Abstract
It is widely accepted that glycoprotein (GP) Ib contains one Ibα and one Ibβ subunit that are connected by a disulfide bond. It is unclear which Cys residue in Ibα, C484 or C485, forms the disulfide bond with Ibβ. Using mutagenesis studies in transfected Chinese hamster ovary (CHO) cells, we found that both C484 and C485 formed a disulfide bond with C122 in Ibβ. In the context of isolated peptides containing the Ibα or Ibβ transmembrane domain and nearby Cys residue, C484 and C485 in the Ibα peptide were both capable of forming a disulfide bond with the Ibβ peptide. Furthermore, coimmunoprecipitation of epitope-tagged subunits showed that at least 2 Ibβ subunits but only 1 Ibα and 1 IX subunit were present in the GP Ib-IX complex. Finally, the size difference between GP Ib from transfected CHO cells and human platelets was attributed to a combination of sequence polymorphism and glycosylation difference in Ibα, not the number of Ibβ subunits therein. Overall, these results demonstrate that Ibα is covalently connected to 2 Ibβ subunits in the resting platelet, necessitating revision of the subunit stoichiometry of the GP Ib-IX-V complex. The αβ2 composition in GP Ib may provide the basis for possible disulfide rearrangement in the receptor complex.
Introduction
Platelet glycoprotein (GP) Ib-IX-V complex binds to von Willebrand factor (VWF) deposited at the injured blood vessel wall, mediating initial platelet rolling and adhesion to the injury site. Ligation of VWF to the GP Ibα subunit in the complex also transmits a signal to the platelet that leads to platelet activation and aggregation.1,2 In addition to mediating outside-in signals, the GP Ib-IX-V complex is also capable of transmitting intracellular signals to the outside.3-7 How this receptor complex mediates signaling in both directions is not clear, but recent work has suggested the likely interaction between the Ibα and Ibβ subunits as a potential key element of the mechanism.8-10 Thus, understanding the interaction between GP Ibα and GP Ibβ, and in general the organizing principle of the GP Ib-IX-V complex, will help to unveil the mechanism underlying transmembrane signaling mediated by the complex.
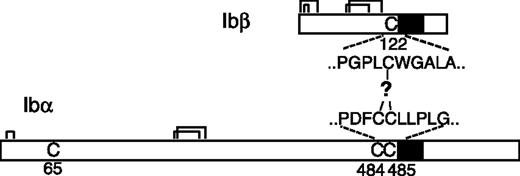
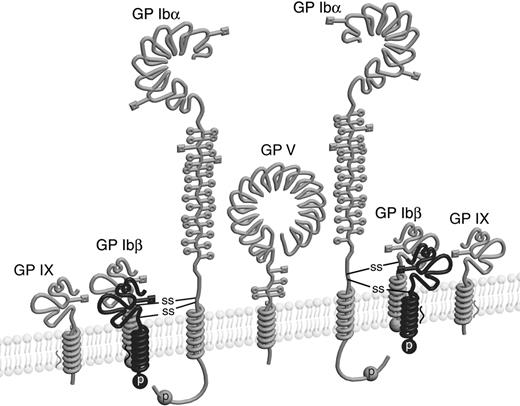
It is widely accepted that the GP Ib-IX-V complex contains 7 subunits composed of 4 different polypeptides: Ibα, Ibβ, IX, and V with a respective stoichiometry of 2:2:2:1.11-15 Each subunit is a type I transmembrane protein. A disulfide bond links GP Ibα and GP Ibβ, forming a complex known as GP Ib, that in turn interacts noncovalently with GP IX and GP V to generate the Ib-IX-V complex. The Ibα extracellular domain contains 9 Cys residues, 7 of which reside in the N-terminal domain that contains the binding site for VWF. The N-terminal domain contains 3 disulfides, C4-C17, C209-C248, and C211-C264.16 C65 is buried in the hydrophobic core of the N-terminal domain and is therefore unpaired.17,18 The other 2 Cys residues in GP Ibα, C484 and C485, are located near the transmembrane (TM) domain and are conserved across species. The Ibα/Ibβ ratio of 1:1 in the receptor complex predicts that only 1 of the 2 Cys residues forms a disulfide bond with the membrane-proximal C122 in GP Ibβ (Figure 1). However, it is not clear which one is paired with C122, and, more intriguingly, what role the free thiol group of the other Cys residue plays. It seems paradoxic that 2 neighboring Cys residues are exposed to presumably similar, if not the same, oxidizing environments, yet they have entirely different fates.
In the present study, we sought to assign the disulfide between GP Ibα and GP Ibβ. To our surprise, we found that both C484 and C485 are linked to GP Ibβ by disulfide bonds. In other words, contrary to the currently prevailing model, 1 Ibα subunit is linked through disulfide bonds to 2 Ibβ subunits.
Materials and methods
Vectors and antibodies
The vector pDX19,20 was used in the expression of GP Ib-IX complex in Chinese hamster ovary (CHO) cells. The CHO K1 cell line was obtained from ATCC (Manassas, VA). The expression vector pGEX-4T-3 was purchased from Amersham Biosciences (Piscataway, NJ). WM23, an anti-Ibα monoclonal antibody, was kindly provided by Dr M. Berndt. Other antibodies against individual subunits of GP Ib-IX complex, including FMC25, Gi27, SZ2, and AK2, were purchased from Axxora (San Diego, CA), Beckman Coulter (Fullerton, CA), Chemicon (Temecula, CA), or Santa Cruz Biotechnology (Santa Cruz, CA). An anti-myc antibody was purchased from Sigma (St Louis, MO), and an HRP-conjugated anti-HA antibody was from Roche (Indianapolis, IN).
