Abstract
The gene encoding the ribosomal protein S19 (RPS19) is frequently mutated in Diamond-Blackfan anemia (DBA), a congenital erythroblastopenia. The consequence of these mutations on the onset of the disease remains obscure. Here, we show that RPS19 plays an essential role in biogenesis of the 40S small ribosomal subunit in human cells. Knockdown of RPS19 expression by siRNAs impairs 18S rRNA synthesis and formation of 40S subunits and induces apoptosis in HeLa cells. Pre-rRNA processing is altered, which leads to an arrest in the maturation of precursors to the 18S rRNA. Under these conditions, pre-40S particles are not exported to the cytoplasm and accumulate in the nucleoplasm of the cells in perinuclear dots. Consistently, we find that ribosome biogenesis and nucleolar organization is altered in skin fibroblasts from DBA patients bearing mutations in the RPS19 gene. In addition, maturation of the 18S rRNA is also perturbed in cells from a patient bearing no RPS19-related mutation. These results support the hypothesis that DBA is directly related to a defect in ribosome biogenesis and indicate that yet to be discovered DBA-related genes may be involved in the synthesis of the ribosomal subunits.
Introduction
Diamond-Blackfan anemia (DBA) is a rare pure red blood cell aplasia of childhood characterized by the absence or decreased numbers of erythroid precursors in the bone marrow but an otherwise normal cellularity. Approximately 40% of the DBA patients present various somatic malformations that mostly occur in the cephalic area but also in the hand and/or limb, urogenital tract, and heart.1-3 Clinical expression in DBA is highly heterogeneous, and evolution of the disease is unpredictable. Treatment includes steroid therapy and transfusion with iron chelation. Bone marrow or cord blood transplantation is the only curative treatment but requires an HLA-matched sibling and is mostly reserved to patients with severe complications.
It has been established that 25% of the DBA patients bear a mutated allele of the gene encoding the ribosomal protein S19 (RPS19).4-6 RPS19 is one of the 32 proteins that assemble with the 18S ribosomal RNA (rRNA) to form the small (40S) ribosomal subunit. RPS19 is an essential protein, as homozygous deletion of RPS19 in the mouse leads to embryonic lethality before implantation at the blastocyst stage.7 A wide range of mutations have been identified in DBA patients, from missense to nonsense mutations and from partial to complete deletion of one allele.3,5,6 Some missense mutations affect both the stability and the intracellular transport of RPS19.8 Consistent with a role in DBA pathogenesis, depletion of RPS19 with specific siRNAs severely alters proliferation and differentiation of erythroleukemic cell lines or CD34+ cells in culture.9-11 Although DBA is to date the only genetic disease linked to mutation of an autosomal ribosomal protein gene, a number of other bone marrow failure symptoms (dyskeratosis congenita, cartilage-hair hypoplasia, and Shwachman-Diamond syndrome) involve genes encoding putative ribosome biogenesis factors.12
Getting insight into the etiology of DBA requires better understanding of RPS19 function. How haploinsufficiency of this protein can lead to DBA is all but clear. Like other ribosomal proteins, RPS19 may be required for efficient translation. Along this line, RPS19 was shown to participate in the interaction of the 40S subunit with the translation initiation factor eIF2α.13 Alternatively, RPS19 may be involved in a nonribosome-related process, as proposed for other ribosomal proteins.14 Several proteins interacting with RPS19 have been identified: the growth factor FGF-2,15 the protein kinase PIM-1,16 and a protein of unknown function named S19-BP.17 In addition, dimers of RPS19 were shown to exert a chemoattractant activity on monocytes when released in the outer milieu upon cell death.18,19
Recently, we have shown that the homolog of RPS19 in yeast Saccharomyces cerevisiae plays an essential role in the biogenesis of the small ribosomal subunit. RPS19 depletion results in a strong defect in the processing of precursors to the 18S rRNA and blocks maturation of the pre-40S particles in the nucleus.20 In eukaryotes, ribosome biogenesis gives rise to a particular structure in the nucleus, the nucleolus, which reflects the high degree of spatial organization of this process. Transcription of ribosomal genes by RNA polymerase I in this domain yields a large 47S precursor transcript that is converted to the 18S, 5.8S, and 28S mature rRNAs after elimination by a series of endonucleolytic and exonucleolytic cleavages of 2 external and 2 internal spacers (respectively, the 5′-ETS and 3′-ETS and the ITS1 and ITS2).21-23 The order of some processing steps within the 5′-ETS and the ITS1 may vary, which results in alternative pre-rRNA maturation pathways (Figure 1). Ribosomal proteins and maturation cofactors assemble with the pre-rRNA, cotranscriptionally and during the entire maturation process, forming highly dynamic ribonucleoparticles. The earliest precursor, called 90S particle, splits into pre-40S and pre-60S particles after cleavage at site 2 within the ITS1. These precursors are eventually exported to the cytoplasm where final assembly and RNA processing steps take place.24,25 The role of the ribosomal proteins in ribosome biogenesis in vertebrates remains poorly documented. In yeast S cerevisiae, several ribosomal proteins were shown to be strictly required for processing, assembly, or nuclear export of preribosomes.26-29 The yeast RPS19 is necessary for recruitment of preribosomal accessory factors and for processing of the ITS1.20 Notably, deletion of only 1 of the 2 genes encoding RPS19 in yeast, which mimics haploinsufficiency in human cells, is sufficient to induce a strong delay in synthesis of the 40S subunits.
