Abstract
Mutations leading to the alteration of cell-cycle checkpoint functions are a common feature of most cancers. Because of the highly regulated nature of the cell cycle, it seems likely that variation in gene dosage of key components due to functional regulatory polymorphisms could play an important role in cancer development. Here we provide evidence of the involvement of promoter single-nucleotide polymorphisms (pSNPs) in the cyclin-dependent–kinase inhibitor genes CDKN2A, CDKN2B, CDKN1A, and CDKN1B in the etiology of childhood pre-B acute lymphoblastic leukemia (ALL). A case-control study, conducted in 240 patients with pre-B ALL and 277 healthy controls, combined with a family-based analysis using 135 parental trios, all of French-Canadian origin, were used to evaluate single-site genotypic as well as multilocus haplotypic associations for a total of 10 pSNPs. Using both study designs, we showed evidence of association between variants CDKN2A −222A, CDKN2B −593A, and CDKN1B −1608A, and an increased risk of ALL. These findings suggest that variable expression levels of cell-cycle inhibitor genes CDKN2A, CDKN2B, and CDKN1B due to regulatory polymorphisms could indeed influence the risk of childhood pre-B ALL and contribute to carcinogenesis.
Introduction
Acute lymphoblastic leukemia (ALL) is the most common pediatric cancer. The etiology of this hematologic malignancy might be explained by a combination of genetic susceptibility and environmental exposure during early development in fetal life and infancy. Assuming that genes modulate individual responses to exogenous and/or endogenous factors, they would thereby also influence an individual's risk of cancer.1 Consistent with this paradigm, it has been shown that childhood leukemogenesis is associated with genetic variability in xenobiotic metabolism,2-10 oxidative stress response,9,11,12 and DNA repair13,14 pathways. However, little is known about the impact of genetic polymorphisms in cell-cycle components, despite the fact that the cell cycle is a highly orchestrated biological process frequently altered in human cancers.15 A critical point in the cell cycle is the G1/S transition checkpoint, during which the cell is irreversibly committed to a new round of division.16 The cyclin-dependent kinase inhibitors (CDKIs) CDKN2A (p16INK4A), CDKN2B (p16INK4B), CDKN1A (p21Cip1/Waf1), and CDKN1B (p27kip1) are key regulators of the G1/S checkpoint, their concerted action preventing cells from undergoing subsequent division in response to oncogenic signaling or DNA damage.17 Accordingly, changes in their expression and/or activity due to polymorphisms might modify the susceptibility to cancer. Correlations between DNA variants in CDKN2A, CDKN2B, CDKN1A, and CDKN1B and cancer susceptibility have been reported, particularly in breast, prostate, and skin carcinomas.18-24 In addition, single-nucleotide polymorphisms (SNPs) in gene promoter sequences (pSNPs) have recently gained much importance because of their quantitative impact on gene expression.25,26 Several studies have suggested that because protein levels regulate many biological pathways, including the cell cycle, pSNPs could influence the overall outcome of these biological processes and thereby modify disease risk.27-33 In this report we performed an association study using both a case-control and family-based design in order to assess the impact of proximal promoter SNPs in CDKN2A, CDKN2B, CDKN1A, and CDKN1B on the susceptibility to childhood pre-B ALL.
Patients, materials, and methods
Study population
The population under study and the inclusion criteria were described previously.6 Incident patients with childhood pre-B ALL (n = 240) were diagnosed in the Division of Hematology-Oncology of the Sainte-Justine Hospital in Montreal (QC, Canada), between October 1985 and November 2003. They comprised 141 boys and 99 girls with a median age of 4.6 years (range, 5 months to 18 years), all of French-Canadian descent, from the province of Quebec. A number of these patients (135), from whom parent DNA was available, were also enrolled in the family-based study. The healthy controls (n = 277) consisted of French-Canadian volunteers recruited while using clinical departments other than Hematology-Oncology of the Sainte-Justine Hospital. The CHU Sainte-Justine Institutional Review Board approved the research protocol, and informed consent was obtained from all participating individuals and/or their parents.