Effect of Cys mutations on the Ibα-Ibβ disulfide in transfected CHO cells
Mutations of the target cysteine residue to serine were carried out in the pDX vectors containing the indicated cDNA19,20 using a Quikchange mutagenesis kit (Stratagene, La Jolla, CA). The mutant gene sequences were confirmed by DNA sequencing (SeqWright, Houston, TX).
Constructs containing the Ibα, Ibβ, and IX cDNAs were transiently cotransfected into CHO K1 cells as previously described.21 At 2 days after transfection, cells from each transfection (∼ 5 × 105 cells) were harvested and lysed in 60 μL cell-lysis buffer (1% Triton X-100, 5 mM CaCl2, 5 mM N-ethylmaleimide, 58 mM sodium borate, pH 8.0) containing 10% (vol/vol) protease inhibitor cocktail for mammalian tissues (Sigma). The supernatant from the cell lysate was mixed with standard Tris-glycine SDS sample buffer (pH 6.8) and resolved using a precast 4-12% Bis-Tris NuPAGE SDS gel with MOPS running buffer (Invitrogen, Carlsbad, CA) or, if necessary, a 5% Tris-glycine SDS gel with standard Tris-glycine running buffer. MagicMark protein standard (Invitrogen) was loaded on each gel as molecular weight markers. After electrophoresis, proteins were transferred to a PVDF membrane, immunoblotted with appropriate antibodies, and detected by chemiluminescence. In parallel, some transfected cells were stained using anti-Ibα or anti-IX antibodies for fluorescence-activated cell sorting (FACS) analysis of surface expression levels of the GP Ib-IX complex.21
Production of Ibα and Ibβ TM peptides
A 10 × His sequence was inserted following the N-terminal Met residue of the glutathione S-transferase (GST) domain encoded in the pGEX-4T-3 vector (Amersham Biosciences), generating an expression vector termed pHex. The genes encoding residues S473 to R542 in GP Ibα and L121 to S181 in GP Ibβ were amplified by polymerase chain reaction (PCR) from the respective cDNAs and inserted into the pHex vector as a BamHI-XhoI fragment. The His-GST-TM fusion protein was overexpressed in the inclusion body of Escherichia coli and purified according to published protocols.22,23 Briefly, the fusion protein was first separated from soluble cytosolic proteins and membrane fractions22 ; solubilized in 50 mM Tris·HCl, pH 8.0, 100 mM NaCl, 0.2% N-lauroyl sarcosine, and 5 mM β-mercaptoethanol; and purified by Ni-affinity chromatography. The purified protein was then cleaved by thrombin (10-20 U/mg protein) overnight at room temperature in 50 mM Tris·HCl, pH 8.0 buffer containing 0.1% N-lauroyl sarcosine and 200 mM imidazole. The TM peptides were separated from the His-GST fragment by preparative reverse-phase high-performance liquid chromatography (HPLC).23 The purity and identity of the Ibα and Ibβ TM peptides was confirmed by SDS–polyacrylamide gel electrophoresis (PAGE), analytic HPLC and MALDI-TOF mass spectrometry (Figure S1, available on the Blood website; see the Supplemental Materials link at the top of the online article). Extinction coefficients of these peptides were calculated according to Pace et al.24
In vitro thiol-disulfide exchange assay
Purified TM peptides were mixed with dodecylphosphocholine (DPC) (Avanti Polar Lipids, Alabaster, AL) in ethanol at a peptide-to-detergent molar ratio of 1:200. The solution was dried by argon stream to a thin film and placed under vacuum overnight. The dried protein-to-detergent mixture was hydrated in 40 μL reaction buffer (100 mM Tris·HCl, pH 8.0, 100 mM KCl, 2 mM EDTA, 1 mM oxidized glutathione, 1 mM reduced glutathione) purged with helium gas, topped with argon gas, sealed, and incubated at room temperature for 6 hours to allow the thiol-disulfide exchange reaction to reach equilibrium. In the control reaction, 2.5 mM freshly prepared dithiothreitol (DTT) was included. The final peptide concentration in the reaction was 25 μM. To quench the reaction, 6.7 μL 0.5 M HCl, followed by 33.3 μL organic solvent containing 0.1% TFA, was added to the glass vial. The reaction products were analyzed by reverse-phase HPLC in a C4 or phenyl analytic column (Grace Vydac, Hesperia, CA) using a linear gradient of buffer A (0.1% TFA) and buffer B (6:3:1 vol/vol 2-propanol-to-acetonitrile-to-water). The assignment of the eluted peaks was assisted by mass determination (Protein Core Facility, Baylor College of Medicine).
C-terminal epitope tagging of GP Ibα, GP Ibβ, and GP IX
The DNA fragment encoding the HA tag (YPYDVPDYA) or myc tag (EQKLISEEDL) was attached, in a step-wise fashion, to the C-terminal end of the Ibα, Ibβ, or IX cDNA using a Quikchange mutagenesis kit (Stratagene). The mutant gene sequences were confirmed by DNA sequencing (SeqWright). For transient transfection, the total amount of DNA encoding the tagged subunits (single tag or 2 tags) was the same as the other 2 subunits. Lysates from transfected cells were pretreated with 40 μL 50% Protein G-agarose beads (Sigma) at 4°C for 1 hour, then immunoprecipitated with 1 μg indicated antibody and 80 μL 50% Protein G-agarose beads at 4°C overnight. The proteins bound to the beads were eluted in Tris-glycine SDS sample buffer, separated in a 4-12% Bis-Tris SDS gel under nonreducing conditions, and immunoblotted using an HRP-conjugated anti-HA antibody.
