Abstract
Platelet integrins α2β1 and αIIbβ3 play critical roles in platelet adhesion and thrombus formation after vascular injury. On resting platelets, both integrins are in a low-affinity state. However, agonist stimulation results in conformational changes that enable ligand binding that can be detected with conformation dependent monoclonal antibodies (mAbs). By using such conformation-dependent mAbs, we could demonstrate that activation of integrin αIIbβ3 is not only sufficient, but also a prerequisite for α2β1 activation. Compared with platelets in plasma, stimulation of washed platelets resulted in only a minor activation of α2β1, as detected with the activation-sensitive mAb IAC-1. Addition of fibrinogen to stimulated washed platelets greatly potentiated activation of this integrin. Also, treatment of αIIbβ3 with the ligand-mimetic peptide RGDS, resulting in outside-in signaling, led to a powerful α2β1 activation, even in the absence of overall platelet activation, involving tyrosine kinase activity but no protein kinase C activation. The absolute necessity of αIIbβ3 for proper α2β1 activation on platelets was demonstrated by using the αIIbβ3 antagonist aggrastat, which was able to completely abolish α2β1 activation, both under static and flow conditions. In addition, analogous experiments with Glanzmann platelets lacking αIIbβ3 confirmed the indispensability of αIIbβ3 for α2β1 activation.
Introduction
Integrins are a large family of heterodimeric transmembrane receptors, each consisting of an α and β subunit, and are key effectors of cell growth, migration, differentiation, and survival.1,2 Integrins possess the unique ability to signal across the plasma membrane in both directions, and since most integrins are not constitutively active, they are expressed on the cell surface as low-affinity receptors. When cells become activated, cytosolic proteins can bind to the cytoplasmic domains of integrins and as a consequence, the integrins are turned into their high-affinity state (“inside-out” signaling). In a process called “outside-in” signaling, ligand-binding of integrins then again activates intracellular pathways via their cytoplasmic domains, which are connected to the cytoskeleton and are associated with several intracellular signaling molecules. Important signaling modulators necessary for the generation of outside-in signals are members of the Src family protein tyrosine kinases, with c-Src as the initiating molecule due to its constitutive interaction with, for example, β3 integrins.3 Such outside-in signals also result in conformational alterations of the integrin (also designated integrin activation), and subsequently these activated integrins can trigger another process of inside-out signaling.4 This integrin activation upon conformational changes is often defined as an increase in integrin “affinity” for its ligand, and has been the topic of many studies.4-6 In addition, cell activation also promotes clustering of integrins contributing to the “avidity” or “valency” regulation of ligand binding.7,8 So in their role as adhesion molecules, integrins signal across the plasma membrane in both directions and are able to switch from an inactive, low-affinity conformation to an active, high-affinity conformation. Integrins can also interact with other receptors, and this sort of “networking” between membrane receptors can be defined as “cross talk.” Numerous examples of cross talk either between an integrin and another membrane receptor or between 2 integrins, resulting in inhibition or activation, have been reported in a variety of cell types.9-17
On platelets, 2 major integrin receptors are expressed, both of which are involved in primary hemostasis. Integrin αIIbβ3 is the most abundant receptor, with 40 000 to 80 000 copies per resting platelet, and acts as a major receptor for fibrinogen and other adhesive molecules.18 Activation of αIIbβ3 enhances adhesion and leads to platelet-platelet interactions and aggregation.19 Integrin α2β1, not uniquely expressed on platelets, is a less abundant receptor, with around 2000 copies per platelet, and serves as a collagen receptor on platelets.20 Together with the signaling collagen receptor GPVI, α2β1 is indispensable for stable adhesion of platelets to the extracellular matrix, which is exposed after vascular injury.21,22 The modulation of the integrin αIIbβ3 on platelets by other receptors has already been extensively studied. For instance, it has been reported that under flow, glycoprotein (GP) Ib binding to von Willebrand factor activates the integrin αIIbβ3, supporting localized platelet adhesion. Subsequently, a second level of αIIbβ3 activation, induced by, for example, ADP, is then necessary in order to allow platelet aggregation.23 Furthermore, earlier work has suggested that αIIbβ3, together with α2β1, also becomes activated under flow following GPVI-induced platelet activation.24 Since activation of both integrins is important during the process of primary hemostasis, it is appropriate to speculate that these 2 major integrin receptors may possibly modulate each other. Recently, our group has developed a monoclonal antibody (mAb), IAC-1, which recognizes an epitope of the α2 I-domain, which is hidden in the resting state and after outside stimulation of α2β1, but is accessible when platelets are stimulated via inside-out signaling, resulting in a fully activated form of α2β1.25,26 Moreover, since binding of this antibody does not require the presence of the receptor ligand, nor does it interfere with platelet collagen binding, it defines a new class of antibodies that is distinct from those belonging to the ligand-induced binding site (LIBS) and ligand mimetic groups.
Here, we investigated whether the 2 major integrin receptors on platelets, αIIbβ3 and α2β1, can modulate each other's activation state. By using the activation-sensitive mAb IAC-1, we report that activation of αIIbβ3 is necessary for proper α2β1activation.
Materials and methods
Antibodies and proteins
The mAb IAC-1, specific for the I-domain of fully activated α2β1, was isolated by our group as previously described25 and labeled with fluorescein isothiocyanate (FITC) according to the manufacturer's instructions (Pierce, Rockford, IL). The anti-α2 I-domain mAbs Gi9 (FITC-conjugated) and 15D7 were from Immunotech (Marseille, France) or made in-house, respectively, and both inhibit collagen-induced platelet aggregation. The anti-GPIb mAb 6B4 has been previously described,27 and the anti-αIIbβ3 mAb AP2 and the anti-β3 mAb AP3 were from GTI (Waukesha, WI). The mAbs AK7 (anti-α2), phycoerythrin (PE)–conjugated CD62P (anti–P-selectin), and FITC-conjugated PAC-1 (anti–activated αIIbβ3) were from BD Biosciences (San Diego, CA). MOPC-21–FITC, an unrelated mouse anti–human immunoglobulin G1 (IgG1), was from Sigma (St Louis, MO). Texas Red–conjugated phalloidin was purchased from Molecular Probes (Eugene, OR), and polyclonal rabbit anti–mouse IgG–FITC was from Dako (Glostrup, Denmark).
Fibrinogen was purchased from Calbiochem (San Diego, CA), human collagen type III was purchased from Sigma, and Horm collagen was purchased from Nycomed (Munich, Germany). Fibrinogen and collagen type III were FITC labeled according to the manufacturer's instructions (Pierce). The FITC labeling was performed in 50 mM borate buffer (pH 8.5), after which an excess of fluorescent dye was removed by overnight dialysis against phosphate-buffered saline (PBS). As a consequence, the collagen-FITC consisted mainly of reconstituted collagen fibrils, which in the presence of Mg2+ specifically interact with α2β1 and not GPVI.