Pre-rRNA processing pathways in HeLa cells. The sequences of the mature 18S, 5.8S, and 28S rRNAs are flanked by external transcribed spacers (5′-ETS and 3′-ETS) and separated by internal transcribed spacers (ITS1 and ITS2) in the 45S primary transcript. Numbers above the 45S pre-rRNA indicate cleavage sites. Temporal order of cleavage at sites 1 and 2 defines the 2 pre-rRNA processing pathways represented here. The major pathway A is characterized by production of the 41S precursor by early removal of the 5′-ETS. The 41S species is then cleaved in the ITS1 sequence to generate the 21S and 32S species that are precursors to the RNA components of the large and small ribosomal subunits, respectively. Arrows indicate the cleavage sites. Nomenclature of the pre-RNAs according to Hadjiolova et al23 and Rouquette et al.24
Pre-rRNA processing pathways in HeLa cells. The sequences of the mature 18S, 5.8S, and 28S rRNAs are flanked by external transcribed spacers (5′-ETS and 3′-ETS) and separated by internal transcribed spacers (ITS1 and ITS2) in the 45S primary transcript. Numbers above the 45S pre-rRNA indicate cleavage sites. Temporal order of cleavage at sites 1 and 2 defines the 2 pre-rRNA processing pathways represented here. The major pathway A is characterized by production of the 41S precursor by early removal of the 5′-ETS. The 41S species is then cleaved in the ITS1 sequence to generate the 21S and 32S species that are precursors to the RNA components of the large and small ribosomal subunits, respectively. Arrows indicate the cleavage sites. Nomenclature of the pre-RNAs according to Hadjiolova et al23 and Rouquette et al.24
To address the function of the human RPS19 protein in ribosome biogenesis and gain insight into the pathophysiologic mechanism of DBA, we have now analyzed the consequences of RPS19 knockdown by siRNA treatment in cultured HeLa cells. A similar approach was shown to efficiently knock down RPS19 production, both in human erythroleukemic cell lines and in CD34+ cells.9-11 Our results indicate that RPS19 is a key protein for 18S rRNA maturation and 40S ribosomal subunit production. In addition, we show that skin fibroblasts from DBA patients have defects in pre-rRNA maturation and nucleolar organization.
Materials and methods
Tissue culture and reagents
Human cervical carcinoma HeLa cells were maintained in culture in Dulbecco modified Eagle medium (DMEM) supplemented with 10% fetal calf serum, 1 mM sodium pyruvate, 100 U/mL penicillin, and 100 μg/mL streptomycin (all from Invitrogen, Carlsbad, CA) in 5% CO2 at 37°C. Fibroblasts from skin biopsies of DBA and non-DBA subjects were grown in Ham's F10 medium supplemented with 10% fetal calf serum. After 2 weeks in culture, fibroblasts were cultured in DMEM-like HeLa cells. For experiments, passage numbers for control and patients' fibroblasts were similar and totaled between 5 and 10. Cis-diamine dichloroplatinum (CDDP) and cycloheximide were purchased from Sigma (St Louis, MO). Antibodies against human RPS19 were produced by injecting the recombinant protein in rabbit (Eurogentec, Seraing, Belgium).
siRNAs
Three different 21-mer siRNAs were designed to knock down expression of the human RPS19 (GenBank accession number BC007615): 5′-AGAGCUUGCUCCCUACGAUdTdT-3′ (rps19-1 siRNA), 5′-AGAGAUCUGGACAGAAUCGdTdT-3′ (rps19-2 siRNA), and 5′-ACUGACACCUCAGGGACAAdTdT-3′ (rps19-3 siRNA). The sequence of the siRNA directed against the human lamin A/C was 5′-CUGGACUUCCAGAAGAACAdTdT-3′. All siRNAs were purchased from Eurogentec.
In most experiments, siRNAs were transfected as described previously using electrotransformation.24 Alternatively, 106 cells in a 100 mm dish were transfected with siRNAs (120 nM) using Oligofectamine (60 μL per dish) in 5 mL OptiMEM medium (both from Invitrogen) according to the manufacturer's instructions. For immunofluorescence, HeLa cells were seeded on coverslips in 24-well plates (3 × 104 cells per well) and transfected with Oligofectamine (3 μL per well) in 1 mL total volume. More than 95% of the cells transfected with rps19 siRNAs displayed redistribution of pre-RNAs by fluorescence in situ hybridization (FISH) (see “Results”), indicating a very efficient transfection rate.
Fractionation and analysis of ribosomes by sucrose density gradient centrifugation
Twenty-four hours or 48 hours after transfection with siRNAs, HeLa cells were treated with 100 μg/mL cycloheximide (Sigma) for 10 minutes, fractionated, and the cytoplasmic fraction analyzed on sucrose gradient as described previously.24
Reverse transcriptase–polymerase chain reaction
RPS19 gene down-regulation induced by RNA interference was analyzed by reverse transcriptase–polymerase chain reaction (RT-PCR). Total RNAs were isolated using TRIzol reagent (Invitrogen), and first-strand synthesis of cDNA was performed from 1 μg RNA at 42°C for 2 hours using AMV Reverse Transcriptase as recommended by the supplier (Promega, Madison, WI). The following primers were used to PCR-amplify the RPS19, β-actin, and P0 mRNAs: 5′-GCGGGATCCGCACGATGCCTGGAGTTACTGT-3′ (RPS19 forward), 5′-AAACTGCAGACGAATGAGGCAATTTATTAACCC-3′ (RPS19 reverse), 5′-AGAGCAAGAGAGGCATCCTCACCCTGAAGTAC-3′ (actin forward), 5′-AGGGATAGCACAGCCTGGATAGCAAC-3′ (actin reverse), 5′-GGCGACCTGGAAGTCCAACT-3′ (P0 forward), and 5′-CCATCAGCACCACAGCCTTC-3′ (P0 reverse). PCR was performed using Taq DNA polymerase (Promega). The annealing temperature was 57°C for coamplification of the RPS19 and β-actin cDNAs and 62°C for the ribosomal protein P0 cDNA.
Northern blot and pulse-chase analysis of pre-rRNA
Pre-rRNA analysis by Northern blot was performed as described previously30 with probes 18S, 28S, 5′-ITS1, ITS2b, and ITS2-d/e.24 For detection of the ITS2, the ITS2-b and ITS2-d/e probes were mixed in equal amounts.
For pulse-chase analysis, fibroblasts were plated at a density of 2.5 × 105 cells in 35 mm–diameter dishes. One day later, they were preincubated for 30 minutes in serum-free methionine-free medium and then incubated for 30 minutes in 1.5 mL of the same medium containing 75 μCi (2.78 MBq) L-[methyl-3H]methionine. The cells were then chased in nonradioactive medium containing 30 μg methionine per milliliter for various times, after which total RNAs were isolated using TRIzol (Invitrogen). RNAs were separated on a 1% agarose gel, transferred to nylon membrane, and exposed to a film using an intensifying screen (Transcreen LE; GE Healthcare Biosciences AB, Uppsala, Sweden).