Preparation of human platelets and Ibα VNTR genotyping
Washed human platelets were obtained from blood freshly donated by healthy persons and prepared as described before.25 The protocol was approved by the Institutional Review Boards of The University of Texas Health Science Center at Houston. Both donors signed informed consent, in accordance with the Declaration of Helsinki, before 10 mL blood was drawn. The platelets were pelleted by centrifugation at 1500g for 10 minutes; washed twice with 10 mL of 30 mM glucose, 120 mM NaCl, 10 mM EDTA, 5 mM sodium citrate, pH 6.5; washed once with 10 mL of 134 mM NaCl, 10 mM EDTA, 10 mM Tris·HCl, pH 7.4; and then lysed in cell-lysis buffer to 1 × 109 platelets/mL. Finally, 1 to 2 μL supernatant was used for SDS-PAGE and Western blot.
Genomic DNA samples that had been identified to contain type A, B, C, or D VNTR in previous studies were used as the templates to amplify the gene fragments containing the Ibα VNTR encoding sequence (V.A.-K., unpublished results, January 2001). The 2 primers used were 5′-TCCACTGCTTCTCTAGACAG and 5′-TTCTCTCAAGGTCCCCAAAC. The PCR fragments were digested with XbaI and XmaI and inserted, respectively, into the Ibα cDNA gene that had been cloned into the pcDNA3.1 vector (Invitrogen). Expression vectors containing various types of VNTR were confirmed by DNA sequencing.
Genomic DNA of the 2 donors in this study was prepared from the buffy coat using the QIAamp DNA blood mini kit (Qiagen, Chatworth, CA), and used as the template for PCR. The 2 primers used for PCR were 5′-CTATTCTACTCATGGTCCACTGCTTCTCTAGAC and 5′-GGAAGCGAACCTCGAAGGAAGAGCAGCCAGGC. The PCR products were analyzed by agarose gel electrophoresis and subsequently sequenced (SeqWright).
Deglycosylation of GP Ibα from platelet and CHO cells
The lysates of either human platelets (50 μL) or transiently transfected CHO cells (500 μL from a 10-cm culture dish) was immunoprecipitated with anti-Ibα antibody SZ2 (30 μg/mL) and protein G-agarose for 2 hours at 4°C. After 3 times of washing by PBS containing 0.1% Triton X-100, GP Ibα bound to the agarose beads was eluted by boiling for 10 minutes in 40 to 50 μL 50 mM sodium phosphate, pH 7.0, containing 0.1% SDS. The eluted GP Ibα was then treated with a mixture of PNGase F, sialidase, β-galactosidase, glucosaminidase, and O-glycosidase (supplied in the Enzymatic CarboRelease Kit; QA-Bio, San Mateo, CA) at 37°C for 3 hours. Afterward, the treated proteins were analyzed by SDS-PAGE and blotted with WM23.
Results
Effects of Cys mutations on the Ibα-Ibβ disulfide
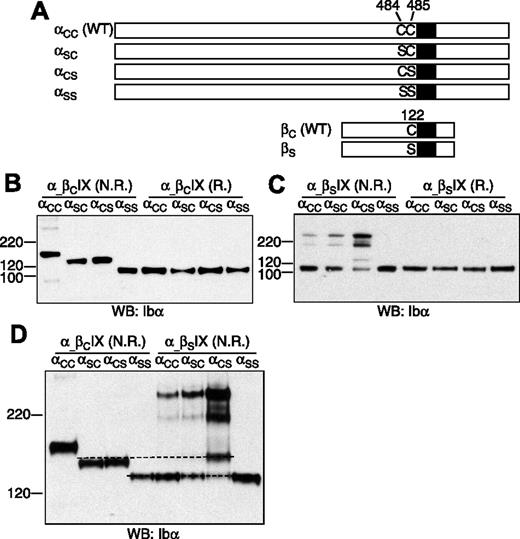
To determine which membrane-proximal Cys residue in GP Ibα forms the disulfide bond with GP Ibβ (Figure 1), 3 Ibα mutant cDNAs, which contain mutation C484S (termed αSC), C485S (αCS), and the double mutation C484S/C485S (αSS), respectively, were constructed (Figure 2A). Each mutant cDNA was transiently cotransfected with the wild-type Ibβ (βC) and IX cDNAs into CHO cells. The expression levels of the GP Ib-IX complex, assayed by FACS to assess surface expression (Figure S2) and by Western blot to measure overall expression (Figure 2B), were not significantly affected by replacement of the Cys residues.
Cysteine residues in GP Ibα and GP Ibβ. The disulfide bonds in each subunit are marked by connected lines. The transmembrane domains are marked by filled boxes. C65 in GP Ibα is buried in the N-terminal domain and is unpaired.17,18 GP Ibα has 2 membrane-proximal Cys residues (C484 and C485), whereas GP Ibβ has one (C122). C122 forms a disulfide bond with a Cys residue in GP Ibα, the identity of which remains unclear.
Cysteine residues in GP Ibα and GP Ibβ. The disulfide bonds in each subunit are marked by connected lines. The transmembrane domains are marked by filled boxes. C65 in GP Ibα is buried in the N-terminal domain and is unpaired.17,18 GP Ibα has 2 membrane-proximal Cys residues (C484 and C485), whereas GP Ibβ has one (C122). C122 forms a disulfide bond with a Cys residue in GP Ibα, the identity of which remains unclear.
Effects of Cys mutations on formation of disulfide bonds between GP Ibα and GP Ibβ. (A) Illustration of wild-type and Cys mutant constructs of GP Ibα and GP Ibβ. (B-C) GP Ibα from the lysates of various transfected CHO cells that had been resolved in a 4-12% Bis-Tris SDS gel (Invitrogen) under nonreducing (N.R.) and reducing (R.) conditions. Molecular markers in kDa are labeled on the left. GP Ibα was blotted by WM23 antibody. (D) GP Ibα from various cell lysates that had been resolved in a 5% Tris-glycine SDS gel under nonreducing (N.R.) conditions. Dashed lines were added to show the difference between the protein bands. The figure is representative of 3 independent experiments.