Inhibiting/activating agents and cells
Convulxin (CVX; Kordia, Leiden, The Netherlands) and ADP (Sigma) were used as platelet activators. Dithiotreitol (DTT; Sigma) was used to activate αIIbβ3 on Chinese hamster ovary (CHO) cells. Antagonists of αIIbβ3 used were aggrastat, obtained from Merck Sharp & Dohme (Whitehouse Station, NJ), and RGDS from Sigma. Prostaglandin E1 (PGE1), staurosporin, genistein, and Ro 31-8220 were all from Sigma. The Src kinase inhibitors PP2, PP1, and their control PP3 compound were from Calbiochem (Darmstadt, Germany).
Blood samples from healthy volunteers, free of antiplatelet agents for the last 10 days, were collected on 3.13% trisodium citrate, and platelet-rich plasma (PRP) was prepared by centrifugation. Alternatively, blood was taken on acid citrate dextrose (ACD), and platelets were washed by several centrifugation steps in the presence of apyrase (75 mU/mL) and PGE1 (100 nM), essentially as previously described.26,28 Washed platelets were finally resuspended in HEPES/Tyrode (137 mM NaHCO3, 2mM KCl, 2 mM MgCl2, 0.3 mM Na2HPO4, 12 mM NaHCO3, 5 mM HEPES, and 0.01% [wt/vol] glucose [pH 7.4]) supplemented with 0.3% BSA at a concentration of 3 × 105/μL and allowed to rest for 20 minutes.
Where indicated, platelets from a previously characterized patient with type I Glanzmann thrombasthenia29 were isolated as described for platelets from the healthy volunteers. A second adult patient with Glanzmann thrombasthenia who had less than 10% surface-expressed αIIbβ3 in his platelets has not been previously reported. Blood was taken from the patients with informed consent and according to institutional guidelines.
CHO-dhfr+ cells and CHO cells, expressing either human αIIbβ3 or α2β1 (both a kind gift from Dr N. Kieffer, Laboratoire de Biologie et Physiologie Intégrée, Université du Luxembourg),30 were cultured in Iscove medium (Cambrex, Verviers, Belgium) supplemented with 10% (wt/vol) fetal calf serum, 1% (vol/vol) penicillin/streptomycin, and 1% (vol/vol) l-glutamine. Cells were grown to confluency and were routinely passaged after detachment using versene (Gibco, Carlsbad, CA).
Flow cytometry
Reaction mixtures of 5 μL PRP or washed platelets (in a total volume of 50 μL), activating agent (CVX at 50 ng/mL or ADP, 50 μM final concentration), anti–P-selectin–PE (1:10 vol/vol), and FITC-labeled Gi9 (5 μg/mL), FITC-labeled IAC-1 (10 μg/mL), or FITC-labeled collagen (40 μg/mL) in HEPES/Tyrode buffer were incubated for 30 minutes at room temperature (RT). When indicated, inhibiting agents were added before platelet activation. When using washed platelets, fibrinogen (0.1-5 mg/mL) was added to the platelet suspensions as indicated. The reaction was stopped by adding 10 vol of 0.2% formaldehyde saline, and a 1:10 dilution was analyzed with an Epics XL-MCL flow cytometer (Becton Dickinson, Miami, FL). Data are presented as the mean value of fluorescence intensity (MFI) (5000-10 000 cells/assay).
αIIbβ3-expressing CHO cells were washed by centrifugation and resuspended in aliquots of 0.5 × 106 cells/mL in XL medium (137 mM NaCl, 5 mM KCl, 1 mM MgCl2, 5 mM HEPES, and 0.01% [wt/vol] glucose [pH 7.4]) supplemented with or without 10 mM DTT for 20 minutes at RT. After incubation, cells were washed twice with an excess of XL medium, and either 5 μg/mL PAC-1–FITC or 40 μg/mL fibrinogen-FITC was added for 30 minutes at RT. In other experiments, cells were stimulated with 10 mM DTT, preincubated with 40 μg/mL of unlabeled fibrinogen for 30 minutes at RT, and then incubated with 10 μg/mL IAC-1–FITC for 30 minutes at RT. After washing, cells were finally resuspended in 400 μL of PBS supplemented with 7-AAD (diluted 1:400; Molecular Probes). Cells were immediately analyzed by flow cytometry. Where indicated, CHO cells expressing α2β1 were used for flow cytometric experiments in a similar manner.
Platelet adhesion under static conditions
Coverslips (18 × 18 mm; Menzel-Glaser, Braunschweig, Germany) were coated with human collagen type III (25 μg/mL in PBS) overnight at 4°C. Coverslips were blocked with 1% BSA and 0.1% glucose in HEPES/Tyrode buffer for 30 minutes at RT. Washed platelets were used at a concentration of 3 × 105/μL in HEPES/Tyrode buffer supplemented with 1 mM MgCl2 and were preincubated for 30 minutes in the presence or absence of RGDS (1 mM final concentration). Coverslips were washed twice with HEPES/Tyrode buffer before incubation with 300 μL of platelet suspension for 60 minutes at RT. Coverslips were washed to remove unbound platelets and stained with IAC-1 and phalloidin–Texas Red as previously described.26 In parallel, coverslips were fixed with 0.5% glutaraldehyde and stained with May-Grünwald-Giemsa. Coverslips were examined using a 40 ×/0.75 numerical aperture (NA) oil objective with an inverted Nikon Eclipse TE200 microscope using standard emission and excitation filters (Nikon, Melville, NY). Platelet adhesion was quantified with a light microscope, at 400 × magnification, connected to a Lucia Image Analyser (Namur, Belgium), and expressed as the percentage of surface coverage. For each experiment, the mean ± SE of 10 images was determined.
Platelet adhesion under flow
Thrombus formation was measured under flow with blood that was perfused over a collagen surface as described before.31,32 Briefly, an area of 20 × 5 mm in the center of a glass coverslip was coated with fibrillar Horm type I collagen (200 μg/mL) for 20 minutes at 37°C. Platelet interaction with immobilized collagen was studied using a parallel plate flow chamber producing a wall shear rate of 1000 s−1. Blood from healthy volunteers was collected on 40 μM d-phenylalanyl-l-propyl-l-arginine chloromethyl ketone (PPACK; Calbiochem) and perfused for 4 minutes. After perfusion, the coverslips were rinsed with HEPES/Tyrode buffer supplemented with 0.1% glucose, 0.1% BSA, 2 mM CaCl2, and 1 U/mL heparin, containing IAC-1–FITC (10 μg/mL), MOPC-21–FITC (5 μg/mL) or Gi9-FITC (5 μg/mL). Adhered platelets were visualized by phase-contrast and fluorescence microscopy using a 40 ×/1.3 NA oil objective with an inverted Nikon Diaphot 200 microscope (Nikon, Tokyo, Japan). The images were analyzed for surface area coverage exactly as previously described, using ImagePro software version 4.1 (Media Cybernetics, Silver Spring, MD).32
Results
Activation of αIIbβ3 is sufficient to activate α2β1
Binding of fibrinogen to αIIbβ3 influences α2β1 activation.