Fluorescence in situ hybridization and immunofluorescence microscopy
Precursors to the 18S rRNA were localized by fluorescence in situ hybridization (FISH) in HeLa cells as described elsewhere24 with the 5′-ITS1 probe conjugated to Cy3 (GE Healthcare Biosciences) on amino-modified deoxythymidine.
For immunostaining, cells grown on glass coverslips were fixed with 4% paraformaldehyde (30 minutes, room temperature) and permeabilized in 0.5% Triton X-100 (5 minutes, room temperature). Immunostaining was performed in phosphate-buffered saline (PBS) containing 0.5% bovine serum albumin using an antifibrillarin monoclonal antibody (clone 72B9, 1:200, overnight, 4°C). Fluorescence detection was achieved by incubating the cells for 1 hour with rhodamine-coupled F(ab′)2 fragment anti–mouse IgG (H+L) antibody (1:200; Immunotech). DNA was counterstained with 0.1 μg/mL DAPI diluted in Mowiol mounting medium. Slides were observed with a 100× objective (NA 1.3) at room temperature and photographed using a DMRB epifluorescence microscope (Leica) equipped with a CoolSnap ES CCD camera (Photometrics). Alternatively, for semiquantitative analysis of the phenotypes in fibroblasts from DBA patients (Figure 6), cells were observed with a 50× objective (NA 0.9) on an Olympus IX-81 microscope equipped with a motorized stage and a CoolSnap HQ camera (Photometrics) and controlled with Metamorph software version 6 (Universal Imaging). For each sample, a randomly chosen portion of the coverslip was automatically scanned in order to acquire images of 100 fields. The nuclei and the nucleoli in each field were then segmented and automatically analyzed with Metamorph.
Electron microscopy
Fibroblasts were cultured on glass coverslips and fixed with 4% paraformaldehyde in 0.1 M Sörensen buffer, pH 7.4. Dehydration was performed with ethanol, and cells were infiltrated in LR White resin. The coverslips were brought in contact with a resin-filled capsules for polymerization.31 Ultrathin sections (50 to 70 nm) were stained with uranyl acetate and lead citrate, and observations were performed at 80 keV on a Jeol (Tokyo, Japan) 1200-EX electron microscope (Electron Microscopy Facility, Institut d'Exploration Fonctionnelle des Génomes).
Results
Inhibition of RPS19 synthesis strongly affects the level of 40S subunits
Requirement of RPS19 for ribosome biogenesis was evaluated with 3 different siRNAs named rps19-1, rps19-2, and rps19-3 and targeting nucleotides 129-147, 385-403, and 366-384 of the human RPS19 open reading frame, respectively. As seen by RT-PCR, all 3 siRNAs reduced the RPS19 mRNA level in human cervical carcinoma HeLa cells albeit with different efficiency—rps19-1 siRNA being the most potent and rps19-2 siRNA the slowest (Figure 2A). In contrast, scramble siRNAs or Oligofectamine alone did not affect the RPS19 mRNA level when compared with nontreated cells. In addition, the levels of the mRNAs encoding β-actin and the ribosomal protein P0 were not modified in RPS19 siRNA-transfected cells. Decrease in the RPS19 mRNA level was paralleled by a strong reduction in the amount of RPS19 protein on Western blot, as shown for the rps19-1 siRNA in Figure 2B. Taken together, these results demonstrate the specific down-regulation of RPS19 expression using siRNAs.
Down-regulation of RPS19 expression blocks maturation of the 18S rRNA. (A) HeLa cells were transfected with either rps19 or scramble siRNAs and cultured for 24 hours or 48 hours. After RNA extraction, the mRNAs encoding the ribosomal proteins RPS19 and P0 were amplified by RT-PCR. The β-actin mRNA was coamplified with RPS19 as an internal control. RT-PCR reactions obtained from untreated cells (control) and Oligofectamine-treated cells are also shown. The 3 siRNAs (rps19-1, rps19-2, rps19-3) differ in their efficacy to down-regulate the RPS19 mRNA level. (B) Knockdown of RPS19 expression upon treatment with siRNA rps19-1 is confirmed by Western blot analysis of total cell extracts. Densitometry shows that up to 90% of the protein is depleted 72 hours after transfection. (C) Analysis on sucrose gradient of the ribosomal subunits from HeLa cells 48 hours after transfection with the rps19-1 or scramble siRNAs shows that depletion of RPS19 strongly affects 40S subunit production, leading to a strong imbalance between the free 60S subunits and 40S subunits. (D) Northern blot analysis of total RNA extracts from cells transfected with rps19 siRNAs using oligonucleotides hybridizing in the mature 18S and 28S rRNAs. The 18S and 28S rRNA levels were evaluated by phosphoimager quantification. (E) Northern blot analysis with probes complementary to the ITS1 (5′-ITS1) or to the ITS2. “Control” indicates untreated cells; “Oligofect.,” transfection treatment without siRNAs; “scrbl,” scramble siRNA. All experiments were repeated at least 3 times with similar results.
Down-regulation of RPS19 expression blocks maturation of the 18S rRNA. (A) HeLa cells were transfected with either rps19 or scramble siRNAs and cultured for 24 hours or 48 hours. After RNA extraction, the mRNAs encoding the ribosomal proteins RPS19 and P0 were amplified by RT-PCR. The β-actin mRNA was coamplified with RPS19 as an internal control. RT-PCR reactions obtained from untreated cells (control) and Oligofectamine-treated cells are also shown. The 3 siRNAs (rps19-1, rps19-2, rps19-3) differ in their efficacy to down-regulate the RPS19 mRNA level. (B) Knockdown of RPS19 expression upon treatment with siRNA rps19-1 is confirmed by Western blot analysis of total cell extracts. Densitometry shows that up to 90% of the protein is depleted 72 hours after transfection. (C) Analysis on sucrose gradient of the ribosomal subunits from HeLa cells 48 hours after transfection with the rps19-1 or scramble siRNAs shows that depletion of RPS19 strongly affects 40S subunit production, leading to a strong imbalance between the free 60S subunits and 40S subunits. (D) Northern blot analysis of total RNA extracts from cells transfected with rps19 siRNAs using oligonucleotides hybridizing in the mature 18S and 28S rRNAs. The 18S and 28S rRNA levels were evaluated by phosphoimager quantification. (E) Northern blot analysis with probes complementary to the ITS1 (5′-ITS1) or to the ITS2. “Control” indicates untreated cells; “Oligofect.,” transfection treatment without siRNAs; “scrbl,” scramble siRNA. All experiments were repeated at least 3 times with similar results.
