Important studies by 2 groups have each independently provided compelling evidence implicating impaired ribosome biogenesis in the molecular pathophysiology of the dominantly inherited pure red cell aplasia, Diamond-Blackfan anemia.
The rarity of a family of disorders known as the inherited bone marrow failure syndromes (IBMFS) belies their importance. Despite obvious differences in their respective molecular lesions, these disorders share not only a predisposition to hematopoietic failure, but also to birth defects and cancer. It is widely accepted that the propensity of the mutated cells in these disorders to apoptosis is the proximate cause of their demise.1 Furthermore, it is theorized that “interdicting” mutations that provide a reprieve from this molecular death sentence may explain the cancer predisposition, both hematopoietic and nonhematopoietic, observed in the IBMFS.2
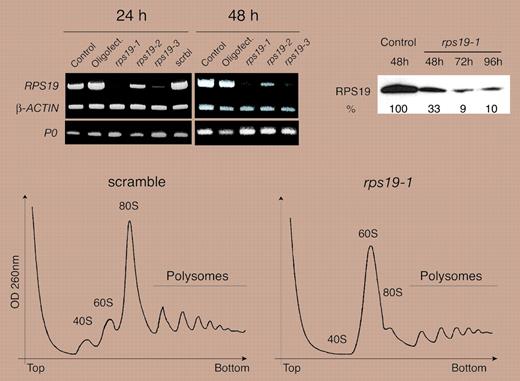
Between 1938, when Diamond and Blackfan first described the clinical syndrome erythrogenesis imperfecta, characterized by pure red cell aplasia,3 and 1997, when the first gene mutated in Diamond-Blackfan anemia (DBA) was reported,4 myriad explanations for the red cell failure of DBA were proposed with great zeal. Various mechanisms ranging from immune mediation to a marrow stromal defect were championed before strong evidence of an intrinsic hematopoietic progenitor disorder emerged.1 Subsequently, although the presence of a mutated gene provided very convincing evidence for a defect intrinsic to the erythroid progenitor, the novel nature of that gene created a fair amount of consternation. Indeed, the developing story line did not, for many, permit the required “willing suspension of disbelief.” The mutation was in a gene, RPS19, which encodes a protein associated with the 40S subunit of the ribosome. That disruption of a fundamental process such as ribosome biogenesis could lead to pure red cell aplasia was not universally accepted, and alternative explanations proposed that the manifestations of DBA might be due to extraribosomal functions of RPS19. Now, 2 new pieces of evidence have emerged almost simultaneously. A second “DBA gene,” RPS24, has been identified,5 and in this issue of Blood, Flygare and colleagues and Choesmel and colleagues describe a functional defect in ribosome biogenesis attributed to RPS19 dysfunction. Thus, Flygare et al and Choesmel et al have coauthored an important chapter in the story of DBA. The authors have clearly demonstrated that a functional defect in ribosome assembly as a consequence of RPS19 protein insufficiency, characterized by faulty cleavage of ribosomal RNA, results in arrested maturation of the 18S rRNA species and culminates in a decreased number of mature ribosomes (see figure).
Down-regulation of RPS19 expression blocks maturation of the 18S rRNA. See the complete figure in the article beginning on page 1275.
Down-regulation of RPS19 expression blocks maturation of the 18S rRNA. See the complete figure in the article beginning on page 1275.
The exact mechanism by which this particular molecular lesion results in a failure to generate red cells will carry the plot forward. The simple explanation is that the high demand on protein synthesis in the developing erythron is the culprit, but other protagonists will no doubt emerge. For some, the final chapter will connect the defect in ribosome assembly with the predisposition to malignancy seen in DBA. Recent evidence provides an interesting theme. The nucleolus has been found consorting with p53. That a failure in protein synthesis may result in p53-mediated cell death6 provides a tantalizing clue that may connect accelerated apoptosis in DBA with the villain in all the IBMFS stories—cancer. For those of us who have been around for a while and for interested newcomers, this multiauthored serialized novel provides a great read.
The author declares no conflicting financial interests. ▪