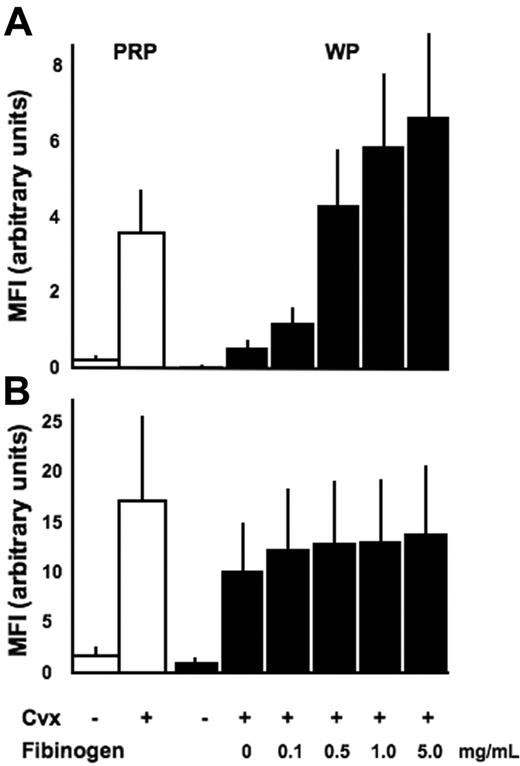
As previously demonstrated,25 stimulation of platelets in plasma with the strong platelet GPVI agonist CVX induces an activated conformation of α2β1 that can be readily recognized by the mAb IAC-1 (Figure 1A). However, an interesting observation was made when similar activation experiments were performed with washed platelets.
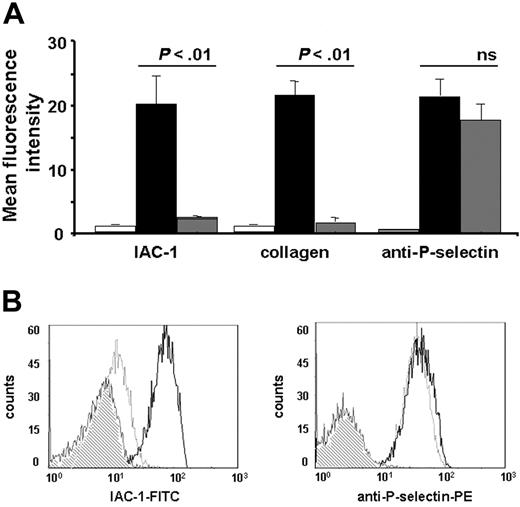
IAC-1 binding to α2β1 on activated washed platelets is decreased compared with platelets activated in plasma and increased by binding of fibrinogen to αIIbβ3. Flow cytometric analysis of IAC-1 (A) or anti–P-selectin (B) binding to resting or CVX-stimulated platelets in plasma (PRP; □) or in buffer in the presence of increasing concentrations of fibrinogen (WP; ▪). All data are expressed as the mean ± SEM of at least 3 independent experiments. In all experiments, saturating concentrations of fluorescent antibodies were used.
IAC-1 binding to α2β1 on activated washed platelets is decreased compared with platelets activated in plasma and increased by binding of fibrinogen to αIIbβ3. Flow cytometric analysis of IAC-1 (A) or anti–P-selectin (B) binding to resting or CVX-stimulated platelets in plasma (PRP; □) or in buffer in the presence of increasing concentrations of fibrinogen (WP; ▪). All data are expressed as the mean ± SEM of at least 3 independent experiments. In all experiments, saturating concentrations of fluorescent antibodies were used.
In contrast to CVX-stimulated platelets present in plasma, IAC-1 bound only moderately to α2β1 present on CVX-stimulated washed platelets. This difference was not donor dependent and was statistically significant (P < .01, n = 3; Figure 1A). The presence of plasma was not responsible for a different degree of platelet activation, since CVX stimulation resulted in either case in a similar P-selectin exposure, indicating equal platelet granule secretion (Figure 1B). In addition, the binding of the control anti-α2 I-domain mAb Gi9 was not notably altered (data not shown), proving (1) that the level of surface exposure of α2β1 was not different in both conditions and (2) that the increased IAC-1 binding to platelets in plasma is most likely not due to trapping of the antibody by microaggregate formation. All experiments were carried out at the optimal concentration of fluorescent IAC-1 antibody and maximal doses of agonist, and similar results were seen when platelets were stimulated with lower agonist doses (data not shown). Similar, but less strong activating effects on IAC-1 binding were seen when platelets were stimulated with ADP (data not shown).
The responsible driving force for the higher binding of IAC-1 to activated platelets in plasma compared with washed platelets was next investigated. In view of the known networking between integrins, we hypothesized that fibrinogen binding to αIIbβ3 on activated platelets could be required for optimal IAC-1 binding to α2β1 and, thus, optimal activation of this integrin. Addition of fibrinogen to CVX-stimulated washed platelets indeed dose-dependently increased α2β1 activation (Figure 1A) with higher levels of exogenous fibrinogen (1 to 5 mg/mL), resulting in even more IAC-1 binding than what was seen in plasma. In contrast, fibrinogen did not influence the binding of the control anti-α2β1 mAb Gi9 (not shown) or the exposure of P-selectin (P > .5, n = 3; Figure 1B), indicating that the increase in α2β1 activation is at least in part a consequence of fibrinogen binding to αIIbβ3.
Another explanation for the increased IAC-1 binding in the presence of fibrinogen could be that this mAb in addition would recognize an epitope on αIIbβ3-bound fibrinogen on platelets, since it has been described previously that anti-α2 mAbs may cross-react with RGD-dependent epitopes in fibrinogen.33 To analyze this, CHO cells expressing human αIIbβ3 were treated with DTT to bring this integrin in an activated conformation,34,35 after which purified fibrinogen was added and IAC-1 binding was determined. The complex fibrinogen bound to the activated αIIbβ3 on CHO cells failed to bind IAC-1, demonstrating that bound fibrinogen and ligand-occupied, activated αIIbβ3 do not contain an epitope recognized by IAC-1 (data not shown).
A conformational change in αIIbβ3 induces a direct signaling to α2β1.
The results thus far suggest that αIIbβ3 activation evoked by stimulation of platelet receptors such as GPVI leads to subsequent activation of α2β1 via an outside-in signaling mechanism of αIIbβ3.
