and with streptavidin (Sigma) at 1:5000 dilution for 1 hour. Plates were then developed and analyzed as described for the PDC-E2-GST ELISA. In each assay, the level of reactivity with GST alone was subtracted from the reactivity with PDC-E2–GST.
Peptide analysis
Eighty-five synthetic overlapping peptides covering the entire length of PDC-E2 were obtained from New England Peptide (Gardner, MA). Peptides were 14 to 20 amino acids long and were reconstituted at 10 mg/mL DMSO and stored at −20°C. Antibody reactivity with PDC-E2 peptides was determined using ELISA plates (VWR Scientific) coated with 50 μL of each peptide at 10 μg/mL in coating buffer overnight at 4°C and subsequently incubated with patient serum at 1:50 or 1:100 dilution as described for ELISA previously. Desalted peptides (60% to 70% purity) were used for initial screening, and each positive reaction was confirmed using high-performance liquid chromatography (HPLC)–purified peptides (more than 85% purity). Serial dilutions were performed using HPLC-purified peptides and serum dilutions ranging from 1:50 to 1:10 000. An irrelevant peptide was used as background control for all dilutions. In several experiments patient serum was preblocked with different amounts of recombinant PDC-E2 or GST protein (25 μg/mL or 50 μg/mL) for 3 hours at room temperature before incubation with the specific peptides.
Statistical analysis
Background levels of reactivity with PDC-E2–GST were determined by analysis of 52 healthy donors, and a cutoff of 0.301 was established based on the 95th percentile of reactivity with samples from healthy donors. To examine whether the level of reactivity with PDC-E2–GST differed among healthy donors and the patient groups analyzed, the Wilcoxon rank-sum test was used for continuous anti–PDC-E2 antibody measures and the Fisher exact test was used for dichotomous measures (using 95% percentile of a healthy donor's mean as a cutoff). No adjustment was made for multiple comparisons. To determine the frequency of reactivity against different domains of PDC-E2 among post-DLI responders and MGUS and PBC patients, the Fisher exact test was used.
Results
Detection of antibody response after DLI
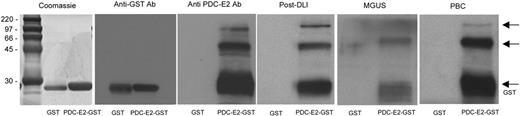
As previously described, a strong antibody response against PDC-E2 was detected in several patients with persistent disease or relapse after allogeneic HSCT who achieved complete remission after DLI.1 To further characterize this antibody response, a full-length PDC-E2–GST fusion protein was generated. This recombinant protein was first used to confirm the specificity of the serum antibody response by Western blot. Antibodies were detected in post-DLI serum (1:500 dilution) as well as in PBC patient serum without evidence of reactivity against the recombinant GST control (Figure 1). Commercially available anti–PDC-E2 and anti-GST antibodies were used as controls. As shown in Figure 1, post-DLI, MGUS, and PBC sera recognized 3 bands that were also reactive with anti–PDC-E2 antibody. Only a lower band of approximately 30 kDa was detected by the anti-GST antibody. Two patients with MGUS had detectable antibodies against PDC-E2, while none of the patients with MM displayed any antibody reactivity pretreatment (data not shown).
Reactivity of patient serum with PDC-E2 demonstrated by Western blot. Purified recombinant PDC-E2–GST or GST proteins were loaded in different lanes and probed with patient serum at 1:500 dilution. Antibodies against GST or PDC-E2 were used as a control. Arrows indicate protein fragments recognized by anti–PDC-E2 antibody.
Reactivity of patient serum with PDC-E2 demonstrated by Western blot. Purified recombinant PDC-E2–GST or GST proteins were loaded in different lanes and probed with patient serum at 1:500 dilution. Antibodies against GST or PDC-E2 were used as a control. Arrows indicate protein fragments recognized by anti–PDC-E2 antibody.
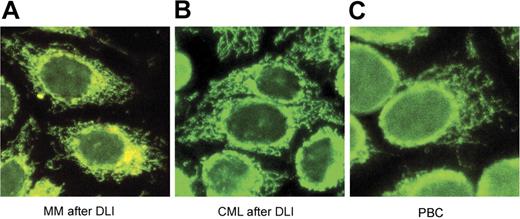
The presence of antibodies against 2 other mitochondrial protein complexes, 2-oxoglutarate dehydrogenase complex (OGDC) and branched-chain 2-oxoacid dehydrogenase complex (BCOADC), representing minor immune reactants in PBC was also tested by Western blot. Confirming published reports, 16% and 44% of PBC samples had detectable antibodies against these 2 complexes, respectively,18,19 while no reactivity was detected in any of the hematologic cancer patient samples (data not shown). The presence of AMAs in the different patient populations was also analyzed by immunofluorescence assays using human Hep-2 cells as substrate. As shown in Figure 2, the post-DLI samples showed the same characteristic antimitochondrial immunofluorescence pattern seen in samples from patients with PBC.