The impact of RPS19 knockdown on ribosome biogenesis was analyzed by separating the ribosomal subunits extracted from RPS19-depleted cells on sucrose gradient. As displayed in Figure 2C, treatment of HeLa cells with rps19-1 siRNA resulted in a marked decrease of the level of free 40S subunits and a parallel buildup of the amount of free 60S subunits, indicating a strong defect in the production of 40S subunits. Consistently, the level of 80S ribosomes and polysomes was also affected. This result indicates that RPS19 is specifically required for synthesis of the small subunit.
RPS19 down-regulation alters pre-RNA processing within the ITS1
Total RNAs were isolated 24 hours after transfection, and Northern blotting experiments were performed using radiolabeled probes complementary to the 18S and 28S rRNAs (Figure 2D, left). Consistent with the analysis on sucrose gradient, the 3 rps19 siRNAs did not affect 28S rRNA synthesis but significantly decreased the level of 18S rRNA when compared with scramble siRNA-transfected cells. Phosphorimager quantification of the 18S and 28S bands revealed that the 18S/28S ratio was 2 times lower in rps19-1 and rps19-3 siRNA-transfected cells than in Oligofectamine- and scramble siRNA-treated cells (Figure 2D, right). The 18S/28S ratio reached an intermediate value in rps19-2 siRNA-transfected cells, consistent with its lesser efficiency in reducing the RPS19 mRNA level 24 hours after transfection (Figure 2A). Thus, siRNA-mediated RPS19 down-regulation impairs 18S rRNA production without affecting 28S rRNA accumulation.
The RNAs were next analyzed by Northern blot using a probe complementary to the ITS1 sequence (5′-ITS1 probe; Figure 2E, left). As expected, this probe revealed several 18S rRNA precursors in control cells, including the 45S, 41S, 30S, and 21S species (Figure 1). We also detected the 18S-E pre-rRNA, a species that comprises the 18S rRNA extended at its 3′ end with about 24 nucleotides of the ITS1.24 Final maturation of this precursor into 18S rRNA occurs after translocation of the pre-40S particles to the cytoplasm. Interestingly, all 3 rps19 siRNAs resulted in a marked buildup of the amount of 21S pre-rRNA when compared with scramble siRNA-transfected cells. In parallel, we observed a decrease of the level of 18S-E pre-rRNA, consistent with a maturation defect of the 21S pre-rRNA. A moderate accumulation of the 41S pre-rRNA was also detected in RPS19-depleted cells, suggesting that RPS19 could participate in the cleavage of this precursor at site 2 within the ITS1. Using a probe complementary to the ITS2, we did not detect major effects on production of the precursors to the 28S and 5.8S rRNAs, the 32S and 12S pre-rRNAs (Figure 2E, right). These data indicate that RPS19 is required for cleavage of the ITS1 at site 2 and for maturation of the 21S pre-rRNA within the pre-40S particle.
RPS19-depleted cells accumulate 18S rRNA precursors in the nucleoplasm
To determine if RPS19 depletion affects nuclear transport of the pre-40S particles, we next performed fluorescence in situ hybridization (FISH) using the 5′-ITS1 probe conjugated to Cy3, which detects all the precursors to the 18S rRNA. As shown in Figure 3A, cells transfected for 24 hours with scramble siRNAs were mostly labeled in the nucleoli, which are not stained with DAPI. Additionally, the cytoplasm displayed a slight signal corresponding to nuclear export of the 18S-E pre-rRNA.24 The distribution of the pre-40S particles was similar in nontreated cells, Oligofectamine-treated cells, or lamin A/C siRNA-transfected cells (data not shown). In contrast, rps19 siRNA-transfected cells were not only labeled in the nucleolus, but numerous bright spots were also evident in the nucleoplasm of the cells. In addition, labeling of the cytoplasm was very faint, indicating that the pre-40S particles were retained in the nucleolus/nucleus. This phenotype was correlated with the efficiency of the siRNAs (Figure 2A): It was observed in 52% of the cells with the rps19-1 siRNA but only in 25% and 36% of the cells with the less efficient rps19-2 and rps19-3 siRNAs, respectively. Notably, this pattern is different from that observed upon knockdown of another ribosomal protein, RPS15, which is required for nuclear export of the pre-40S particles.24 In addition, when the cells were treated for 24 hours with 100 μg/mL cycloheximide, a well-known protein synthesis inhibitor, the nucleoplasm was not labeled with the ITS1 probe (Figure 3A). Along the same line, the rps19-1 siRNA did not affect distribution of fibrillarin, whereas treatment with cycloheximide resulted in the relocation of this protein from the nucleolus to nucleoplasmic dots (Figure 3B). Fibrillarin is a core protein of the C/D snoRNPs, which are involved in pre-rRNA methylation, an early event in ribosome biogenesis. These different phenotypes indicate that the pattern observed with rps19 siRNAs is not a mere consequence of translation inhibition secondary to the lack of 40S subunits.
RPS19 depletion results in nuclear retention of pre-40S particles.(A) HeLa cells were transfected with different siRNAs, as indicated, and grown for 24 hours. The intracellular location of the 18S rRNA precursors was then analyzed by FISH using the 5′-ITS1 probe conjugated to Cy3. In rps19-1 siRNA-treated cells, accumulation of 18S rRNA precursors in the nucleoplasm is indicated by arrows; the inset shows the boxed region at higher magnification. Comparison is made with cells treated for 24 hours with 100 μg/mL cycloheximide. (B) Detection of fibrillarin, a nucleolar protein, by immunofluorescence. Cells were treated with cycloheximide or transfected with the rps19-1 siRNA as in panel A. “Control” indicates untreated cells.