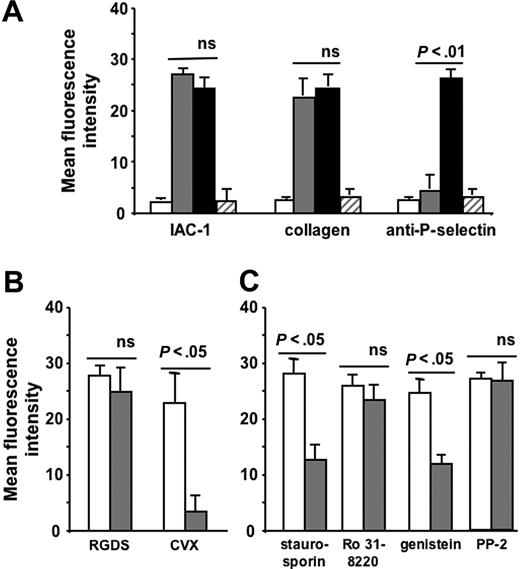
Next, we investigated whether a conformational change in αIIbβ3 per se could influence the activation state of α2β1 independent of other platelet agonists. Therefore, we incubated platelets with the small peptide RGDS, which is known to bind to αIIbβ3 on nonactivated platelets and to provoke conformational changes in both the αIIb and β3 subunits.36-38 Remarkably, the addition of 1 mM RGDS peptide to washed platelets stimulated the binding of fluorescent IAC-1 and collagen to a comparable degree as stimulation in the presence of the strong platelet agonist CVX and fibrinogen (P < .005, n = 6; Figure 2A). In this case, the α2β1 activation was not accompanied by P-selectin exposure, indicating that RGDS did not cause an overall platelet activation with secretion responses (Figure 2A). To demonstrate that RGDS has no direct effect on activation of α2β1, CHO cells expressing human α2β1 were incubated with 1 mM RGDS, and IAC-1 binding was determined. These RGDS-triggered CHO cells failed to bind IAC-1, confirming that α2β1 is not an RGD-sensitive integrin (data not shown). In contrast, IAC-1–binding to these α2β1-expressing CHO cells was readily achieved by stimulation with a cell-permeable α2-cytosolic peptide, as recently described.26 Similar experiments with the peptide-mimetic antagonist aggrastat, also known to bind αIIbβ3 on nonactivated platelets and to induce a conformational change in the αIIb, but not the β3 subunit of αIIbβ3,39 did not result in IAC-1 binding, collagen binding, or P-selectin exposure (Figure 2A). Binding of RGDS and aggrastat to αIIbβ3 was specific since binding of PAC-1, which competes for the same binding site on αIIbβ3,40 was almost completely abolished (data not shown).
Increased IAC-1 binding to α2β1 on RGDS-treated washed platelets and involvement of tyrosine kinases and actin in this signaling. (A) Flow cytometric experiments detecting IAC-1, collagen, and anti–P-selectin binding to resting washed platelets (□), to RGDS-treated washed platelets (⊡), and to CVX-stimulated washed platelets in the presence of 100μg/mL fibrinogen (▪) or to aggrastat-treated washed platelets (▨). (B) Flow cytometric experiments of IAC-1 binding to RGDS- and CVX-stimulated platelets in the absence (□) or presence (⊡) of PGE1 (5 μM). (C) Flow cytometric experiments of IAC-1 binding to RGDS-triggered platelets incubated with 5 μM PGE1 in the absence (□) or presence (⊡) of staurosporin (2 μM), Ro 31-8220 (10 μM), genistein (50 μg/mL), or PP2 (10 μM). All data are expressed as the mean ± SEM of at least 3 independent experiments.
Increased IAC-1 binding to α2β1 on RGDS-treated washed platelets and involvement of tyrosine kinases and actin in this signaling. (A) Flow cytometric experiments detecting IAC-1, collagen, and anti–P-selectin binding to resting washed platelets (□), to RGDS-treated washed platelets (⊡), and to CVX-stimulated washed platelets in the presence of 100μg/mL fibrinogen (▪) or to aggrastat-treated washed platelets (▨). (B) Flow cytometric experiments of IAC-1 binding to RGDS- and CVX-stimulated platelets in the absence (□) or presence (⊡) of PGE1 (5 μM). (C) Flow cytometric experiments of IAC-1 binding to RGDS-triggered platelets incubated with 5 μM PGE1 in the absence (□) or presence (⊡) of staurosporin (2 μM), Ro 31-8220 (10 μM), genistein (50 μg/mL), or PP2 (10 μM). All data are expressed as the mean ± SEM of at least 3 independent experiments.
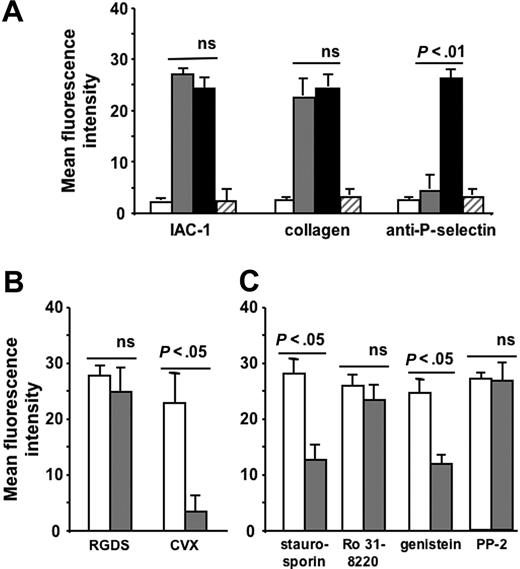
To investigate whether the cellular pathway responsible for the observed signaling from αIIbβ3 to α2β1 is similar to the pathways described for integrin signaling, washed platelets were incubated with RGDS and a chemical inhibitor of signaling pathways. Since incubation of the RGDS-treated platelets with PGE1 (5 μM final concentration), which elevates cAMP and thereby antagonizes many platelet processes, did not influence the degree of RGDS-induced α2β1 activation (unaltered IAC-1 binding; P > .5, n = 4; Figure 2B), all further experiments were performed in the presence of PGE1 to exclude additional general activation effects. The proper working of PGE1 was demonstrated by adding PGE1 to CVX-stimulated platelets, which resulted in a significant decrease of IAC-1 binding (P < .5, n = 3; Figure 2B). The IAC-1 binding on RGDS-triggered washed platelets was significantly reduced by staurosporin (2 μM), a broad-spectrum inhibitor of serine-threonine protein kinases, and was also able to inhibit tyrosine phosphorylation. Addition of the general tyrosine kinase inhibitor genistein (50 μg/mL) also decreased IAC-1 binding (P < .05, n = 3; Figure 2C) in contrast to the protein kinase C inhibitor Ro 31-8220 (10 μM; P > .5, n = 4; Figure 2C). The specific Src kinase inhibitor PP2 (10 μM), however, did not influence IAC-1 binding to RGDS-triggered platelets (P > .5, n = 3; Figure 2C). Using another Src kinase inhibitor, PP1 (10 μM), gave similar results (data not shown). All inhibitors used were active at their respective concentration, as determined by a significant decrease in PAC-1 binding of ADP-stimulated platelets (data not shown).