Effects of Cys mutations on formation of disulfide bonds between GP Ibα and GP Ibβ. (A) Illustration of wild-type and Cys mutant constructs of GP Ibα and GP Ibβ. (B-C) GP Ibα from the lysates of various transfected CHO cells that had been resolved in a 4-12% Bis-Tris SDS gel (Invitrogen) under nonreducing (N.R.) and reducing (R.) conditions. Molecular markers in kDa are labeled on the left. GP Ibα was blotted by WM23 antibody. (D) GP Ibα from various cell lysates that had been resolved in a 5% Tris-glycine SDS gel under nonreducing (N.R.) conditions. Dashed lines were added to show the difference between the protein bands. The figure is representative of 3 independent experiments.
The effects of cysteine mutations on the Ibα-Ibβ disulfide were initially analyzed after resolution by SDS-PAGE under nonreducing conditions (Figure 2B). In CHO cells expressing the wild-type Ib-IX complex (αCCβCIX cell), the majority of GP Ibα was in complex with GP Ibβ, resulting in a dominant GP Ib band in the gel. Replacing both Cys residues with Ser rendered GP Ibα unable to form any intermolecular disulfide bond in αSSβCIX cells. When either one, but only one, of the 2 Cys residues was replaced with Ser, GP Ibα was linked to another protein by a disulfide bond to generate a complex that had an apparent molecular weight approximately averaging those of GP Ib and GP Ibα.
To test whether GP Ibα was still covalently linked to GP Ibβ in this new complex, a mutant Ibβ cDNA containing the mutation C122S (βS) was constructed, and a series of transiently transfected CHO cells was analyzed. In αCCβSIX cells, GP Ib was absent, and the majority of GP Ibα was not covalently linked to any proteins, confirming that C122 is required for the Ibα-Ibβ disulfide (Figure 2C). A small portion of GP Ibα formed disulfide bond(s) with not-yet-identified proteins to produce complexes of apparent molecular weights around 220 kDa (Figure 2C). A similar distribution of GP Ibα was observed in αSCβSIX cells, indicating that C485 is still linked with C122 of GP Ibβ when C484 was replaced by Ser. In αCSβSIX cells, the majority of GP Ibα was present in protein complexes of approximately 220 kDa. An additional band was observed, but it was not the same as in αSCβCIX or αCSβCIX cells (Figure 2D), indicating that C122 was involved in an intermolecular disulfide with C484 in αCSβCIX cells.
Overall, these results showed that, in transfected CHO cells, both C484 and C485 of GP Ibα form a disulfide bond with C122 of GP Ibβ. Mutating C484 or C485 did not affect the ability of the other residue to form a disulfide bond with C122. In the absence of C122, the Cys residues in GP Ibα, especially C484, formed disulfide bond(s) with not-yet-identified proteins. In the GP Ib-IX complex, the only explanation for both C484 and C485 to form disulfide bonds with C122 is that GP Ibα forms covalent links with 2 copies of GP Ibβ.
In vitro formation of the Ibα-Ibβ disulfide bond
We next used the thiol-disulfide exchange assay to test whether we could replicate formation of the Ibα-Ibβ disulfide bond(s) between model peptides in vitro. In an earlier study we showed that replacing either the Ibα or Ibβ TM domain with an unrelated sequence affected formation of the Ibα-Ibβ disulfide bond in transfected CHO cells.21 Given the close proximity of the Ibα-Ibβ disulfide to the TM domains (Figure 1), it is possible that the interaction between the Ibα and Ibβ TM domains favorably positions the membrane-proximal Cys residues for disulfide bond formation.
The thiol-disulfide exchange reaction has been used qualitatively and quantitatively to measure protein interactions, protein folding, and stability.26-29 It was adapted to monitor association of TM helices in detergents and lipid bilayers.30,31 In this assay, Cys-containing peptides or proteins were placed in an environment in which the thiol-disulfide exchange occurs reversibly and quickly. Thus, disulfide formation depends largely on protein-protein association to bring 2 Cys residues into proximity and the intrinsic propensity of the Cys residue to form a disulfide bond. The extent of disulfide formation, in the form of new covalent structure, can be readily monitored by HPLC.
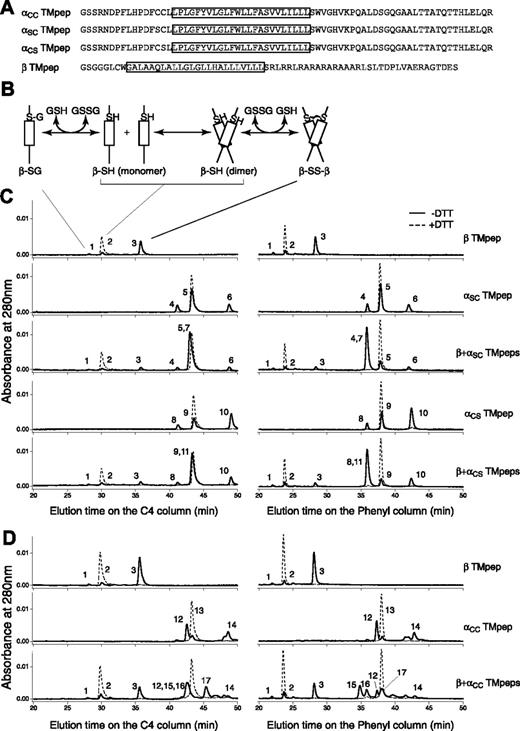
Three Ibα TM peptides and 1 Ibβ TM peptide were obtained (Figure S1). They contained the membrane-proximal Cys residue(s) in question, the TM domain, and a part of or the entire cytoplasmic domain of the respective subunit (Figure 3A). The simplest reaction scheme, consisting of a TM peptide with one Cys residue, contains thermodynamically coupled dimerization and disulfide formation steps (Figure 3B). In DPC micelles, greater than 80% of the Ibβ TM peptide formed a disulfide-linked homodimer; by contrast, a significantly lower portion of the Ibα TM peptide formed a homodimer (Figure 3C). Because the dimerization step was the same for both αSC and αCS peptides, more αCS homodimer products indicated that C484 is more likely than C485 to form a disulfide bond between 2 Ibα peptides.