Genotyping of promoter SNPs
DNA was isolated from either buccal epithelial cells, peripheral blood, or bone marrow in remission as described previously.34 DNA segments containing the polymorphic sites were amplified by polymerase chain reaction (PCR) using a “touchdown” thermal cycling protocol.35 The resulting PCR products were dot-blotted in duplicate on a nylon membrane and assayed for the presence or absence of variants by hybridization in parallel with allele-specific oligonucleotides (ASOs) as described in Labuda et al.36 The amplimers and oligonucleotide probes used for ASO analysis are given in Table 1
EMSAs
Double-stranded oligonucleotide probes corresponding to the sequences surrounding the polymorphic sites were radiolabeled and allowed to interact with nuclear extracts prepared from HepG2 (hepatoma), Jeg-3 (choriocarcinoma), and HeLa (cervical carcinoma) cells as described in Belanger et al.38 Briefly, protein was quantified with the Bradford protein assay (Bio-Rad, Mississauga, ON, Canada). Nuclear extracts (5 μg) were incubated with 35 fmol radiolabeled double-stranded DNA probes and a buffer containing 50 mM Tris-HCl (pH 7.5), 5 mM MgCl2, 2.5m M EDTA, 2.5 mM DTT, 250 mM NaCl, 0.25 μg/μL poly deoxyinosinate-deoxycytidylate, and 20% glycerol, in a total volume of 10 μL for 20 minutes at room temperature. Complexes were separated in a 6% nondenaturing polyacrylamide gel (acrylamide-bisacrylamide, 60:1) in 0.5 × Tris-borate-EDTA buffer (190 V at 4°C). For competition of binding, a 50-fold molar excess of competitor (either the unlabeled probe oligonucleotide or the corresponding mutant oligo) was included. The oligonucleotides used in the electrophoretic mobility shift assays (EMSAs) are as follows (only the 5′-3′ top strand of the double-stranded oligonucleotides are shown): CDKN2A –222T>A, ACAACCTTCC(T/A)AACTGCCAAATTGAATCGGGGTGT; CDKN2B −1270C>T, AATGCTACCCGGTTCCCTT(C/T)CCTGTCCAGGTGGATTT, −593A>T, C, GGATCTCAGATTCTTA(A/T/C)AGTATAATTTTTTTT, and −287C>G ATCTTAAGAAA(C/G)ACGGAGTTATTTTGA; CDKN1A −1284T>C, TTCTGTTTTT(T/C)AGTGGGATTT, −899T>G, TGGGGAAAC(T/G)GGGGCTC, and −791T>C, ACAGAAGAAA(T/C)CCCTGTGGTT; and CDKN1B −1857C>T, TACCACAGGCTCAAGA(C/T)AGCTGCATTTAA, −1608G>A, GGTTTCCTGTCCAGG(G/A)ACATGCA, and −373G>T, TAAGCCCCGACCTCCCTCCCGCTCCTC(G/T)CCCGGGAAGCCGGGAC.
Statistical analysis
Hardy-Weinberg equilibrium was examined using the χ2 test for goodness of fit. Fisher exact tests (2-sided) were used to compare allele/genotype/haplotype carriership in patients and controls. Crude odds ratios (ORs) are given with 95% confidence intervals (CIs). All analyses were carried out using STATA statistical software (release 9.1; StataCorp, College Station, TX). CDKN2B, CDKN1A, and CDKN1B haplotypes and corresponding frequencies were estimated using the PHASE software (version 2; University of Washington, Seattle).39 Linkage disequilibrium between SNPs was tested with the Arlequin linkage utility software (version 2.00; University of Geneva, Switzerland).40 An omnibus χ2 test, implemented in the Evolutionary-Based Haplotype Analysis Package (eHap version 2.0; Carnegie Mellon University and University of Pittsburgh, PA),41 was used to examine overall haplotype associations with disease phenotype. Transmission disequilibrium from parents to children of individual SNPs and corresponding haplotypes was assessed with FBAT (family-based association test) software (version 1.5.1; Program for Population Genetics, Harvard School of Public Health, Boston, MA).42 A multiallelic test was also carried out in FBAT to obtain the global haplotype association significance in the family-based setting. Correction for multiple testing errors was performed using the false discovery rate (FDR) principle,43 with a predetermined type I error rate set at 10%.