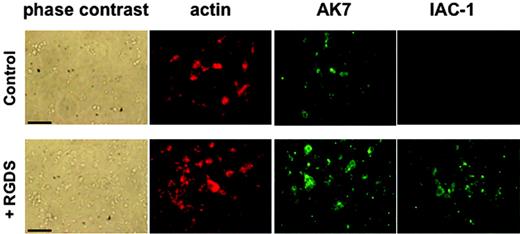
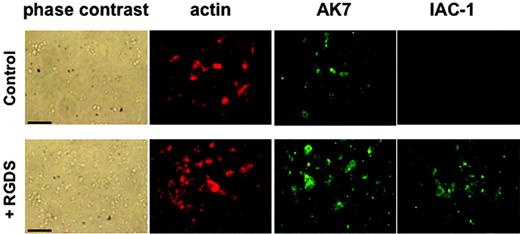
RGDS was also able to induce IAC-1 binding under static adhesion conditions. Washed platelets in the presence of MgCl2 that were added to a collagen type III surface did adhere, but this did not result in visually detectable levels of IAC-1 binding, indicating absence of major α2β1 activation on the adhered platelets in the absence of fibrinogen (Figure 3). However, preincubation with RGDS for 30 minutes resulted in (1) a significant increase in number of adhered platelets to the collagen, and (2) a clearly positive IAC-1 staining of the adhered platelets (Figure 3). The control anti-α2 mAb AK7 gave similar fluorescence signals of the platelets, independent of RGDS addition (Figure 3A). The adhesion of platelets to coated collagen was α2β1 specific, since addition of the anti-α2 mAb 15D7 almost completely abolished platelet adhesion (data not shown). All together, these results demonstrate that activation of αIIbβ3 induced by a conformational change can directly activate α2β1, resulting in collagen binding of platelets in suspension and more binding to collagen of platelets in adhesion.
IAC-1 binding to α2β1 on RGDS-treated washed platelets under static adhesion conditions. Unstimulated or RGDS-treated washed platelets were allowed to adhere to immobilized collagen type III–coated coverslips for 60 minutes. After fixation and permeabilization, cells were incubated with phalloidin–Texas Red (200 nM) to stain actin and AK-7 (5 μg/mL), followed by a 1:50 dilution of rabbit anti–mouse IgG–FITC, to stain α2β1 or IAC-1–FITC (10 μg/mL) to stain activated α2β1. Scale bars indicate 25 μm.
IAC-1 binding to α2β1 on RGDS-treated washed platelets under static adhesion conditions. Unstimulated or RGDS-treated washed platelets were allowed to adhere to immobilized collagen type III–coated coverslips for 60 minutes. After fixation and permeabilization, cells were incubated with phalloidin–Texas Red (200 nM) to stain actin and AK-7 (5 μg/mL), followed by a 1:50 dilution of rabbit anti–mouse IgG–FITC, to stain α2β1 or IAC-1–FITC (10 μg/mL) to stain activated α2β1. Scale bars indicate 25 μm.
Activation of αIIbβ3 is a prerequisite to activate α2β1
Inhibition of fibrinogen binding to αIIbβ3 influences α2β1 activation in suspension.
To further substantiate the importance of αIIbβ3 activation for proper activation of α2β1, experiments were performed with platelets in plasma in the presence of aggrastat, an αIIbβ3 antagonist that blocks the binding of fibrinogen.
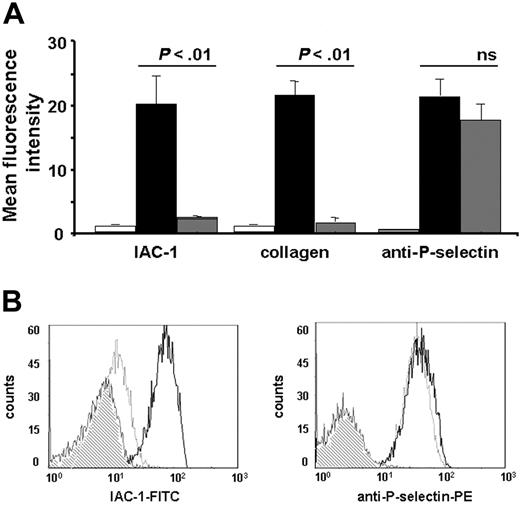
Addition of aggrastat (1 μg/mL) greatly reduced the binding of IAC-1 to CVX-stimulated platelets and thus α2β1 activation by more than 90% (P < .01, n = 4; Figure 4A). In addition, aggrastat also inhibited the binding of α2β1 on platelets to fluorescent monomeric collagen (Figure 4A), confirming the importance of fibrinogen binding to activated αIIbβ3 for the adhesive properties of α2β1. The reduced IAC-1 binding with aggrastat was not due to a decreased platelet activation, as the exposure of P-selectin was not significantly altered in the presence of the αIIbβ3 blocker (P > .5, n = 4; Figure 4A). Similar results were obtained with abciximab (Reopro; Eli Lilly, Brussels, Belgium), another αIIbβ3 antagonist. As a control, no binding of abciximab to human α2β1-expressing CHO cells could be observed by flow cytometry (not shown), excluding the possibility of a direct blocking of α2β1 by αIIbβ3 antagonists. Similar results at low platelet concentrations further excluded the possibility that formation of (micro-) aggregates under nonstirring conditions interfered with IAC-1 binding (data not shown). Interestingly, the reduction in IAC-1 binding to α2β1 on CVX-stimulated platelets in plasma where αIIbβ3 is blocked by aggrastat (Figure 4A) was notably lower than the observed IAC-1 binding to α2β1 on CVX-stimulated washed platelets (Figure 1A). This suggests that autocrine fibrinogen, present in platelet α-granules (about 1 mg fibrinogen/1010 platelets) and released upon platelet activation,41 contributes to αIIbβ3-mediated IAC-1 binding to washed platelets.
Inhibition of fibrinogen binding to αIIbβ3 reduces IAC-1 binding to α2β1 on CVX-stimulated platelets in plasma. (A) Flow cytometric experiments with FITC-labeled IAC-1, FITC-labeled monomeric collagen, and PE-labeled anti–P-selectin, binding to resting platelets (□) or to CVX-stimulated platelets in the absence (▪) or presence (⊡) of 1 μg/mL aggrastat. Data are expressed as the mean ± SEM of at least 3 independent experiments. (B) Representative tracings of the experiments depicted in panel A with the binding of FITC-labeled IAC-1 (left panel) and PE-labeled anti–P-selectin (right panel) to resting platelets (⊡) or to CVX-stimulated platelets in the absence (black line) or presence (gray line) of aggrastat. In all experiments, saturating concentrations of fluorescent antibodies were used.
Inhibition of fibrinogen binding to αIIbβ3 reduces IAC-1 binding to α2β1 on CVX-stimulated platelets in plasma. (A) Flow cytometric experiments with FITC-labeled IAC-1, FITC-labeled monomeric collagen, and PE-labeled anti–P-selectin, binding to resting platelets (□) or to CVX-stimulated platelets in the absence (▪) or presence (⊡) of 1 μg/mL aggrastat. Data are expressed as the mean ± SEM of at least 3 independent experiments. (B) Representative tracings of the experiments depicted in panel A with the binding of FITC-labeled IAC-1 (left panel) and PE-labeled anti–P-selectin (right panel) to resting platelets (⊡) or to CVX-stimulated platelets in the absence (black line) or presence (gray line) of aggrastat. In all experiments, saturating concentrations of fluorescent antibodies were used.
Inhibition of fibrinogen binding to αIIbβ3 influences α2β1 activation under flow conditions.
The necessity of αIIbβ3 activation for the activation state of α2β1 was also studied under dynamic flow conditions, representing a more physiologic condition of platelet activation during thrombus formation.42 The αIIbβ3 antagonist aggrastat was again used to inhibit fibrinogen binding to platelet αIIbβ3 in whole blood.
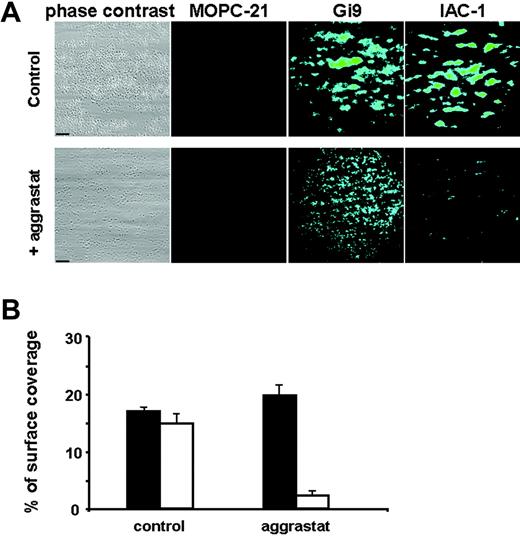
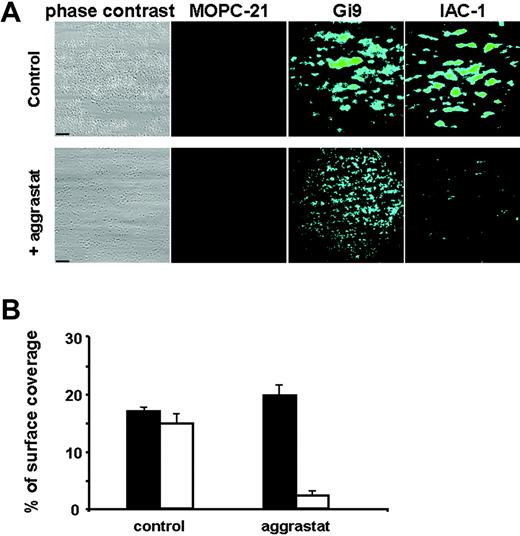
Perfusion of the blood over a collagen surface at 1000 s−1 resulted in platelet adhesion and aggregate formation, with a surface coverage of 18.0% ± 2.2% after 4 minutes of perfusion, as measured by phase-contrast microscopy (Figure 5A). These aggregates stained positive for IAC-1 binding, with a surface coverage measured by fluorescence microscopy of 16.4% ± 5.5%. Treatment with the αIIbβ3 antagonist aggrastat completely inhibited aggregate formation, although still many single platelets adhered, diffusely spread over the collagen surface (Figure 5A). IAC-1 binding to these platelets was significantly decreased by more than 80% (P < .001, n = 5; Figure 5B). Staining of the adherent platelets with the unrelated mouse anti–human IgG MOPC-21 did not result in a fluorescence signal. However, staining with the anti-α2 I-domain mAb Gi9 resulted in fluorescent staining of the platelets, regardless of the presence or absence of aggrastat, indicating that the detection system used was sensitive enough to visualize single platelets (Figure 5A). These experiments under flow conditions also demonstrate that inhibition of ligand binding to αIIbβ3 prevented activation of α2β1.
Inhibition of fibrinogen binding to αIIbβ3 decreases IAC-1 binding to adhering platelets under flow conditions. Anticoagulated whole human blood was perfused over a collagen-coated surface at a wall shear rate of 1000 s−1. Postperfusion was performed with either FITC-labeled MOPC-21 (5 μg/mL), FITC-labeled Gi9 (5 μg/mL), or FITC-labeled IAC-1 (10 μg/mL). (A) Representative phase-contrast (left column) and fluorescence (right columns) microscopic images of adhered platelets after 4 minutes of perfusion in the presence (bottom row) or absence (top row) of aggrastat. Scale bars indicate 25 μm. (B) Platelet adhesion in the presence or absence of aggrastat, determined as a percentage of surface coverage. Data obtained from phase-contrast images are in black; fluorescence data of IAC-1–FITC images are in white. Data are expressed as the mean ± SE of 10 images of at least 3 independent experiments.
Inhibition of fibrinogen binding to αIIbβ3 decreases IAC-1 binding to adhering platelets under flow conditions. Anticoagulated whole human blood was perfused over a collagen-coated surface at a wall shear rate of 1000 s−1. Postperfusion was performed with either FITC-labeled MOPC-21 (5 μg/mL), FITC-labeled Gi9 (5 μg/mL), or FITC-labeled IAC-1 (10 μg/mL). (A) Representative phase-contrast (left column) and fluorescence (right columns) microscopic images of adhered platelets after 4 minutes of perfusion in the presence (bottom row) or absence (top row) of aggrastat. Scale bars indicate 25 μm. (B) Platelet adhesion in the presence or absence of aggrastat, determined as a percentage of surface coverage. Data obtained from phase-contrast images are in black; fluorescence data of IAC-1–FITC images are in white. Data are expressed as the mean ± SE of 10 images of at least 3 independent experiments.
Absence of αIIbβ3 does not result in α2β1 activation.
Finally, we wanted to prove that activation of αIIbβ3 is a prerequisite for proper activation of α2β1, and therefore we stimulated PRP obtained from a patient with type I Glanzmann thrombasthenia, with no expression of αIIbβ3 on the cell surface,29 and determined IAC-1 and collagen binding.