Thiol-disulfide exchange assay of the TM peptides derived from GP Ibα and GP Ibβ. (A) Sequences of the Ibα and Ibβ TM peptides. The TM domain in each construct is boxed. Residue C148 in the Ibβ cytoplasmic domain was changed to Ser (the residue in the rat sequence) to avoid complication in data analysis. A GGG sequence was added between the thrombin cleavage site and the Ibβ sequence to facilitate thrombin digestion. (B) A reaction scheme for the Ibβ TM peptide. The TM-TM interaction mediates the dimerization step, which is coupled to formation of a disulfide bond between 2 Ibβ peptides. GSH indicates reduced glutathione; GSSG, oxidized glutathione; β-SG, Ibβ peptide linked to glutathione; β-SH, reduced monomeric Ibβ peptide; β-SS-β, disulfide-linked Ibβ homodimer. (C) The thiol-disulfide exchange reaction of the Ibβ TM peptide with the Ibα TM peptides containing one Cys residue. The TM peptides were mixed with DPC micelles and incubated at room temperature for the reaction to reach equilibrium. Acid was then added to quench the reaction, and the reaction products were analyzed by reverse-phase analytic HPLC using 2 different columns. DTT in the reaction prevented formation of any disulfides, and their chromatograms (dashed line) helped to assign the eluted peaks. Peak 1 indicates β-SG; peak 2, β-SH; peak 3, β-SS-β; peak 4, αSC-SG; peak 5, αSC-SH; peak 6, αSC-SS-αSC; peak 7, αSC-SS-β; peak 8, αCS-SG; peak 9, αCS-SH; peak 10, αCS-SS-αCS; peak 11, αCS-SS-β. (D) The thiol-disulfide exchange reaction of the Ibβ TM peptide with the αCC TM peptide, at the 2:1 molar ratio. Peak 12 indicates oxidized monomeric αCC with an intramolecular disulfide; peak 13, reduced monomeric αCC; peak 14, oxidized dimeric αCC with 2 intermolecular disulfides; peak 15, β-SS-αCC-SS-β; peak 16, αCC-SS-β; peak 17, β-SS-αCC-SS-αCC-SS-β.
Thiol-disulfide exchange assay of the TM peptides derived from GP Ibα and GP Ibβ. (A) Sequences of the Ibα and Ibβ TM peptides. The TM domain in each construct is boxed. Residue C148 in the Ibβ cytoplasmic domain was changed to Ser (the residue in the rat sequence) to avoid complication in data analysis. A GGG sequence was added between the thrombin cleavage site and the Ibβ sequence to facilitate thrombin digestion. (B) A reaction scheme for the Ibβ TM peptide. The TM-TM interaction mediates the dimerization step, which is coupled to formation of a disulfide bond between 2 Ibβ peptides. GSH indicates reduced glutathione; GSSG, oxidized glutathione; β-SG, Ibβ peptide linked to glutathione; β-SH, reduced monomeric Ibβ peptide; β-SS-β, disulfide-linked Ibβ homodimer. (C) The thiol-disulfide exchange reaction of the Ibβ TM peptide with the Ibα TM peptides containing one Cys residue. The TM peptides were mixed with DPC micelles and incubated at room temperature for the reaction to reach equilibrium. Acid was then added to quench the reaction, and the reaction products were analyzed by reverse-phase analytic HPLC using 2 different columns. DTT in the reaction prevented formation of any disulfides, and their chromatograms (dashed line) helped to assign the eluted peaks. Peak 1 indicates β-SG; peak 2, β-SH; peak 3, β-SS-β; peak 4, αSC-SG; peak 5, αSC-SH; peak 6, αSC-SS-αSC; peak 7, αSC-SS-β; peak 8, αCS-SG; peak 9, αCS-SH; peak 10, αCS-SS-αCS; peak 11, αCS-SS-β. (D) The thiol-disulfide exchange reaction of the Ibβ TM peptide with the αCC TM peptide, at the 2:1 molar ratio. Peak 12 indicates oxidized monomeric αCC with an intramolecular disulfide; peak 13, reduced monomeric αCC; peak 14, oxidized dimeric αCC with 2 intermolecular disulfides; peak 15, β-SS-αCC-SS-β; peak 16, αCC-SS-β; peak 17, β-SS-αCC-SS-αCC-SS-β.
The reaction scheme for the thiol-disulfide exchange reaction between 2 different peptides is more complicated. It was nonetheless clear that when the Ibβ TM peptide was mixed with the Ibα TM peptide containing only one Cys residue, either C484 or C485, the dominant product in the reaction, was the disulfide-linked αβ heterodimer (Figure 3C). In other words, both αSC and αCS TM peptides were able to out-compete with the Ibβ TM peptide for association with the Ibβ TM peptide, suggesting that the TM-TM interaction between the Ibα and Ibβ TM domains and their neighboring residues is strong and specific. The reactivities of C484 and C485 with C122 to form the intermolecular disulfide bond were similar, judged by the extent of heterodimer formation in each reaction (Table S1).