RPS19 depletion results in nuclear retention of pre-40S particles.(A) HeLa cells were transfected with different siRNAs, as indicated, and grown for 24 hours. The intracellular location of the 18S rRNA precursors was then analyzed by FISH using the 5′-ITS1 probe conjugated to Cy3. In rps19-1 siRNA-treated cells, accumulation of 18S rRNA precursors in the nucleoplasm is indicated by arrows; the inset shows the boxed region at higher magnification. Comparison is made with cells treated for 24 hours with 100 μg/mL cycloheximide. (B) Detection of fibrillarin, a nucleolar protein, by immunofluorescence. Cells were treated with cycloheximide or transfected with the rps19-1 siRNA as in panel A. “Control” indicates untreated cells.
siRNA-mediated RPS19 shutdown induces apoptosis
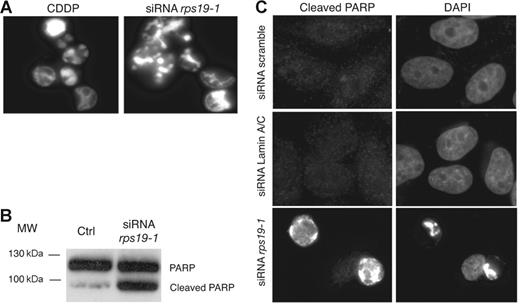
In the course of our experiments, we observed that a significant amount of cells detached from the dish 48 hours after transfection with rps19 siRNAs using Oligofectamine. These cells did not exclude the trypan blue vital dye, indicating that loss of RPS19 induced cell death (data not shown). Upon DAPI staining, these floating cells displayed condensed DNA and a fragmented nucleus, a pattern similar to that observed in cells treated for 24 hours with 10 μM cisplatin (CDDP), a well-known inductor of apoptosis (Figure 4A). To ascertain that these cells were undergoing apoptosis, we looked for the presence of the 85 kDa cleaved fragment of the PARP protein, a caspase substrate. Western blot analysis revealed cleavage of PARP in cells treated with rps19-1 siRNAs for 48 hours but not in cells transfected with scramble siRNAs (Figure 4B). This result was confirmed by immunofluorescence with an antibody specific for cleaved PARP. Cells transfected with rps19-1 siRNA displayed a strong labeling together with condensed DNA and fragmented nuclei (Figure 4C). No signal was visualized in cells transfected with scramble or lamin A/C siRNAs, consistent with the absence of dead cells with these control siRNAs. These results suggest that alteration of ribosome biogenesis in RPS19-depleted cells may trigger apoptosis.
Apoptosis in HeLa cells transfected with rps19 siRNAs. (A) DAPI staining of cells detaching from the culture dish 48 hours after transfection with siRNA rps19-1 shows hypercondensed chromatin, similar to treatment with 10 μM cis-platin (CDDP) for 24 hours. (B) Immunodetection of the 85 kDa fragment of cleaved PARP on Western blot. “Ctrl” indicates untransfected cells. (C) Cleaved PARP is detected by immunofluorescence in RPS19-depleted cells but not in cells transfected with scramble or lamin A/C siRNAs.
Apoptosis in HeLa cells transfected with rps19 siRNAs. (A) DAPI staining of cells detaching from the culture dish 48 hours after transfection with siRNA rps19-1 shows hypercondensed chromatin, similar to treatment with 10 μM cis-platin (CDDP) for 24 hours. (B) Immunodetection of the 85 kDa fragment of cleaved PARP on Western blot. “Ctrl” indicates untransfected cells. (C) Cleaved PARP is detected by immunofluorescence in RPS19-depleted cells but not in cells transfected with scramble or lamin A/C siRNAs.
Fibroblasts from DBA patients display defects in pre-rRNA processing
We next examined ribosome biogenesis in dermal fibroblasts from 3 DBA patients. Two of these patients, MUTS-A and MUTS-B (mutation in S19), bear mutations in the RPS19 gene: Q12Stop and R62Q, respectively. The genetic disorder affecting patient NOTS-A (notS19) is unknown. Consistent with the presence of only one normal RPS19 allele, quantitative RT-PCR showed that half of the RPS19 mRNAs in MUTS-A and MUTS-B cells corresponded to the mutated alleles (Figure S1, available on the Blood website; see the Supplemental Figure link at the top of the online article). Cells from the 3 patients grew slowly in culture with a generation time of 35 to 40 hours, whereas the doubling time of control dermal fibroblasts was close to 24 hours (Figure S1).
We analyzed total RNAs from exponentially growing cells by Northern blot with the 5′-ITS1 probe (Figure 5A). The intensity profiles of each lane, normalized to the amount of actin mRNA, showed consistent differences in patient cells (Figure 5B). In cells derived from patients with a mutation in RPS19 (MUTS-A and MUTS-B), we observed a reproducible increase in 21S pre-rRNA relative to the downstream species 18S-E (Figure 5C), consistent with a delayed maturation of the ITS1 in these cells, as observed with rps19 siRNAs. Higher amounts of upstream precursors (45S, 41S) were also detected. Interestingly, the NOTS-A fibroblasts, which are derived from a patient with no mutation in RPS19, also showed a clear but distinct defect in rRNA maturation: in this case, the 21S species was underaccumulated relative to the 18S-E pre-rRNA, whereas upstream precursors (45S, 41S, 30S) were more abundant than in control cells, suggesting deficient maturation of the 5′-ETS. Probing of the Northern blots with an oligonucleotide complementary to the ITS2 showed no conspicuous change in the amount of precursors to the 5.8S and 28S rRNA (32S, 12S; not shown).