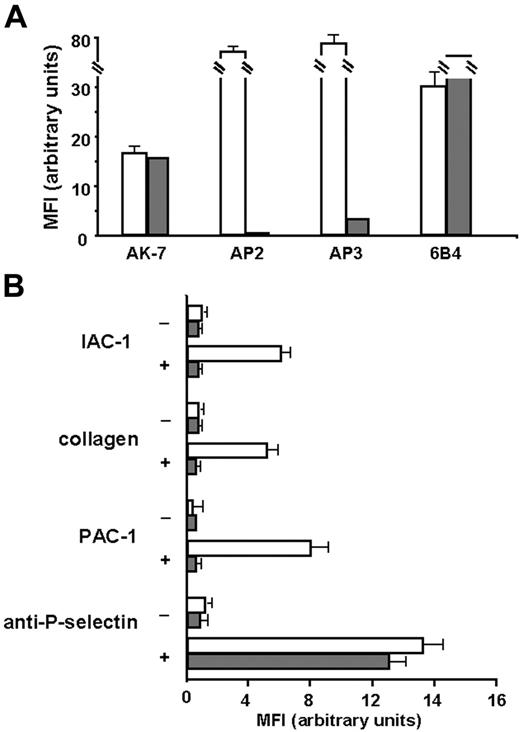
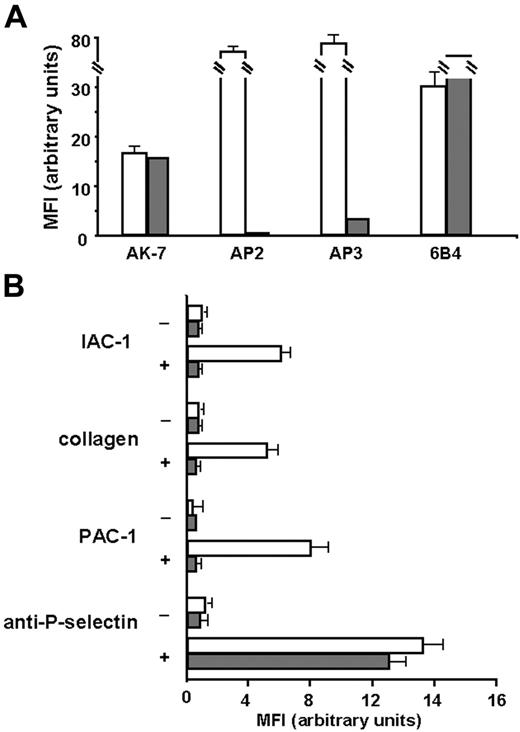
First, we checked for α2β1 expression, and as shown in Figure 6A, α2β1 was expressed in similar amounts on platelets of the patient with Glanzmann thrombasthenia compared with those of the control. The absence of binding of the anti-αIIbβ3 mAb AP2 confirmed the Glanzmann phenotype, and the residual binding of the anti-β3 mAb AP3 has been described previously for this patient, since these platelets normally express the vitronectin receptor αVβ3.29,43 When the Glanzmann platelets were stimulated with CVX, no IAC-1 binding nor collagen binding could be observed, in contrast to control platelets (Figure 6B). Stimulation of the Glanzmann platelets did not result in PAC-1 binding as expected, and general platelet activation, detected by P-selectin exposure, was comparable with that of control platelets (Figure 6B). Similar results were observed after stimulation with ADP and with platelets from another patient with Glanzmann thrombasthenia (data not shown). These experiments with Glanzmann platelets demonstrate that proper α2β1 activation on platelets cannot occur in the absence of αIIbβ3, and therefore reveal the absolute requirement of αIIbβ3 activation for activation of α2β1.
IAC-1 binding to α2β1 on CVX-stimulated platelets in plasma is not observed in the absence of αIIbβ3. (A) Flow cytometric analysis of membrane glycoprotein expression of the Glanzmann platelets. Control platelets in plasma (□) or platelets from a patient with Glanzmann platelets in plasma (⊡) were incubated with AK-7 (anti-α2; 2 μg/mL), AP2 (anti-αIIbβ3; 5 μg/mL), AP3 (anti-β3; 5 μg/mL), or 6B4 (anti-GPIb; 2 μg/mL), followed by a 1:10 dilution of rabbit anti–mouse IgG–FITC. (B) Flow cytometric experiments detecting IAC-1, collagen, PAC-1, and anti–P-selectin binding to resting platelets (−) or CVX-stimulated platelets (+) of control (□) or Glanzmann patient (⊡). Data are expressed as the mean ± SEM of at least 2 independent experiments on control platelets and 1 or 2 experiments on Glanzmann platelets.
IAC-1 binding to α2β1 on CVX-stimulated platelets in plasma is not observed in the absence of αIIbβ3. (A) Flow cytometric analysis of membrane glycoprotein expression of the Glanzmann platelets. Control platelets in plasma (□) or platelets from a patient with Glanzmann platelets in plasma (⊡) were incubated with AK-7 (anti-α2; 2 μg/mL), AP2 (anti-αIIbβ3; 5 μg/mL), AP3 (anti-β3; 5 μg/mL), or 6B4 (anti-GPIb; 2 μg/mL), followed by a 1:10 dilution of rabbit anti–mouse IgG–FITC. (B) Flow cytometric experiments detecting IAC-1, collagen, PAC-1, and anti–P-selectin binding to resting platelets (−) or CVX-stimulated platelets (+) of control (□) or Glanzmann patient (⊡). Data are expressed as the mean ± SEM of at least 2 independent experiments on control platelets and 1 or 2 experiments on Glanzmann platelets.
Discussion
We previously characterized the mAb IAC-1 as an antibody specific for the α2 I-domain on fully activated integrin α2β1.25,26 Here we have used this novel antibody to demonstrate that ligand binding to integrin αIIbβ3 is a key regulator of α2β1 activation. First, a significant reduction in IAC-1 binding to α2β1 was observed when working with washed stimulated platelets, where plasma proteins like fibrinogen are absent. Second, this low IAC-1 binding to washed stimulated platelets could be restored upon supplementation with purified fibrinogen. Third, blockage of the fibrinogen binding to αIIbβ3 by aggrastat dramatically decreased IAC-1 binding to α2β1, both under static and flow conditions. Fourth, addition of the αIIbβ3-specific peptide RGDS induced IAC-1 binding to α2β1 on resting platelets to a comparable degree to that of stimulation of platelets with CVX in the presence of fibrinogen, but without general platelet activation. Fifth, no IAC-1 binding to α2β1 on Glanzmann platelets with no αIIbβ3 expression on the surface was observed upon platelet stimulation. All of these data suggest that stimulation of αIIbβ3 is indispensable for the activation of the collagen-binding integrin α2β1.