Results
Promoter SNPs
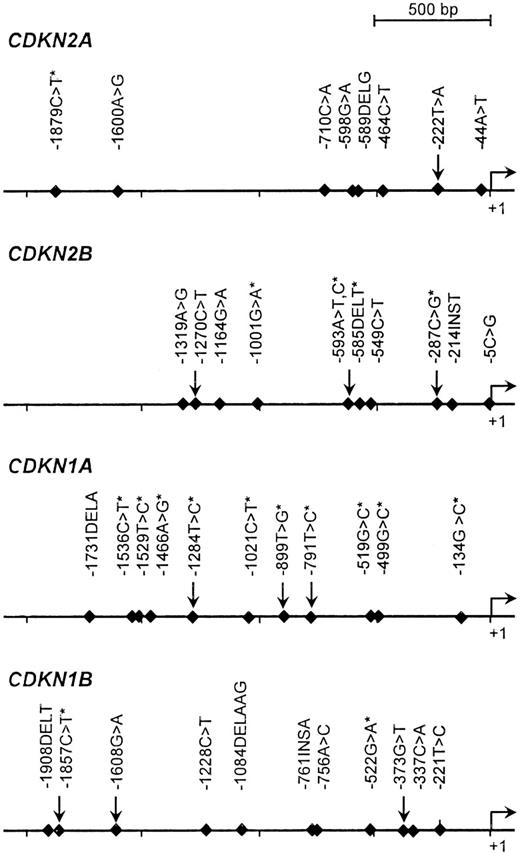
The detection of proximal promoter variants in a population panel consisting of 40 unrelated individuals (8 Africans, 8 Europeans, 8 Asians, 8 Middle-Easterners, and 8 Amerindians) was performed by PCR-based denaturing high-performance liquid chromatography (dHPLC) analysis followed by direct sequencing as described in Sinnett et al.44 The targeted promoter region was arbitrarily defined as the 2-kb sequence upstream of the transcriptional initiation site. A total of 40 sequence variants were identified for the genes CDKN2A, CDKN2B, CDKN1A, and CDKN1B, including 21 that were previously reported in public databases (Figure 1). For the purpose of this study targeting French-Canadians, we considered only pSNPs that were common among Europeans; in other words, those that were found at least twice among the 8 European patients initially screened. This led to the genotyping of 10 pSNPs: CDKN2A, −222T>A; CDKN2B, −1270C>T, −593A>T,C and −287C>G; CDKN1A, −1284T>C, −899T>G, and −791T>C; and CDKN1B, −1857C>T, −1608G>A, and −373G>T (Table 1). Of note, polymorphism −222T>A has been detected in the promoter region of CDKN2A in previous studies.19,45,46 The observed allele and genotype frequencies in children with ALL and in healthy controls are reported in Tables 2 and 3. In controls, the frequencies of 6 of these pSNPs were in agreement with those reported in other populations of European descent as per the National Center of Biotechnology Information (NCBI) database of SNPs (dbSNP)37 and the International Haplotype Mapping (HapMap) Project47 databases. All distributions were in Hardy-Weinberg equilibrium.
Polymorphisms detected in the promoter regions of CDKN2A, CDKN2B, CDKN1A, and CDKN1B. pSNPs that were genotyped in this report are identified by arrows; those reported in public databases are marked by an asterisk. The promoter positions were numbered with respect to the first nucleotide of the first exon as +1, and the nucleotide immediately upstream as −1.
Polymorphisms detected in the promoter regions of CDKN2A, CDKN2B, CDKN1A, and CDKN1B. pSNPs that were genotyped in this report are identified by arrows; those reported in public databases are marked by an asterisk. The promoter positions were numbered with respect to the first nucleotide of the first exon as +1, and the nucleotide immediately upstream as −1.
Predicted functional impact of the pSNPs
The screening of the promoter region for predicted transcription factor–binding sites (TFBSs) using matInspector (http://www.genomatix.de/products/index.html)48 led to the identification of pSNPs that might create and/or disrupt some of these TFBSs (Table 4). The putative impact of these pSNPs on DNA-protein–binding capacity was further validated by EMSAs. In the latter, double-stranded oligonucleotide probes corresponding to the sequences surrounding the polymorphic sites (“Patients, materials, and methods”) were radiolabeled and allowed to interact with nuclear extracts prepared from HepG2, Jeg-3, and HeLa cells, and differential allelic shifts were assessed (see Figure 2 for representative data). As indicated in Table 4, 7 of the tested pSNPs showed differential allelic shifts in at least 1 of the cell lines tested.
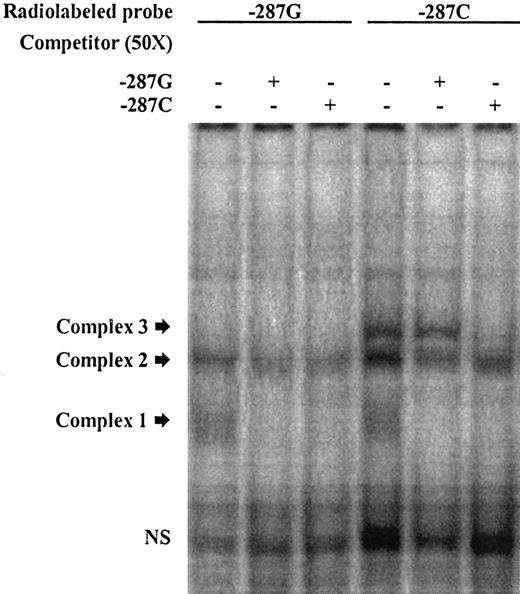
EMSA illustrating allelic DNA-protein interactions in the promoter region of CDKN2B. Labeled double-stranded oligonucleotide (ds-oligo) probes corresponding to the CDKN2B −287C>G alleles were incubated with HeLa nuclear extracts. Lanes 1 to 3 represent labeled −287G ds-oligos; lanes 4 to 6, labeled −287C ds-oligos. The unlabeled probes used to compete DNA-protein interactions (in 50-fold molar excess) are indicated (+) at the top of each lane. Probe sequences are listed in Table 1. Fast migrating unbound probes can be seen at the bottom of the gel (NS indicates nonspecific), and the position of the DNA-protein complexes of slower mobility are marked by arrows. In this experiment, 3 distinct complexes were found following incubation of the probes with HeLa nuclear extract. Complex 1 was observed with both labeled ds-oligos −287G and −287C (lanes 1 and 4) but was competed by both unlabeled probes, indicating unstable DNA-protein interactions. Complex 2 was also found with both alleles; the −287C-derived complex was competed by both unlabeled probes; however, the −287G-derived complex seemed to be less affected by competitors and thus more stable, suggesting higher binding affinity. Complex 3 appeared only when −287C was present (lane 4 vs lane 1). The specificity of this interaction was illustrated through competition with the specific unlabeled probe, which did not occur with the mismatched −287G probe (lane 6 vs lane 5).
EMSA illustrating allelic DNA-protein interactions in the promoter region of CDKN2B. Labeled double-stranded oligonucleotide (ds-oligo) probes corresponding to the CDKN2B −287C>G alleles were incubated with HeLa nuclear extracts. Lanes 1 to 3 represent labeled −287G ds-oligos; lanes 4 to 6, labeled −287C ds-oligos. The unlabeled probes used to compete DNA-protein interactions (in 50-fold molar excess) are indicated (+) at the top of each lane. Probe sequences are listed in Table 1. Fast migrating unbound probes can be seen at the bottom of the gel (NS indicates nonspecific), and the position of the DNA-protein complexes of slower mobility are marked by arrows. In this experiment, 3 distinct complexes were found following incubation of the probes with HeLa nuclear extract. Complex 1 was observed with both labeled ds-oligos −287G and −287C (lanes 1 and 4) but was competed by both unlabeled probes, indicating unstable DNA-protein interactions. Complex 2 was also found with both alleles; the −287C-derived complex was competed by both unlabeled probes; however, the −287G-derived complex seemed to be less affected by competitors and thus more stable, suggesting higher binding affinity. Complex 3 appeared only when −287C was present (lane 4 vs lane 1). The specificity of this interaction was illustrated through competition with the specific unlabeled probe, which did not occur with the mismatched −287G probe (lane 6 vs lane 5).
Single-locus analysis
First we assessed the involvement of the selected 10 pSNPs in childhood ALL by performing a case-control study. The estimated ORs and 95% CIs for the corresponding alleles and genotypes are given in Tables 2 and 3. The CDKN2A −222A allele was overrepresented in patients when compared with controls (7.5% vs 3.6%), as was the heterozygous −222TA genotype (13.2% versus 6.5%). Evidence of an increased risk of ALL among carriers of the CDKN2A −222A variant was demonstrated (OR = 2.2, 95% CI, 1.2-4.0, P = .008) and remained significant after correction for potential multiple testing errors. In contrast, the CDKN2B −593T allele was underrepresented among patients (30.9% vs 37.9%), suggesting a protective effect of this variant (OR = 0.7, 95% CI, 0.6-1.0, P = .02). The frequency of carriers of the −593T-associated genotypes (AT, TT, and TC) was lower in patients with ALL compared with that in controls, but failed to reach statistical significance (Table 3). In CDKN1B, we found that −1608GA heterozygotes were more frequent among patients than controls (21.4% vs 13.6%), conferring an increased risk of ALL in children (OR = 1.7, 95% CI, 1.0-2.8, P = .03), but this result did not remain significant after correction for multiple testing. The initial case-control assessment did not reveal any additional noteworthy associations for the other CDKN2B and CDKN1B variants, and the frequency of CDKN1A variants did not differ between patients and controls (Tables 2–3), indicating that these pSNPs alone do not appear to modify the risk of childhood pre-B ALL in our dataset.
Haplotype analysis
Because single variants in a candidate gene might not be sufficient to capture the genetic variability relative to a given phenotype, promoter haplotypes were constructed for CDKN2B, CDKN1A, and CDKN1B. Haplotype phase was estimated and the corresponding frequencies and distributions among patients with ALL and controls were assessed (Table 5). Omnibus χ2 tests were performed to determine whether the effects of the haplotypes differed significantly between patients and controls. We found evidence for overall association between the 6 CDKN2B-derived haplotypes and ALL (χ2 = 19.1 [5 degrees of freedom], P < .001), which remained significant after multiple testing corrections. When comparing individual haplotype distributions, the largest differences were observed for haplotypes 2B-2, 2B-3, and 2B-6. Haplotype 2B-2 (CTG), carrying the protective −593T allele (Table 2), occurred at a lower frequency among patients with ALL (31.6% vs 37.9%), suggesting an inverse correlation with the disease (OR = 0.8, 95% CI, 0.6-1.0, P = .04). In contrast, haplotype 2B-3 (CAG), carrying the high-risk −593A allele, was overrepresented in patients as opposed to in controls (20.2% vs 13.2%) and was significantly associated with an increased risk of ALL (OR = 1.7, 95% CI, 1.2-2.4, P = .004). Interestingly, haplotype 2B-6 (TAC) was found exclusively in patients with ALL. For CDKN1A and CDKN1B, we were able to construct 7 and 5 haplotypes, respectively, but the omnibus tests did not suggest overall association, nor did any of the individual haplotypes show significant association with the risk of ALL (Table 5).
Family-based analysis
To further assess the impact of these polymorphisms on childhood pre-B ALL risk, we performed a family-based study by genotyping all 10 pSNPs in 148 patient-parental trios. We either analyzed each variant independently (single-marker; Table 6) or as haplotypes (Table 7) using FBATs. In the univariate FBATs, we found a significant preferential transmission of the high-risk CDKN2A −222A variant to affected offspring (Z = 2.60, P = .009; Table 6). The protective CDKN2B −593T allele was shown to be transmitted to affected patients less often than expected (Z = −2.64, P = .008), whereas the high-risk −593A allele was shown to be overtransmitted (Z = 2.778, P = .005; data not shown). In addition, these findings held true under the dominant and recessive models as well (data not shown). These results, which remained significant after correction for multiple testing, are consistent with the case-control results discussed in “Single-locus analysis” (Tables 2–3). For CDKN1A and CDKN1B, no single variant was found to be significantly associated with childhood ALL using the additive model (Table 6).
The global haplotype FBATs revealed no significant transmission disequilibrium of the promoter variants of CDKN2B, CDKN1A, and CDKN1B across all trios (Table 7). However FBAT analysis of individual haplotypes showed that CDKN2B-1 (CAC), bearing the high-risk A-593 allele, was preferentially transmitted to affected offspring (Z = 2.22, P = .03), whereas haplotype 2B-2 (CTG) was associated with a protective effect and shown to be undertransmitted to patients with ALL (Z = −2.44, P = .01). No other significant associations were detected for the CDKN1A and CDKN1B promoter haplotypes under the additive model (Table 7). Haplotype CDKN1B-2 (CGG) did show evidence of increased transmission under the recessive model (Z = 2.44, P = .01), suggesting the possibility that this haplotype carrying the variant −373G is associated with an increased risk of childhood ALL (data not shown). However, it should be noted that though the additive model is expected to perform well even when the true model is nonadditive, misspecification of a recessive or dominant mode of inheritance can lead to a decrease in power of the FBAT.49-51
Discussion
CDKIs are key regulators of the G1/S checkpoint.52 Their strict control and concerted action are crucial to maintain cell homeostasis and genomic integrity during cellular division. It is therefore plausible that variation in gene dosage of such critical cell-cycle regulators due to functional regulatory polymorphisms could influence cancer susceptibility by altering cell-cycle checkpoints. In the present study, we tested this hypothesis in childhood ALL by assessing the genotype and haplotype distributions associated with 10 common pSNPs found in the genes CDKN2A, CDKN2B, CDKN1A, and CDKN1B. This genetic epidemiology study was performed in French-Canadians, a population known for its relative genetic homogeneity due to particular demographic and historic characteristics.53 Using 2 distinct yet complementary study designs (case-control and parental trios), we identified putative associations between pSNPs in CDKN2A (−222T>A), CDKN2B (593A>T,C), and CDKN1B (−1608G>A) and a modified risk of childhood ALL, supporting the idea that DNA variants leading to variable CDKI levels might contribute at least to childhood leukemogenesis.
In the case of CDKN2A, earlier work led to the identification of a critical region containing functional promoter activity within the 869 bases immediately upstream of its coding domain.54 It is conceivable that the variant −222T>A might alter promoter function, perturbing CDKN2A expression and therefore cell-cycle control. Interestingly, our in silico analysis showed that this pSNP leads to the loss of a predicted c-Myb binding site, a transcription factor required for proliferation, differentiation, and survival of hematopoietic cells.55,56 Furthermore, reduced CDKN2A expression due to regulatory polymorphisms has been suggested to contribute to arrested lymphoblast differentiation, which is characteristic of leukemic disorders.57 Additional studies are required however to confirm whether this particular −222T>A nucleotide change has an effect on CDKN2A expression. We cannot rule out the possibility of other linked functional SNPs within (or beyond) the CDKN2A sequence that could influence childhood ALL predisposition and account for the observed association, especially given the fact that no haplotypic data were available in this particular study. To this effect, variant −222T>A has been shown to be in complete linkage disequilibrium with another common CDKN2A variant, an alanine-to-threonine substitution at codon 148 (Ala148Thr) shown to be associated with malignant melanoma.19,45,58-60
For CDKN2B, although we failed to show a significant positive association with −593AT heterozygous or −593AA homozygous individuals, the haplotype-specific analysis did support evidence of an association between allele −593A and childhood leukemia. Haplotype 2B-3 (CAG) carrying the −593A allele was overrepresented among patients with ALL, and 2B-1 (CAC) was overtransmitted more frequently from parents to affected offspring, suggesting a potential positive association with the disease. The differential binding detected at the −593A>T,C site in both HeLa and HepG2 nuclear extracts further supports this hypothesis. The fact that these associations were observed for given haplotypes rather than at individual SNPs could reflect the benefit of haplotype-based analysis. Haplotypes provide an advantage because they contain more information as to the genetic variability in the surrounding locus when the contributing SNPs are not all directly observed, providing they are within linkage units (haplotype blocks) with the SNP under study.61 Using the information generated by the International HapMap Project, we were able to confirm that the CDKN2B −593A>T, C variant is found within a 33-kb haplotype block alongside 22 other tagged SNPs. It is possible that at least 1 of these SNPs in linkage disequilibrium with the −593 variant might contribute to the observed modified risk of childhood ALL.
Both CDKN2A and CDKN2B are well-characterized tumor suppressors, and their implication in human cancers and various hematologic malignancies, including ALL,62 has been demonstrated. So far, deletion events have been the main cause of somatic inactivation of CDKN2B in certain ALL subtypes,63 leading to deregulation of the cell cycle and subsequent tumor genesis. Here we have provided evidence of the implication of CDNK2B germline mutations in childhood leukemogenesis.
CDKN1B also plays a critical role in regulating cell proliferation, and studies of knockout mice suggest that CDKN1B acts as a tumor suppressor as well.64-67 Furthermore, a familial study on prostate cancer revealed an association with the regulatory SNP rs34330 found in the 5′ untranslated region of CDKN1B, providing evidence that germline variants of this gene may indeed play a role in cancer susceptibility.18 In this report, we observed an increased risk of childhood ALL among carriers of the CDKN1B −1608GA genotype. However, the FBATs failed to corroborate this association, indicating random transmission of the −1608A allele from parents to affected children. To this effect, previous work by Labuda et al68 suggested that parental genetics might be important in predicting the risk of cancer (at least childhood leukemia). In other words, if at certain loci the parental genotypes rather than the offspring's own combination of genotypes are responsible for genetic susceptibility to a disease, analyses such as FBAT would fail to detect the disease-susceptibility allele(s) since the parent-to-child transmission would essentially be random. We tested this hypothesis in our dataset for CDKN1B −1608G>A by substituting the parents for the patients and comparing fathers with male controls and mothers to female controls, but we failed to detect any significant associations (data not shown). Although this hypothesis remains speculative and requires further analysis to rule out the possibility that the observed case-control association is simply a spurious result, it illustrates the importance of considering the effect of parental genotypes and of combining various study designs when assessing complex disease susceptibility.
In conclusion, our new findings suggest that germline variants in CDKI genes CDKN2A, CDKN2B, and CDKN1B may play a role in susceptibility to childhood leukemia. Also, we cannot rule out the possibility that these polymorphisms might act in combination with other disease modifiers in the same or any other related biological pathways. Further studies that evaluate interaction effects with genes involved in xenobiotic metabolism and DNA repair will be interesting because these may build upon previous findings of the implication of such disease-susceptibility genes in childhood leukemia and allow us to further understand the biological mechanism underlying the observed associations.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Conflict-of-interest statement: The authors declare no competing financial interests.
Contribution: J.H. carried out most of the molecular genetic studies and statistical analyses, and drafted the manuscript; H.B. was involved in pSNP discovery; M.L. carried out the EMSAs; P.B. performed in silico studies; D.L. was involved in haplotype construction; D.S. conceived the study, participated in its design and coordination, and helped to draft the manuscript. All authors have read and approved the final manuscript.
Acknowledgments
We are indebted to all the patients and their parents who consented to participate in this study.
This work was supported by research funds provided by the Canadian Institutes of Health Research (CIHR), as well as Genome Canada/Quebec. J.H. is the recipient of a CIHR studentship. D.S. holds the François-Karl Viau Chair in Pediatric Oncogenomics and is a scholar of the Fonds de la Recherche en Santé du Québec (FRSQ).