Because of its 2 Cys residues, the αCC TM peptide generated more products in the thiol-disulfide exchange reaction (Figure 3D). Consistent with the self-oligomerizing ability of the αSC and αCS peptides, the majority of the αCC peptide, when incubated by itself, was in the oxidized monomeric form (with an intramolecular disulfide). A number of products were generated when the αCC peptide was mixed with the Ibβ TM peptide. An αβ2 heterotrimeric complex containing 2 intermolecular disulfides was a major product (Figure 3D). Note that a significant amount of oxidized αCC monomer and a disulfide-linked ββ homodimer were also present. Together, these products are consistent with the αβ2 model, albeit with a different disulfide pattern among the 3 peptides.
Overall, these results matched those obtained by cell transfection remarkably well. Both C484 and C485 were capable of forming a disulfide bond with Ibβ; C484 was more likely than C485 to form a disulfide bond with other proteins. C122 in GP Ibβ prefers to make a disulfide bond with Ibα. Finally, the association between the Ibα and Ibβ TM domains and their flanking regions supports the αβ2 configuration, facilitating formation of the intermolecular disulfides.
GP Ib-IX complex expressed in CHO cells contains 2 copies of GP Ibβ but only 1 copy of GP Ibα and IX
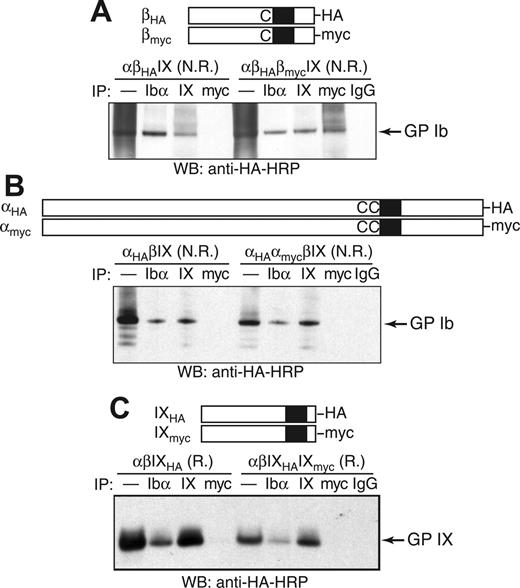
As an alternative test of whether 2 copies of GP Ibβ are present in GP Ib, coimmunoprecipitation (co-IP) experiments were used to determine whether HA-tagged GP Ibβ (βHA) was in the same protein complex with myc-tagged GP Ibβ (βmyc) in transfected CHO cells (Figure 4A). Although addition of the HA or myc tag to GP Ibβ led to a moderate decrease in surface expression of the GP Ib-IX complex (Figure S2), its expression level was sufficiently high to permit co-IP studies. In transfected CHO cells, a significant amount of GP Ibβ was in a presumably denatured form, resulting in a “smearing” background in the nonreducing SDS gel (Figure 4A). To block out false-positive co-IP signals that may result from nonspecific interactions or simple aggregation of misfolded GP Ibβ, only the Ibβ subunits that had been properly assembled into the Ib-IX complex were monitored. Thus, both co-IP and SDS-PAGE steps were carried out under nonreducing conditions so that GP Ib could be kept intact and subsequently visualized by Western blot.
Co-IP of tagged GP Ibα, GP Ibβ, and GP IX in the GP Ib-IX complex from CHO cells. The HA and myc tags were, respectively, appended to the C-terminus of (A) GP Ibβ, (B) GP Ibα, or (C) GP IX. The CHO cells were transiently transfected with indicated subunits, and cell lysates were immunoprecipitated with antibodies, resolved in a 4% to 12% Bis-Tris SDS gel under nonreducing (N.R.) or reducing (R.) conditions, and blotted with HRP-conjugated anti-HA antibody. In the lanes marked by —, the lysates were loaded directly onto the SDS gel.
Co-IP of tagged GP Ibα, GP Ibβ, and GP IX in the GP Ib-IX complex from CHO cells. The HA and myc tags were, respectively, appended to the C-terminus of (A) GP Ibβ, (B) GP Ibα, or (C) GP IX. The CHO cells were transiently transfected with indicated subunits, and cell lysates were immunoprecipitated with antibodies, resolved in a 4% to 12% Bis-Tris SDS gel under nonreducing (N.R.) or reducing (R.) conditions, and blotted with HRP-conjugated anti-HA antibody. In the lanes marked by —, the lysates were loaded directly onto the SDS gel.
We were able to detect the GP Ib band in lysates of αβHAIX cells by blotting for the HA tag amid the smearing background (Figure 4A). Further, HA-containing GP Ib coprecipitated with anti-Ibα (AK2) and anti-IX (FMC25) antibodies (Figure 4A), indicating that attachment of the HA tag to GP Ibβ did not affect assembly of the GP Ib-IX complex or formation of the Ibα-Ibβ disulfide bonds. The same results were observed after the addition of the myc tag (data not shown).
In the CHO cells transfected with both tagged Ibβ subunits as well as the wild-type Ibα and IX subunits (αβHAβmycIX cells), the HA-containing GP Ib coprecipitated with anti-myc antibody but not with nonspecific IgG antibody, indicating that both βHA and βmyc subunits were in the same Ib-IX complex (Figure 4A). Several unidentified HA-containing protein complexes larger than GP Ib also coprecipitated with anti-myc antibody, which may be due to the nonspecific interactions of misfolded GP Ibβ. In contrast to the cocomplexation of βHA and βmyc subunits, HA-tagged GP Ibα (αHA) did not coprecipitate with anti-myc antibody in αHAαmycβIX cells (Figure 4B), nor did HA-tagged GP IX with anti-myc antibody in αβIXHAIXmyc cells (Figure 4C). Therefore, there are only 1 Ibα, 1 IX, and at least 2 Ibβ subunits in the GP Ib-IX complex expressed in CHO cells.