Indirect immunofluorescence analysis. Sera from MM after DLI (A), CML after DLI (B), and PBC (C) were diluted 1:40 in PBS and applied to human Hep-2 slides. FITC-conjugated secondary antibodies were used as detectors. All slides display the characteristic antimitochondrial immunofluorescence pattern.
Indirect immunofluorescence analysis. Sera from MM after DLI (A), CML after DLI (B), and PBC (C) were diluted 1:40 in PBS and applied to human Hep-2 slides. FITC-conjugated secondary antibodies were used as detectors. All slides display the characteristic antimitochondrial immunofluorescence pattern.
Quantification of anti–PDC-E2 antibodies in healthy donors and patient groups
The PDC-E2–GST fusion protein was used for ELISA to detect and quantify the antibody response in 68 patients with hematologic malignancies. The level of nonspecific background reactivity was determined in a group of 52 healthy donors. No reactivity against PDC-E2 compared with healthy donors was detected in the control groups of patients with chronic GVHD (P = .99), patients with hematologic malignancies after myeloablative conditioning and reconstitution with T-cell–depleted allogeneic bone marrow (P = .99), and patients with MM at diagnosis (P = .99). Although no significant differences were detected in the MGUS group compared with the healthy donors (P = .16), 2 MGUS patients displayed weak antibody responses against PDC-E2 above background levels (Figure 3). Significantly elevated levels of PDC-E2 antibodies were detected in the MM and leukemia DLI responders (P = .03 and P = .006, Fisher exact test; and P = .002 and P < .001, Wilcoxon rank-sum test, respectively) when compared with healthy donors. As shown in Figure 3, only 2 post-DLI samples (1 MM DLI responder and 1 leukemia after DLI) had a high level of reactivity when tested at 1:100 serum dilution. These 2 samples maintained reactivity at serum dilution up to 1:10 000, while most of the PBC samples were still reactive at 1:100 000 serum dilution (data not shown).
Quantitative analysis of PDC-E2 antibodies in post-DLI, post-HSCT, MGUS, PBC, and MM patients compared with healthy donors. PDC-E2 antibodies were measured by ELISA in patient serum (1:100 dilution). Results are shown for 95 patient samples in 7 groups. The dashed line represents the cutoff limit based on the 95th percentile of reactivity in 52 healthy donors.
Quantitative analysis of PDC-E2 antibodies in post-DLI, post-HSCT, MGUS, PBC, and MM patients compared with healthy donors. PDC-E2 antibodies were measured by ELISA in patient serum (1:100 dilution). Results are shown for 95 patient samples in 7 groups. The dashed line represents the cutoff limit based on the 95th percentile of reactivity in 52 healthy donors.
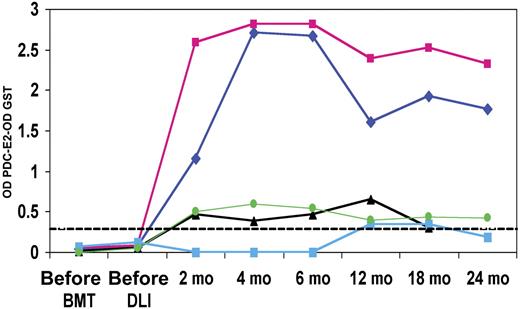
Serial reactivity in PDC-E2–positive DLI responders
The PDC-E2–GST ELISA was used to quantify antibody reactivity at serial time points before treatment and up to 2 years after DLI. Of the 5 DLI responders, 3 displayed weak antibody responses (Figure 4), while the remaining 2 patients displayed elevated antibody titers starting at 2 months after DLI that persisted for at least 2 years after transplantation. In these 2 patients, reactivity against PDC-E2 was maintained at serum dilutions of 1:10 000 at all time points tested. Although 3 of the 5 DLI responders that developed PDC-E2 antibodies also developed some evidence of acute or chronic GVHD after DLI, none showed evidence of chronic liver disease. The clinical characteristics of the 5 DLI responders as well as the 54 PBC patients are summarized in Table 1. Liver function tests in the first 2 years following DLI showed no evidence of cholestatic liver disease.
Serial analysis of post-DLI patient serum. PDC-E2 antibody reactivity was measured by ELISA in 5 patients before HSCT, before DLI, and at several times after DLI. GST background reactivity was subtracted for each sample. The dashed line represents the cutoff limit based on the 95th percentile of reactivity in 52 healthy donors.
Serial analysis of post-DLI patient serum. PDC-E2 antibody reactivity was measured by ELISA in 5 patients before HSCT, before DLI, and at several times after DLI. GST background reactivity was subtracted for each sample. The dashed line represents the cutoff limit based on the 95th percentile of reactivity in 52 healthy donors.
Analysis of anti–PDC-E2 reactivity in PBC
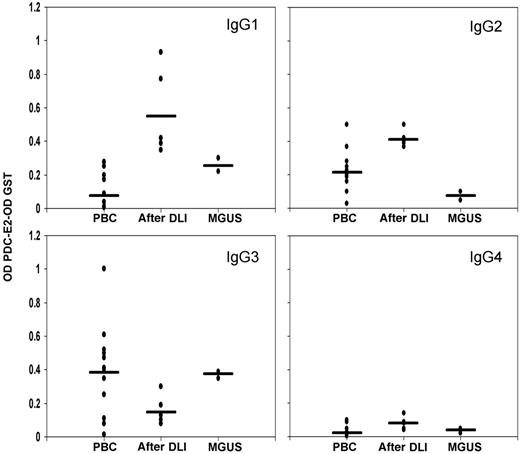
Isotype of the anti-PDC IgG antibodies was determined to define potential differences in the antibody production mechanisms. Results were compared with the reactivity found in 12 PBC serum samples known to be positive for anti–PDC-E2 by Western blot (1:1000 dilution). In accordance with previous reports, PBC samples were predominantly IgG3 positive (Figure 5), 20,21 a feature shared by MGUS samples. In striking contrast, the antibody response detected after DLI was predominantly of the IgG1 and IgG2 isotypes.
IgG subclass analysis. IgG subclasses were measured by ELISA in post-DLI, MGUS, and 12 positive PBC samples. GST background reactivity was subtracted for each sample. Black bars represent the mean reactivity for each group.
IgG subclass analysis. IgG subclasses were measured by ELISA in post-DLI, MGUS, and 12 positive PBC samples. GST background reactivity was subtracted for each sample. Black bars represent the mean reactivity for each group.
Enzyme inhibition of anti-PDC antibodies in PBC and post-DLI patient serum
AMAs from sera of patients with PBC are characterized by the ability to inhibit the pyruvate dehydrogenase enzyme function in vitro. Sera from post-DLI patients that tested positive for anti–PDC-E2 antibodies by Western blotting were analyzed in parallel with sera from patients with PBC and healthy controls. Overall, the inhibitory activity of sera from post-DLI patients was in the same range of enzyme inhibition (47.9%) as observed for sera from patients with PBC (56.12%); sera from healthy controls run in parallel did not have the same activity (7.6%). Post-DLI sera were tested at different time points after treatment (2 months to 2 years), but no statistically significant variations in the level of enzyme inhibition could be detected (data not shown).
Comparative peptide analysis in PBC, MGUS, and post-DLI patient serum
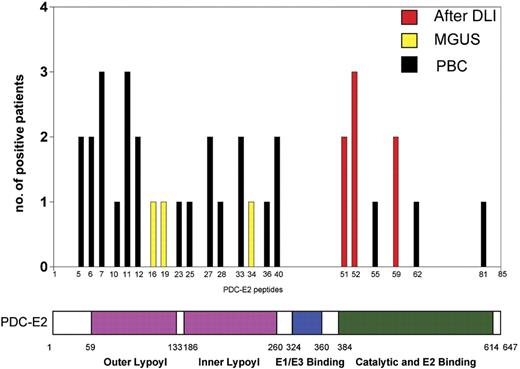
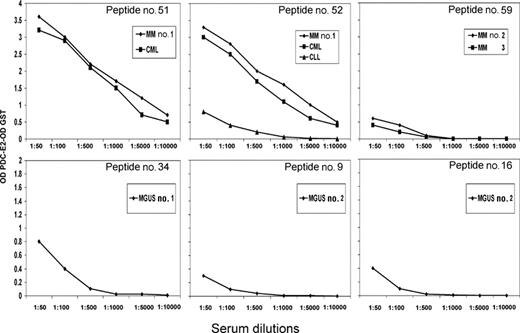
Using ELISA, serum (or plasma) from the DLI responders, MGUS patients and PBC patients were tested against a series of 85 overlapping peptides representing the entire amino acid sequence of PDC-E2. Overall, patient samples recognized only 6 of the 85 PDC-E2 peptides (Figure 6). Post-DLI patients demonstrated conserved antibody responses, with 3 patients reacting with peptide n52 and 2 patients reacting with each of the peptides n51 and n59. Serial analysis of these sera confirmed that the antibody response was directed against these same peptides for more than 2 years after DLI (data not shown). Importantly, all post-DLI antibodies were directed against peptides located in the catalytic domain of the protein. As described in previous studies, AMAs from patients with PBC are primarily directed against epitopes in the inner and outer lipoyl domain of the PDC-E2 protein.5,9–11 We confirmed these reports, finding PBC sera predominantly directed against peptides located in the lipoyl domain and only 3 samples reactive with peptides located in the catalytic domain (peptides n55, n62, and n81) (Figure 6). The frequency of reactivity against different domains of PDC-E2 between post-DLI responders and PBC patients was statistically significant (P = .01, Fisher exact test). Antibodies in patients with MGUS were directed against different peptides but shared the lipoyl domain targeting. Peptides n16, n19, and n34 were each recognized by MGUS patients. Reactivity against specific peptides was successfully blocked after incubating patient serum with different concentrations of recombinant PDC-E2–GST protein but not when incubating with the same amount of GST (data not shown). Antibody titers against each of these purified peptides were also determined by serial dilution and closely reflected the titers measured against recombinant PDC-E2–GST fusion protein, confirming the specificity of the immune response (Figure 7).
Comparison of antibody specificity in post-DLI, MGUS, and PBC patient serum. Serum samples (1:100 dilution) were analyzed for peptide specificity using ELISA and a set of 85 overlapping peptides representing the entire amino acid sequence of PDC-E2. The graph shows the number of samples reactive with each peptide and the corresponding functional domain of the protein.
Comparison of antibody specificity in post-DLI, MGUS, and PBC patient serum. Serum samples (1:100 dilution) were analyzed for peptide specificity using ELISA and a set of 85 overlapping peptides representing the entire amino acid sequence of PDC-E2. The graph shows the number of samples reactive with each peptide and the corresponding functional domain of the protein.
PDC-E2 peptide titers in post-DLI and MGUS patients. Serum samples were analyzed at serial dilutions against individual PDC-E2 peptides. Reactivity was measured by ELISA against each purified peptide. Reactivity with an irrelevant peptide was subtracted in each assay.
PDC-E2 peptide titers in post-DLI and MGUS patients. Serum samples were analyzed at serial dilutions against individual PDC-E2 peptides. Reactivity was measured by ELISA against each purified peptide. Reactivity with an irrelevant peptide was subtracted in each assay.
Discussion
PBC is an autoimmune disease of the liver in which more than 95% of patients develop AMAs directed against several members of the 2-oxoacid dehydrogenase complex, in particular against the PDC-E2 subunit.5,7 Although the B- and T-cell epitopes of PDC-E2 have been well characterized, the immunologic mechanism that results in specific reactivity against a limited region of the protein complex remains elusive. The expression of PDC-E2 is not limited to biliary cells or hepatocytes. Nevertheless, the development of coordinated B- and T-cell immunity to PDC-E2 is highly characteristic of PBC, and this autoimmune response appears as a major contributing factor in the development of progressive cholestatic liver disease in these patients. Because AMAs are primarily directed against the lipoyl domain of PDC-E2, several recent studies have proposed a role for this region in the breakdown of self-tolerance in PBC,22 but no conclusive data are available.
Donor lymphocyte infusion can be an effective therapy for patients with MM or other hematologic malignancies who relapse after HSCT.17,23–25 Patients frequently develop acute and chronic GVHD after DLI, significantly increasing morbidity and mortality rates.26,27 GVHD lesions are characterized by infiltrating CD3+ T cells, expression of major histocompatibility complex (MHC) class II, and up-regulation of adhesion molecules in epithelial cells resembling some of the immunopathologic characteristics of PBC.28 Nonetheless, although patients with PBC and GVHD share similar immunopathologic features in liver biopsies, on the weight of evidence, patients with GVHD do not develop antibodies against PDC-E2. The present study, in which no antibody response to PDC-E2 could be detected in 10 patients who developed GVHD after HSCT, our previous analysis,1 and a report by Quaranta and colleagues29 all confirmed this data.
The initial identification of an antibody response against PDC-E2 in patients who underwent DLI, a different therapy than the ones leading to GVHD in the previous studies, prompted us to better define the characteristics of this dissimilar immune reaction. Antibody responses were studied in several groups of patients both before any treatment and after allogeneic HSCT and DLI. While some patients with MGUS showed evidence of low-titer antibody response at time of diagnosis, only patients with MM and chronic leukemias who responded to DLI displayed significantly elevated levels of anti–PDC-E2 antibodies when compared with a group of 52 healthy donors (P = .03 and P = .006, respectively). Nonetheless, although reacting with the same molecule, the antibody responses to PDC in the 2 groups of patients—namely, the PBC and post-DLI groups—were distinct in every other aspect. Specifically, the subsequent peptide analysis demonstrated how post-DLI response targeted different epitopes of the PDC-E2 protein than the spontaneous response in PBC. Antibodies from post-DLI patients specifically reacted against peptides in the catalytic domain of the molecule, in striking contrast with the typical response in PBC directed against the lipoyl domain. Although the dominant epitope in PBC has previously been shown to be directed predominantly against the inner lipoyl domain,9 our samples from patients with PBC were reactive with both inner and outer lipoyl domain peptides. This broader range of reactivity found in our peptide analysis could reflect antibody reactivity against conformational epitopes of the larger protein.30 Moreover, the antibody response in hemato-oncologic patients was directed exclusively against PDC-E2 with no reactivity toward OGDC or BCOADC, the other immunogenic reactants identified in PBC. Differences were also confirmed analyzing the involved IgG subclasses, confirming the previously described prevalence of IgG3 in PBC20,21 and prevalently detecting IgG1 and IgG2 in patients after DLI arguing in favor of a potentially T-cell–dependent immunoglobulin production.31,32 Most importantly, the absence of detectable liver disease in all of the examined patients over time furthermore distinguishes the population, although a delayed onset of a potential liver disease cannot be excluded considering the presence of AMAs for years in otherwise asymptomatic patients who eventually develop PBC.33
Although reacting with the same molecule, the important differences in epitope specificity and antibody characteristics lead us to hypothesize a different origin of the processed molecule and potentially different processing. The initial study describing the antibody response to PDC demonstrated an overexpression of the protein in several MM tumor cell lines and in primary MM cells if compared with normal plasma cells.1 Differently, no evidence of overexpression of PDC in biliary cells of patients with PBC could be detected,34 arguing against the presentation of an overexpressed molecule in PBC. Moreover, different hypotheses have been generated recently about the origin of the immunogenic epitope in PBC, considering both candidates for molecular mimicry35 and the potential modification of the native protein by xenobiotics36 ; in both cases, though, antibody reactivity against the inner lipoyl domain could only be confirmed. Finally, the presence of antibody responses for only a limited time in certain patients strikingly disagrees with the typical finding of long-term AMA positivity in PBC.33 Hence, the differential reactivity of sera from patients with PBC compared with HSCT-DLI suggests that the loss of tolerance has occurred via different mechanisms. This hypothesis is based not only on differences in epitope recognition but also on the likelihood that when cells undergo apoptosis the fragments of PDC-E2 produced are different among various cell types.37,38 For example, biliary epithelial cells, which are the target in PBC, continue to retain the full antigenic profile following apoptosis. However, other cell types, following apoptosis, produce different fragments of PDC-E2. We submit, therefore, that in patients with HSCT-DLI the immune response is produced against the different spatial or conformational presentation of PDC-E2. In contrast, in patients with PBC, the biliary epithelial cell is more than an innocent bystander, and its unique immunobiology during metabolism and apoptosis leads to the differential antimitochondrial response seen herein.
In conclusion, we were able to detect a specific high-titer antibody response against the immunodominant autoantigen PDC-E2 of PBC in a number of patients with hematologic malignancies who underwent allogeneic HSCT and DLI. Considering the described overexpression of this molecule within the tumor cells and the temporal association of antibody production and DLI, this immune response appears to be associated with tumor rejection rather than the development of a spontaneous autoimmune disease. Because a variety of autoantibodies are frequently detected in GVHD and after DLI, it will be of interest to further characterize the epitope specificity of these responses. Comparing the epitope specificity of antibody responses with those in different autoimmune diseases may distinguish those epitopes that contribute to specific autoimmune tissue damage.
Authorship
Contribution: R.B. designed and performed research and wrote the paper; S.O. and M.G. performed research; S.L. and E.W. analyzed data; E.Z. and F.P. contributed vital new reagents; E.P.A., R.J.S., and N.C.M. provided clinical data and samples; M.E.G. contributed vital new reagents, clinical data, samples, and analytical tools and edited the paper; and J.R. designed research, coordinated the work, and edited the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Jerome Ritz, Dana-Farber Cancer Institute, 44 Binney St - M530, Boston, MA, 02115; e-mail: jerome_ritz@dfci.harvard.edu.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by National Institutes of Health (NIH) grants CA078378, AI29530, DK39588, and DK037003, and the Ted and Eileen Pasquarello Research Fund.