Activation of αIIbβ3 upon cellular activation (inside-out signaling) or by binding to its ligand fibrinogen induces a conformational change in αIIbβ3 and leads to outside-in signaling, resulting in tyrosine kinase activity, Ca2+ responses, cytoskeletal reorganization, and pseudopod formation.3,5,44-46 In addition, fibrinogen causes αIIbβ3 clustering, which also triggers signaling pathways, most likely by concentrating intracellular integrin-associated proteins.47,48 The answers to the questions of how and if monovalent αIIbβ3 ligands like RGDS induce outside-in signaling of αIIbβ3 has remained elusive. One study reported that in platelets, monovalent peptidomimetic αIIbβ3 antagonists could not increase intracellular Ca2+ concentrations used as a marker for outside-in signaling, although they induced LIBS epitopes.39 Here, platelet experiments with the monovalent αIIbβ3 ligand RGDS resulted in a significant IAC-1 binding, indicating that RGDS can mediate outside-in signaling of αIIbβ3, which results in activation of α2β1. Moreover, the IAC-1 binding induced upon RGDS addition was similar in degree to that seen in platelets stimulated with the strong agonist CVX and fibrinogen, but clearly without evoking overall platelet activation, as is known for RGDS. Similar experiments with another monovalent ligand, the αIIbβ3 antagonist aggrastat, did not result in IAC-1 binding. This is similar to the study of Honda et al,39 where aggrastat was not able to increase intracellular Ca2+ concentrations in nonactivated platelets. Since RGDS induces conformational changes in both the αIIb and β3 subunits, whereas aggrastat results only in a conformational change of the αIIb subunit,36,39 our results point out that a conformational change in the β3 subunit, but not in the αIIb subunit, is a prerequisite for αIIbβ3-mediated outside-in signaling. This is consistent with previous reports, where it has been described that the cytoplasmic domain of the β3 subunit plays a critical role in αIIbβ3-mediated outside-in signaling.49-51
When washed platelets were allowed to adhere to coated collagen type III under static conditions, some adhesion was observed, but this was not accompanied by fluorescent IAC-1 staining, or, in other words, α2β1 activation. This α2β1-dependent cell adhesion to coated collagen without IAC-1 binding has already been described for α2β1-expressing CHO cells under static conditions and for platelet adhesion in vitro.24,26 Here, treatment of the platelets with RGDS resulted in significantly more adhesion of the platelets to collagen, and this was accompanied by IAC-1 binding. This is also similar to our previous data, where direct inside-out stimulation of α2β1 by using a cell-permeable α2-cytosolic peptide resulted both in enhanced adhesion and IAC-1 binding.26
The observed direct signaling from αIIbβ3 to α2β1 upon RGDS addition involves tyrosine kinase activity, as shown by the sensitivity to genistein; however, we were not able to find evidence for the involvement of the major integrin-signaling tyrosine kinase Src. Also, we could not observe a contribution from protein kinase C, but this has already been described for platelets where both αIIbβ3 and α2β1 activation can occur via PKC-dependent and -independent cAMP-insensitive pathways, depending on the agonist used.52,53 Hence, for the moment it remains elusive whether the signaling from αIIbβ3 to α2β1 will be similar to previously described integrin-signaling pathways,5,46,54 or whether this signaling occurs via another, yet undescribed, pathway.
Linking the observation that ligand binding to αIIbβ3 results in α2β1 activation with the observation that blockage of αIIbβ3 activation can almost completely abolish IAC-1 binding resulted in the hypothesis that αIIbβ3 is indispensable for the activation of α2β1. To test this, platelets from patients with type I Glanzmann thrombasthenia, without any expression of αIIbβ3 on the cell surface,29 were isolated and stimulated with platelet agonists such as CVX and ADP. Stimulation of these platelets did not result in IAC-1 or collagen binding to α2β1 in contrast to wild-type platelets. This confirms that αIIbβ3 activation is essential for proper activation of α2β1.
Lecut et al24 demonstrated that the collagen receptor GPVI, along with autocrine ADP and thromboxane, mediates α2β1 and αIIbβ3 integrin activation during collagen-induced thrombus formation under flow. Under control conditions of a moderate high shear of 1000s−1, both PAC-1 (for activated αIIbβ3) and IAC-1 (for activated α2β1) binding was observed, whereas blockade of the platelet receptor GPVI reduced PAC-1 binding and thus αIIbβ3 activation. This reduction was accompanied by a reduced IAC-1 binding, indicating that the activation of α2β1 was also severely impaired.24 The observations in the present study now suggest for the first time that the affinity regulation of α2β1 and αIIbβ3 by GPVI during thrombus formation does not occur independently, but rather in a 2-step model: first, stimulation of GPVI activates the integrin αIIbβ3; second, ligand binding to the activated αIIbβ3 will in turn activate the integrin α2β1. Although the physiologic importance of this 2-step model of integrin activation during human platelet thrombus formation in vivo is not yet known, we hypothesize that the 2 collagen receptors GPVI and α2β1, the latter in an inactive or intermediate active conformation, are necessary for platelets to bind to exposed immobilized collagen upon vessel wall damage. GPVI will next activate αIIbβ3, which will result in platelet aggregation on the one hand, and activation of α2β1 to its fully activated conformation on the other hand. This fully activated conformation of α2β1 may then reinforce the adhesion of the adhered platelets, which is in line with recent data showing that the fully active conformation of α2β1 results in significantly more adhesion.26 Moreover, in parallel with resting αIIbβ3 that is only able to bind to immobilized fibrinogen but which requires activation to bind to soluble fibrinogen, resting α2β1 also can interact only with its immobilized ligand and not with the soluble form. Because, however, no soluble collagen is expected to be present physiologically, our results imply that the role of α2β1 activation is to allow for faster platelet adherence and for platelet spreading: indeed, activation of α2β1 on CHO cells allowed them to spread,26 whereas it is known that Glanzmann platelets attach but do not spread on collagen.55
In summary, we here demonstrate for the first time that activation of αIIbβ3 is both a sufficient and required condition for full activation of α2β1 on platelets, a process which may have physiologic importance during platelet thrombus formation in vivo.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Conflict-of-interest disclosure: the authors declare no competing financial interests.
Contribution: G.R.V.d.W., A.S., J.M.E.M.C., B.F.I., and K.V. participated in performing the research; G.R.V.d.W., A.S., K.V., J.W.M.H., M.F.H., and H.D. participated in designing the research, providing relevant apparatus, and in writing the paper; A.N. helped in the study of the Glanzmann patients; and all authors checked the final version of the manuscript.
Acknowledgments
The authors are grateful to Dr N. Kieffer (Laboratoire de Biologie et Physiologie Intégrée, Université du Luxembourg, Luxembourg) for the αIIbβ3- and α2β1-expressing CHO cells, and to Dr Paquita Nurden for obtaining the blood from the patients with Glanzmann thrombasthenia. We also thank R. Combrié for analyzing the data on the Glanzmann platelets.
This work was supported by a Concerted Research Grant (GOA) from the University of Leuven (GOA/2004/09). K.V. is a postdoctoral fellow of the Fonds voor Wetenschappelijk Onderzoek (FWO) Vlaanderen, Belgium.