GP Ib from human platelets and CHO cells are equivalent
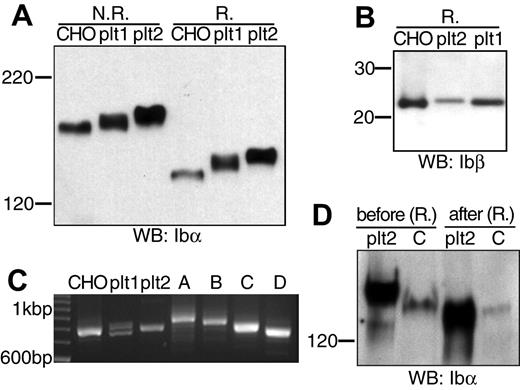
The absence of a nucleus precludes any simple transfection studies on the platelet. To shed light on the Ibα/Ibβ ratio in GP Ib expressed in the platelet, we compared the apparent molecular weights of GP Ib, GP Ibα, and GP Ibβ derived from transfected CHO cells and freshly prepared resting human platelets. We obtained human platelets from 2 healthy donors, according to the protocol approved by the Institutional Review Board. Lysates from platelets and from CHO cells expressing the wild-type Ib-IX complex were resolved by SDS-PAGE and then probed with anti-Ibα (WM23) or anti-Ibβ (Gi27) antibodies.
The apparent molecular weights of GP Ib and GP Ibα from platelets were higher than those from CHO cells, respectively (Figure 5A). Because GP Ibβ from CHO cells and platelets had the same apparent molecular weights (Figure 5B), the molecular weight difference in GP Ib was due to that in GP Ibα. To pinpoint the difference in GP Ibα from platelets and CHO cells, we first analyzed the number of 13-residue tandem nucleotide repeat sequence (VNTR) in the Ibα genes from the 2 donors. These repeats in the macroglycopeptide region of GP Ibα contain multiple glycosylation sites, and the VNTR polymorphism contributes to variation in the Ibα molecular weight.32,33 As shown in Figure 5C and later confirmed by DNA sequencing (not shown), the Ibα cDNA gene that had been used in this study had type D VNTR (1 repeat), whereas donor no. 1 had heterozygous type C/D VNTR alleles and donor no. 2 type C. Subsequent direct comparison of type C VNTR-containing GP Ibα from platelets and CHO cells showed that the VNTR polymorphism did not account for all the difference in the Ibα molecular weight. Further, after being partially deglycosylated under the same condition, GP Ibα from donor no. 2 and CHO cells had the same molecular weights (Figure 5D). Thus, the size difference between GP Ib from human platelets and transfected CHO cells was due to a combination of VNTR polymorphism and glycosylation difference in GP Ibα, not the number of Ibβ subunits therein. We conclude that in the resting platelet GP Ib contains 1 copy of GP Ibα along with 2 copies of GP Ibβ, just as in CHO cells.
Comparison of GP Ib, GP Ibα, and GP Ibβ from transfected CHO cells and human platelets. (A) GP Ib and GP Ibα from CHO cells and platelets of 2 donors (plt1, plt2) that had been resolved in a 5% Tris-glycine SDS gel under either nonreducing (N.R.) or reducing (R.) conditions and blotted by WM23. (B) GP Ibβ from CHO cells and platelets that had been resolved in a 4% to 12% Bis-Tris SDS gel under reducing (R.) conditions and blotted by Gi27. (C) Analysis of genomic Ibα gene sequences from the 2 donors. Genomic DNA was prepared from the buffy coat, and the VNTR-containing gene fragment was amplified by PCR along with various Ibα expression vectors. The amplified fragments were resolved in a 2.3% agarose gel. The 100 bp (base pair) DNA ladder was shown on the left. Four pcDNA-based vectors expressing GP Ibα with type A-D VNTR were included as standards on the right. (D) GP Ibα (type C VNTR) from platelets (plt2) and CHO cells before and after deglycosylation. Both Ibα were coimmunoprecipitated with SZ2 antibody, eluted from protein G-agarose beads, treated with a mixture of glycanases, resolved in a 5% Tris-glycine SDS gel under reducing conditions, and blotted by WM23.
Comparison of GP Ib, GP Ibα, and GP Ibβ from transfected CHO cells and human platelets. (A) GP Ib and GP Ibα from CHO cells and platelets of 2 donors (plt1, plt2) that had been resolved in a 5% Tris-glycine SDS gel under either nonreducing (N.R.) or reducing (R.) conditions and blotted by WM23. (B) GP Ibβ from CHO cells and platelets that had been resolved in a 4% to 12% Bis-Tris SDS gel under reducing (R.) conditions and blotted by Gi27. (C) Analysis of genomic Ibα gene sequences from the 2 donors. Genomic DNA was prepared from the buffy coat, and the VNTR-containing gene fragment was amplified by PCR along with various Ibα expression vectors. The amplified fragments were resolved in a 2.3% agarose gel. The 100 bp (base pair) DNA ladder was shown on the left. Four pcDNA-based vectors expressing GP Ibα with type A-D VNTR were included as standards on the right. (D) GP Ibα (type C VNTR) from platelets (plt2) and CHO cells before and after deglycosylation. Both Ibα were coimmunoprecipitated with SZ2 antibody, eluted from protein G-agarose beads, treated with a mixture of glycanases, resolved in a 5% Tris-glycine SDS gel under reducing conditions, and blotted by WM23.