Alterations of pre-rRNA processing in skin fibroblasts from DBA patients. (A) Northern blot analysis of total RNA extracts from control and DBA fibroblasts with the 5′-ITS1 probe. The β-actin mRNA is detected as a loading control. (B) Intensity profiles of the hybridization signal in each lane (phosphoimaging) normalized to the level of actin mRNA. (C) Variation of the relative levels of 21S and 18S-E pre-rRNAs as compared with control cells. The amounts of the 2 species were measured after Northern blotting with the 5′-ITS1 probe by phosphoimaging. The results were analyzed with the Student t test. Error bars indicate standard deviation; n, number of experiments. (D) Pulse-chase analysis of pre-rRNA processing with 3H-methylmethionine. The cells were labeled for 30 minutes with 3H-methylmethionine, and chase was performed for the indicated time. Contrast in the panel corresponding to MUTS-A cells was enhanced by image processing (“enhanced”). All other panels correspond to identical exposure and processing. The 28S rRNA is shown as a loading control.
Alterations of pre-rRNA processing in skin fibroblasts from DBA patients. (A) Northern blot analysis of total RNA extracts from control and DBA fibroblasts with the 5′-ITS1 probe. The β-actin mRNA is detected as a loading control. (B) Intensity profiles of the hybridization signal in each lane (phosphoimaging) normalized to the level of actin mRNA. (C) Variation of the relative levels of 21S and 18S-E pre-rRNAs as compared with control cells. The amounts of the 2 species were measured after Northern blotting with the 5′-ITS1 probe by phosphoimaging. The results were analyzed with the Student t test. Error bars indicate standard deviation; n, number of experiments. (D) Pulse-chase analysis of pre-rRNA processing with 3H-methylmethionine. The cells were labeled for 30 minutes with 3H-methylmethionine, and chase was performed for the indicated time. Contrast in the panel corresponding to MUTS-A cells was enhanced by image processing (“enhanced”). All other panels correspond to identical exposure and processing. The 28S rRNA is shown as a loading control.
We also analyzed the kinetics of pre-rRNA maturation by pulse-chase labeling with 3H-methylmethionine. In this procedure, pre-rRNAs are labeled through methylation by box C/D snoRNPs early in the biogenesis pathway. After autoradiography (Figure 5D), DBA fibroblasts all showed delayed processing of the early pre-rRNAs when compared with control cells. Intensity of pre-rRNA labeling in MUTS-A cells was much lower than in the other cells, indicating a lower rate of ribosome biogenesis; this may be correlated with these cells having the slowest growth rate (Figure S1). The 21S and 30S pre-rRNAs were detected after a longer chase time in MUTS-A and MUTS-B cells, consistent with a delayed cleavage of the ITS1. In NOTS-A cells, the most conspicuous phenotype was the accumulation of 30S or 32S pre-rRNA, in accordance with the high steady-state level of 30S pre-rRNA on Northern blots. These results indicate that pre-rRNA processing is altered both qualitatively and quantitatively in cells from DBA patients.
Altered nucleolar organization in fibroblasts from DBA patients
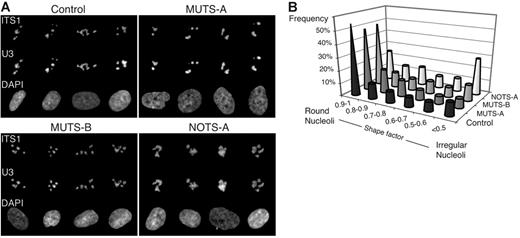
We next localized the pre-40S particles in these cells. FISH was performed with 2 probes in parallel: the 5′-ITS1 probe to detect the precursors to the 18S rRNA and a probe complementary to the U3 box C/D snoRNA (Figure 6A). The U3 snoRNP is involved in early processing of the pre-rRNA in the 5′-ETS and in the ITS1. Phenotypes were quantified by systematically analyzing the number of nucleoli, their size, labeling intensity, and shape. In DBA cells, the mean number of nucleoli was close to 3, as in control cells, but fluorescence with the 5′-ITS1 probe was more intense, suggesting accumulation of precursors (Table 1)In addition, nucleoli in NOTS-A cells were often of irregular shape, as indicated by distribution of the shape factor (Figure 6B); in addition, the total surface occupied by the nucleoli in the x,y plane was also significantly higher in these cells (Table 1). In contrast, the nucleoli in MUTS-A cells were often rounded and looked more condensed than in control cells. Morphology of the nucleoli in MUTS-B cells did not differ much from that in control cells at this resolution, consistent with the less pronounced rRNA maturation defect observed by Northern blot.
Nucleolar disorganization in skin fibroblasts from DBA patients. (A) Galleries of nuclei after codetection by FISH of the U3 snoRNA and the precursors to the 18S rRNA. Hybridization was performed in parallel with probes complementary to U3 (conjugated to Cy5) and to the ITS1 (conjugated to Cy3). (B) Distribution of the shape factor calculated for the nucleoli. This factor (4π.S/P2; S, surface; P, perimeter) reflects the structure roundness: Its value is equal to 1 for a circle and decreases for flat or irregular objects. The nucleolar shape is much more irregular in NOTS-A cells when compared with control cells (Student t test, P < .001).
Nucleolar disorganization in skin fibroblasts from DBA patients. (A) Galleries of nuclei after codetection by FISH of the U3 snoRNA and the precursors to the 18S rRNA. Hybridization was performed in parallel with probes complementary to U3 (conjugated to Cy5) and to the ITS1 (conjugated to Cy3). (B) Distribution of the shape factor calculated for the nucleoli. This factor (4π.S/P2; S, surface; P, perimeter) reflects the structure roundness: Its value is equal to 1 for a circle and decreases for flat or irregular objects. The nucleolar shape is much more irregular in NOTS-A cells when compared with control cells (Student t test, P < .001).
Nucleolar disorganization in skin fibroblasts from DBA patients
| . | No. of cells analyzed . | No. of nucleoli per cell . | Surface of nucleoli per cell, μm2 . | Average fluorescence in nucleoli for ITS1, gray level per pixel . | Average fluorescence in nucleoli for U3, gray level per pixel . |
|---|---|---|---|---|---|
| Control | 568 | 3.3 ± 1.2 | 18.0 ± 4.9 | 558 ± 136 | 818 ± 227 |
| MUTS-A | 182 | 2.7 ± 1.1 | 19.7 ± 7.9 | 627 ± 153 | 977 ± 282 |
| MUTS-B | 168 | 3.0 ± 1.2 | 20.6 ± 6.5 | 641 ± 169 | 783 ± 232 |
| NOTS-A | 91 | 2.6 ± 1.1 | 29.4 ± 8.2 | 638 ± 160 | 711 ± 202 |
| . | No. of cells analyzed . | No. of nucleoli per cell . | Surface of nucleoli per cell, μm2 . | Average fluorescence in nucleoli for ITS1, gray level per pixel . | Average fluorescence in nucleoli for U3, gray level per pixel . |
|---|---|---|---|---|---|
| Control | 568 | 3.3 ± 1.2 | 18.0 ± 4.9 | 558 ± 136 | 818 ± 227 |
| MUTS-A | 182 | 2.7 ± 1.1 | 19.7 ± 7.9 | 627 ± 153 | 977 ± 282 |
| MUTS-B | 168 | 3.0 ± 1.2 | 20.6 ± 6.5 | 641 ± 169 | 783 ± 232 |
| NOTS-A | 91 | 2.6 ± 1.1 | 29.4 ± 8.2 | 638 ± 160 | 711 ± 202 |
Semiquantitative analysis of FISH labeling was performed on randomly taken images (see “Material and methods”). Comparison of any measure in DBA cells relative to the corresponding control with Student t test shows a very significant difference (P < .01). Data shown as mean ± standard deviation.
Labeling with the U3 probe mostly overlapped the 5′-ITS1 signal, showing the same morphologic differences. However, the mean fluorescence density with this probe was significantly higher in MUTS-A cells than in control cells, whereas it was lower in NOTS-A cells. This result suggested different distributions of the pre-rRNA processing machinery in the nucleoli of these cells and, thus, changes in the nucleolar organization. This hypothesis was also supported by detection of fibrillarin (not shown).
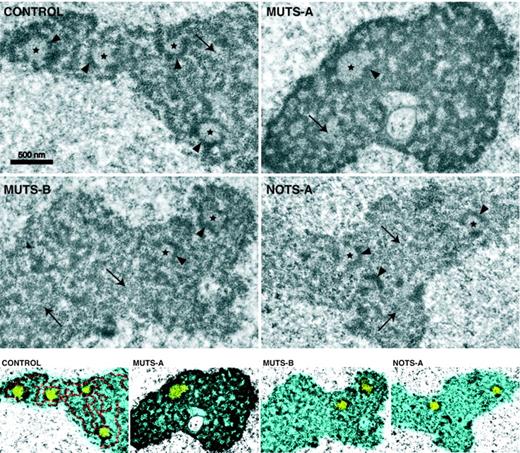
Nucleolar morphology was observed at higher resolution by transmission electron microscopy (Figure 7). As classically described, nucleoli in control fibroblasts displayed light fibrillar centers (FCs), which contain rDNA and the transcription machinery, surrounded by electron-dense material forming the dense fibrillar component (DFC) in which the early preribosomal maturation steps take place. The DFC extends as a meshwork in the nucleoli. Interspersed in the nucleolus, the late preribosomal particles form a granular component (GC), which was not very abundant in control fibroblasts. Strikingly, the nucleoli in MUTS-A cells were much more contrasted due to an abundant and very compact DFC. This result is consistent with the high labeling of U3 by FISH in these cells, because the steps taking place in the DFC involve U3 and fibrillarin. In contrast, NOTS-A cells displayed elongated and sometimes fragmented nucleoli, with a scarce DFC and an extended GC, suggesting accumulation of ill-matured preribosomes in the nucleolus. In addition, the FCs in these cells were often incompletely surrounded by a DFC, unlike in control cells. Interestingly, electron microscopy also revealed FCs of irregular shape and a disorganized DFC in MUTS-B cells, although to a lesser extent than in NOTS-A cells, showing a subtle alteration of the ultrastructural organization of the nucleoli.
Visualization of nucleolar organization in DBA fibroblasts by electron microscopy. The 3 nucleolar components classically described are visible in control cells: fibrillar centers (stars), the meshwork of the highly contrasted dense fibrillar component (arrowheads) and, in between, the granular component (arrows). The dense fibrillar component appears abnormally compact and reticulated in MUTS-A cells, whereas it is scarce and disorganized in NOTS-A cells. Contrast enhancement and colors were applied to stress the differences in nucleolar organization (bottom): nucleolus in blue with fibrillar centers in yellow. The highly contrasted dense fibrillar component is highlighted in red in control cells. The nucleolus appears less fibrillar and more granular in NOTS-A and MUTS-B cells.
Visualization of nucleolar organization in DBA fibroblasts by electron microscopy. The 3 nucleolar components classically described are visible in control cells: fibrillar centers (stars), the meshwork of the highly contrasted dense fibrillar component (arrowheads) and, in between, the granular component (arrows). The dense fibrillar component appears abnormally compact and reticulated in MUTS-A cells, whereas it is scarce and disorganized in NOTS-A cells. Contrast enhancement and colors were applied to stress the differences in nucleolar organization (bottom): nucleolus in blue with fibrillar centers in yellow. The highly contrasted dense fibrillar component is highlighted in red in control cells. The nucleolus appears less fibrillar and more granular in NOTS-A and MUTS-B cells.
These biochemical and cytologic data both indicate that the nucleolar function is affected in cells from DBA patients, including cells with no mutation in RPS19.
Discussion
The results presented here demonstrate that RPS19 is required for maturation of the 18S rRNA in human cells and thus for production of the 40S subunit. Absence of RPS19 results in accumulation of 21S pre-rRNA, which indicates a defect in ITS1 processing at the 3′ end of the 18S rRNA. The pre-40S particles are blocked in the nucleus in RPS19-depleted cells and accumulate in nucleoplasmic dots, which may represent degradation sites for the ill-matured pre-40S particles. The 41S species also accumulates in the absence of RPS19, which may be interpreted as a delayed cleavage at site 2 in the ITS1. Thus, these data indicate that RPS19 is required for efficient processing of the ITS1. This conclusion is further supported by the low level of 18S-E pre-rRNA in RPS19-depleted cells, a pre-rRNA downstream of the 21S species.
These data from HeLa cells display a strong parallel with our recent observations in yeast S cerevisiae,20 which suggests that the role of RPS19 in preribosomal RNA processing is conserved through evolution. As in HeLa cells, depletion of RPS19 in yeast alters ITS1 cleavage at site A2 (equivalent of site 2 in vertebrates) and inhibits processing of the 18S rRNA 3′ end, leading to the accumulation of unprocessed pre-40S particles in the nucleus. In yeast, RPS19 is required for recruitment of maturation proteins specific for the pre-40S particles.20 Because these proteins have mammalian homologs, we expect mammalian RPS19 to fulfill a similar role in the assembly of the preribosomal particles.
Consistent with our observations in RPS19-depleted HeLa cells, ribosome biogenesis is altered in skin fibroblasts from DBA patients bearing a mutation in RPS19, as indicated both by pre-rRNA processing defects and nucleolus disorganization. The defects observed are rather mild, as one might expect considering the central role of ribosomes in cell metabolism. Because ribosome biogenesis is highly sensitive to the cell physiologic state, steady-state defects in pre-rRNA maturation may result from a combination of indirect effects. However, these fibroblasts precisely accumulate the same precursors as cells treated with rps19 siRNAs, suggesting that maturation of the pre-40S particles is directly affected by RPS19 haploinsufficiency. We observe a more severe phenotype in MUTS-A cells, which bear an early stop codon (Q12Stop) in RPS19, than in MUTS-B cells characterized by the missense mutation R62Q, located in the DBA mutation hot spot. A mutation equivalent to R62Q in yeast RPS19 only partially affects cell viability, suggesting that this mutant is partially functional.20 One might speculate that severity of the phenotype is correlated with the degree of haploinsufficiency of RPS19, which is expected to be more pronounced in MUTS-A than in MUTS-B cells. Examination of cells from more patients is necessary to draw a more definitive conclusion regarding this correlation.
Most interestingly, fibroblasts from patient NOTS-1, who has no mutation in RPS19, also display a clear but distinct alteration of pre-rRNA processing and nucleolar organization. Thus, this patient might bear a mutation in a gene whose protein product is also involved in ribosome biogenesis but at a different step than RPS19. Confirmation of this hypothesis would strengthen the idea that DBA is a consequence of a defect in ribosome biogenesis rather than linked to the loss of a nonribosomal function of RPS19. Given the large number of proteins involved in ribosome biogenesis, one would then expect to find a variety of genes mutated in DBA.
Alteration of RPS19 function in DBA skin fibroblasts is consistent with the wide variety of symptoms unrelated to the hematopoietic system displayed by patients. However, erythropoiesis stands out as the process primarily affected by RPS19 haploinsufficiency. Consistently, RPS19 knockdown with siRNAs was shown to decrease proliferation and differentiation of CD34+ cells.9,11 Because of the high proliferation rate associated with their differentiation, erythroblasts may be especially sensitive to the stress induced by a defect in ribosome biogenesis, which may perturb their response to mitogenic, differentiating, or apoptotic signals. Erythroid progenitors from DBA patients were reported to rapidly undergo apoptosis upon erythropoietin deprivation, unlike control cells,32 and expression of several genes involved in mitogenic and apoptotic pathways were found to be deregulated in several types of hematopoietic progenitors.33 Indeed, alteration of rDNA transcription or preribosomal assembly is known to trigger activation of the tumor suppressor p53 and cell-cycle arrest in cultured cells34-36 and in vivo.34,37,38 This may be correlated with the delayed growth rates observed here in DBA fibroblasts. We also find that a severe lack of RPS19 induced with siRNAs favors apoptosis in HeLa cells. However, RPS19 loss of function is much milder in DBA patients that in rps19 siRNA-treated cells and may only be deleterious in cells with high growth and division rates. Accordingly, tissue-specific heterozygous knockout of the RPS6 gene in mouse T cells does not affect initial maturation of these cells in thymus but partially blocks their activation and accumulation in peripheral lymphoid organs.38 Similarly, sensitization of the cells to cell-cycle arrest or apoptosis by the constitutive moderate defect in preribosomal assembly observed in DBA cells may only become decisive in very defined physiologic processes, the most critical of which is erythroid differentiation. The decreased expression of RPS19 and other ribosomal proteins observed in differentiating erythroblasts11,39 may even aggravate the defect in ribosome biogenesis and favor a fatal stress response.
Authorship
Contribution: V.C. and D.B. designed part of the experiments, performed research, and analyzed data; J.R., J.N.-D., and A.C. performed research and analyzed data; S.F. and T.L. contributed essential reagents; G.T. and L.D.C. participated in research design; and P.-E.G. designed research, analyzed data, and wrote the paper with the assistance of D.B. and V.C.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
V.C. and D.B. contributed equally to this study.
Correspondence: Pierre-Emmanuel Gleizes, LBME-UMR5099, 118 route de Narbonne, 31062 Toulouse Cedex, France; gleizes@ibcg.biotoul.fr.
The online version of this article contains a data supplement.
An Inside Blood analysis of this article appears at the front of this issue.
This work was supported by the Agence Nationale pour la Recherche (RIBODBA project), the Association pour la Recherche contre le Cancer (ARC), the CNRS and the Université Paul Sabatier, National Institutes of Health (NIH) grant 1R01HL079565 (L.D.C., A.C., and G.T.), and a fellowship from the Ligue contre le Cancer du Gers (J.R.).
The authors are grateful to Nicole Gas for constant support. We also wish to thank Laurent Barricault, Yves Henry, Olivier Vaute, Didier Trouche, Michèle Caizergues-Ferrer, Jérôme Cavaillé, Hélène Duplan, and Isabelle Ceruti for discussion, technical advice, and reagents and Marie-George Come (Roper Scientifics, Evry, France) for assistance with image analysis.





This feature is available to Subscribers Only
Sign In or Create an Account Close Modal