Discussion
The evidence presented in this study supports the conclusion that GP Ibα is connected by disulfide linkages to 2 Ibβ subunits in the GP Ib-IX-V complex in the resting platelet. This finding differs from the widely accepted stoichiometry of the complex, in which the Ibα/Ibβ ratio was thought to be 1:1. The stoichiometry of the GP Ib-IX-V complex was previously estimated primarily by quantitating the number of subunits present on the platelet surface. Using subunit-specific antibodies, a ratio of 2:2:1 was estimated for the Ibα, IX, and V subunits in the complex.12-14 GP Ibβ was not included in these estimates because of the lack of suitable Ibβ-specific antibodies. Instead, a Ibα/Ibβ ratio of 1:1 was deduced from the apparent molecular weights determined by SDS-PAGE. An apparent molecular weight of 170 kDa for GP Ib, 135 kDa for GP Ibα, and 25 kDa for GP Ibβ is most consistent with a αβ heterodimeric model.11,12 As far as we are aware, this is the only evidence supporting a Ibα/Ibβ ratio of 1:1.
Results presented here support a Ibα/Ibβ ratio of 1:2. First, mutating either C484 or C485 in GP Ibα resulted in the same Ibα- and Ibβ-containing complex in CHO cells with an apparent molecular weight averaging those of GP Ib and GP Ibα. Furthermore, both C484 and C485 consistently formed disulfide bonds with GP Ibβ in the context of isolated TM peptides. Finally, co-IP of differently tagged subunits showed the presence of at least 2 Ibβ subunits but only 1 copy of Ibα and IX in the GP Ib-IX complex. Therefore, we propose that the molar ratio among the Ibα, Ibβ, IX, and V subunits in the complex is 2:4:2:1 (Figure 6).
Schematic drawing of the GP Ib-IX-V complex with the revised stoichiometry. Four GP Ibβ subunits, as well as 4 intermolecular disulfides, are included in the complex. Illustrations of individual subunits are adapted from earlier publications.43,44
During early characterization of the purified GP Ib-IX complex 2 minor bands of slightly lower molecular weight than GP Ib were observed in the nonreducing SDS gel after co-IP by Ibα- or IX-specific antibodies.13,34 These bands were attributed to possible “intramolecular disulfide cleavages in the α-chain of glycoprotein Ib”34 and/or “partial reduction of GP Ib.”13 However, reduction of intramolecular disulfides in GP Ibα did not affect its mobility in the SDS gel (Figure 2B, compare Ibα from αSSβCIX cells in the nonreducing condition with the reducing condition). Generation of 2 products (Ibα-Ibβ and Ibα) from partial reduction of GP Ib can be readily explained by a 1:2 ratio but not by a 1:1 ratio. Indeed, the lower band of the 2 minor bands seemed to match well with GP Ibα under reducing conditions.
The revised stoichiometry of the GP Ib-IX-V complex will require reexamination of some data related to GP Ib. More importantly, it will help shed light on the mechanism by which this receptor complex carries out its function. For instance, the cytoplasmic signaling protein 14-3-3ζ can interact with both Ibα and Ibβ cytoplasmic domains.35-39 Both interactions are important to the function of the GP Ib-IX-V complex.3-5,8,9,40 14-3-3ζ is a dimer, and disrupting 14-3-3ζ dimerization decreases its affinity for the GP Ib-IX complex but not the isolated peptide containing the Ibα cytoplasmic domain.41 On the basis of the αβ heterodimeric model for GP Ib, it was thought that 2 monomers in the 14-3-3ζ dimer interact with the Ibα and Ibβ cytoplasmic domains, respectively, with the implicit assumption that one 14-3-3ζ dimer interacts with one GP Ib complex.41 Now that the αβ2 heterotrimeric model posits an additional Ibβ cytoplasmic domain and consequently a total of four 14-3-3ζ–binding sites in GP Ib, the Ib-14-3-3ζ interaction and its likely alteration in response to intracellular signals may be more complicated than previously thought.
The αβ2 heterotrimeric model of GP Ib also opens a door for possible disulfide rearrangement in the GP Ib-IX-V complex. There are 2 theoretically possible disulfide arrangements among the 4 membrane-proximal Cys residues in GP Ib: C122-C484/C485-C122 and C122-C122/C484-C485. The former is the de facto one in the resting platelet. Our characterization of the TM peptides suggests, however, that the latter could be achieved if given favorable conditions (Figure 3D). Moreover, when GP Ibα is free of disulfides with GP Ibβ (as in αCCβSIX cells), it could form a disulfide-linked complex with other proteins through C484, C485, or both (Figure 2C). Although we have not determined all the components of the Ibα-containing complex of more than 220 kDa that is present in αCCβSIX, αSCβSIX, and αCSβSIX cells, its apparent molecular weight is consistent with that of a Ibα homodimer. We also note that a Ibα-containing polypeptide of 210 kDa was found as the target of an autoantibody in a patient with prolonged bleeding time.42 It remains to be seen whether GP Ibα actually forms a disulfide with a protein other than GP Ibβ in the platelet and, more importantly, if it does, whether it correlates with ligand binding as well as downstream signaling.
The online version of this article contains a data supplement.
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Conflict-of-interest disclosure: the authors declare no competing financial interests.
Contribution: S.-Z.L., X.M., and R.L. designed and performed the research, analyzed the data, and wrote the paper; V.A.-K., S.S. and J.A.L. contributed vital new reagents.
S.-Z.L. and X.M. contributed equally to this study.
We thank Drs Michael Berndt and Xiaoping Du for their generous gifts of antibodies, Scott Holmes for graphic assistance on Figure 6, Zhou Zhou for assistance in platelet preparation, and Drs Jing-Fei Dong and Julia Lever for critical reading of the manuscript.
This work was supported by grants from the National Institutes of Health (HL082808), the American Heart Association-Texas Affiliate (0565078Y), and by the Welch Foundation (AU-1581) (R.L.) and the Howard Temin Award (CA096706) from the National Cancer Institute (R.L.).

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal