Abstract
αv integrins are thought to play an important role in tumor angiogenesis. However, discrepancies between findings with Arg-Gly-Asp (RGD) mimetics, which block angiogenesis in animal models, and knockout mice, in which loss of some αv integrins enhances tumor angiogenesis, raise questions concerning the function of these integrins and the precise role of αv substrate mimetics in antiangiogenic therapies. We have examined the effects of a novel non–peptide RGD mimetic, S 36578-2, on human endothelial cells to elucidate its antagonist activity and to identify possible agonist functions. S 36578-2 is highly selective for αvβ3 and αvβ5 integrins and induces detachment, caspase-8 activation, and apoptosis in human umbilical endothelial cells (HUVECs) plated on vitronectin. Importantly, the compound has no effect on the morphology or survival of cells plated on interstitial matrix components such as fibronectin, and it does not potentiate the apoptotic process in suspended cells. Identical results were obtained with a cyclic RGD peptide with similar target specificity. In microvascular endothelial cells, S 36578-2–induced death was also linked to its antiadhesive effect, with established lines markedly more resistant than primary cultures to the antiadhesive and proapoptotic effects. Altogether, these findings have important implications for the development of this class of antiangiogenics.
Introduction
The formation of new blood vessels from existing vasculature, or angiogenesis, is essential for successful tumor growth and for the development of metastases. Previous work has suggested that certain endothelial cell integrins, including αvβ3 and αvβ5, actively contribute to the angiogenic process.1,2 More recently, endogenous inhibitors of angiogenesis have been shown to target these integrins.3 Integrins are transmembrane receptors for extracellular matrix (ECM) and basement membrane proteins that are composed of 2 noncovalently associated subunits, α and β.4 To date, 18 α and 8 β subunits have been identified in mammals, and their association in various combinations leads to the formation of at least 24 receptors with distinct ligand specificity. Besides mediating stable adhesion, integrins transmit signals that regulate cell survival, growth, motility, and remodeling of their extracellular environment.4-6
The integrins αvβ3 and αvβ5 bind to ECM molecules through an Arg-Gly-Asp (RGD)–binding site. Based on the concept that they are proangiogenic receptors, specific inhibitors, including blocking monoclonal antibodies, RGD peptides, and RGD peptidomimetics, have been developed and evaluated in vivo.7 Indeed, pharmacologic agents targeted to αvβ3, αvβ5, or both, have been reported to block tumor and retinal angiogenesis.8-14 Some of these angiogenesis inhibitors, including a humanized monoclonal anti-αvβ3 (Vitaxin; MedImmune, Gaithersburg, MD) and an αvβ3/αvβ5–selective RGD-based cyclic peptide (cilengitide), have entered clinical trials.15,16
Early experiments show that a monoclonal antibody directed against αvβ3 inhibits angiogenesis by inducing the apoptosis of angiogenic blood vessels.17 Subsequent studies based on results obtained with adhesion-blocking antibodies or RGD peptides have led to several models of antagonist-induced cell death. According to the first and most classic model, integrin engagement by an ECM ligand provides essential survival signals, and the loss of cell contact with the ECM induced by adhesion-blocking agents results in a type of caspase-dependent apoptosis known as anoikis.18 Activation of caspase-8 (extrinsic) and caspase-9 (intrinsic) pathways have been described in different cell types after integrin antagonism or disruption of adhesion.19-22 In a second model, RGD-based peptides enter cells and directly activate caspases in an integrin-independent manner.23-26 A third model, integrin-mediated death (IMD), has also been proposed in which unligated or antagonized integrins can directly recruit caspase-8 and trigger the apoptosis of adherent cells.27-29
Despite the compelling arguments for a proangiogenic role of αv integrins, studies on genetically altered mice models raise serious questions regarding the notion that αvβ3 and αvβ5 integrins are proangiogenic or are even necessary for angiogenesis.30 Mice lacking αv, which denotes the inactivation of all 5 αv integrins—α5β1, αvβ3, αvβ5, αvβ6, and αvβ8—develop normally until embryonic day 9.5. Thereafter, only 20% of the mice survive until birth.31 The liveborn αv-null mice consistently exhibit extensive vasculogenesis, angiogenesis, and intracerebral and intestinal hemorrhage. Conditional deletion of αv integrin expression in vascular endothelial cells was more recently found to have no detectable effect on the development of cerebral blood vessels. Rather, conditional deletion in glial cells was found to lead to the cerebral hemorrhage phenotype observed in the complete αv knockout.32 Like mice lacking β533 or a combination of β3 and β5,34 mice lacking β335 are viable and fertile and have no obvious defects in vascular development. Moreover, β3-and β3/β5-null mice display enhanced tumor growth and enhanced angiogenesis within these tumors compared with wild-type controls.34,36,37 The apparent contradiction between the data obtained with αv antagonists and genetic ablations of the genes encoding these integrins can be reconciled if αv integrins are considered negative regulators of angiogenesis, as proposed by Hynes.30 Alternatively, if these integrins are regarded as sensors (or dependence receptors) that actively promote endothelial cell death when unoccupied or in an inappropriate ECM environment, IMD could occur.27 According to the latter hypothesis, the increased pathologic angiogenesis in mice lacking β3 or β3 and β5 integrins could be explained by the genetic loss of proapoptotic stimuli.38 These results underscore the complexity of the role of αv integrins in angiogenesis and highlight the need for further evaluation of the mechanism of action of αv integrin–targeting agents.
In the present study, we have examined the direct effects on human endothelial cells of a small heterocyclic non–peptide RGD mimetic (S 36578-2) selective for αvβ3 and αvβ5 integrins. Particular attention was paid to the ability of this compound to activate the cellular apoptotic machinery in cells plated on different substrates for which cell adhesion depends on the engagement of αvβ3 and αvβ5 integrins (anoikis) or does not depend on it (IMD). In addition to studying its adhesion-blocking activity, we sought to identify possible agonist functions of the RGD mimetic. As a model, the well-characterized human umbilical vein endothelial cells (HUVECs) were used, as were human microvascular endothelial cells, which represent a more relevant target in potential antiangiogenic therapeutic strategies. Our results preclude a scenario in which molecules devised to target αvβ3 and αvβ5 integrins induce direct, integrin-independent activation of caspases. Rather, cell death is dependent on the disruption of cell-matrix adhesion, and no evidence for IMD was observed. Finally, compared with primary cultured cells, immortalized cell lines displayed enhanced resistance to S 36578-2.
Materials and methods
Cell culture
Human umbilical vein endothelial cells (HUVECs) were obtained from fresh human umbilical veins.39 Cells were maintained in human endothelial-SFM (Invitrogen, Cergy Pontoise, France) supplemented with 20% fetal calf serum (FCS) PAN (Biotech GmbH, Aidenbach, Germany), epidermal growth factor (EGF, 10 ng/mL; Invitrogen), fibroblast growth factor (bFGF, 10 ng/mL, prepared in the laboratory), and heparin (10 ng/mL; Sigma, Saint Quentin Fallavier, France). For all experiments, cells were used up to the 6th passage. The human microvascular endothelial cell line CDC.HMEC-1 was provided by F. J. Candal (Centers for Disease Control and Prevention, Atlanta, GA). Cells were maintained in MCDB131 (Invitrogen) supplemented with 12.5% Hyclone FCS (Perbio Science, Brebieres, France), glutamine (10 mM; Invitrogen), EGF (10 ng/mL), bFGF (10 ng/mL), heparin (10 ng/mL), and hydrocortisone (1 μg/mL; Sigma). Cells were used between passages 15 and 22. Primary cultures of human dermal and lung microvascular endothelial cells (Promocell, Heidelberg, Germany) were cultivated according to the manufacturer's recommendations. For experiments described in this paper, cells were cultured in M199 (Invitrogen) supplemented with 5% FCS (referred to as experimental medium).
Coating
Tissue culture plates or coverslips were incubated overnight at 4°C with 50 ng/cm2 vitronectin (VN; BD Biosciences, Le Pont de Claix, France) or 10 μg/mL fibronectin (FN; Invitrogen). Then they were blocked with 0.5% heat-inactivated fatty acid–free bovine serum albumin (BSA; Sigma) for 1 hour at 37°C and washed in phosphate-buffered saline (PBS). Tissue culture plates were incubated at 37°C with PolyHEMA (PH; 12 mg/mL ethanol; Sigma) until total evaporation and then were washed in PBS.
Antibodies
Primary antibodies used were rabbit polyclonal anti–α5 integrin (Chemicon, Temecula, CA); mouse monoclonal anti–integrin β3 (kindly provided by J. Gonzalez, Madrid, Spain), αvβ3 (clone LM609; Chemicon), αvβ5 (clone P1F6; Chemicon), or α5β1 (clone JBS5; Chemicon); mouse monoclonal anti-PARP (Biomol Research Laboratories, Plymouth Meeting, PA); mouse monoclonal anti–caspase-8 (Calbiochem, La Jolla, CA) or anti–caspase-9 (MBL, Nagoya, Japan); rabbit polyclonal anti–cleaved caspase-3 (Cell Signaling, Beverly, MA); rabbit polyclonal anti–Erk1/2 (E1B4, produced in the laboratory); and IgG isotope control (BD Biosciences PharMingen France, Le Pont de Claix). Secondary antibodies used for immunocytochemistry were fluorescently conjugated anti–mouse IgG (Molecular Probes, Invitrogen) or anti–mouse IgM (BD Biosciences PharMingen). Those used for Western blotting were peroxidase- or alkaline phosphatase–conjugated anti–mouse IgG or anti–rabbit IgG (Promega, Charbonnieres, France). The secondary antibody used for detection of integrins by flow cytometry was a fluorescently conjugated anti–mouse IgG (Santa Cruz Biotechnology, Santa Cruz, CA).
Compounds
S 36578-2, the sodium salt of (5-(S)-7-{[4-(pyridin-2-ylamino)-butyryl amino]-methyl}-6,9-dihydro-5H-benzocyclohepten-5-yl) acetic acid, was separated on a chiral column from the racemate compound (compound 1.2 synthesized as described by Perron-Sierra et al40 ). The RGD peptide cyclo(Arg-Gly-Asp-D-Phe-[NMe]Val) (cilengitide or EMD-121974, hereafter referred to as cyclic RGD) was synthesized as described.41 Both compounds were solubilized to a concentration of 10 mM in dimethyl sulfoxide (DMSO). The broad-spectrum caspase inhibitor Z-VAD-FMK (Alexis Biochemicals, San Diego, CA) was solubilized to a concentration of 30 mM in DMSO.
Binding activities
Purified human integrins (αvβ3, αvβ5, α5β1 from placenta, and αIIbβ3 from platelets) were adsorbed onto 96-well plates. S 36578-2 or cyclic RGD was added to displace biotinylated vitronectin from αvβ3 and αvβ5, biotinylated fibronectin from α5β1, or biotinylated fibrinogen from αIIbβ3. Ligand binding was evaluated as previously described.40
Cell adhesion assays
Cells grown in stock medium for 24 hours were detached with trypsin/EDTA. After rinsing in RPMI without phenol red containing 0.5% BSA, cells were preincubated with S 36578-2 or cyclic RGD (0.01-10 μM), anti-αvβ3, anti-αvβ5, or anti-α5β1 antibodies (20 μg/mL) for 30 minutes at 4°C before adhesion for 20 minutes at 37°C on VN- or FN-coated wells (Terasaki plates). Adhesion was evaluated as previously described.40
Quantification of integrin expression
Cells grown on VN- or FN-coated plates in experimental medium were detached with trypsin/EDTA. After rinsing in cold PBS–0.5% BSA, they were incubated with antibody anti-αvβ3, anti-αvβ5, or anti-α5β1 or with IgG istotype control (0.1 mg/mL) for 1 hour at 4°C. Cells were washed in cold PBS–0.5% BSA, incubated with fluorescently conjugated secondary antibodies for 1 hour at 4°C, washed in cold PBS–0.5% BSA and then in cold PBS, and analyzed by flow cytometry (Epics XL/MCL flow cytometer; Beckman Coulter, Roissy, France).
Immunofluorescence
Cells plated on VN- or FN-coated coverslips in experimental medium were fixed with 3% paraformaldehyde 100 mM sucrose, permeabilized with 0.2% Triton X-100 in PBS, and blocked with PBS-5% FCS before incubation with primary antibody diluted in PBS-5% FCS for 90 minutes at room temperature. After incubation with fluorescently labeled secondary antibodies and phalloidin (Molecular Probes), cells were washed and mounted (Gel Mount Aqueous Mounting Medium; Sigma). Fluorescence was observed on an inverted microscope (Axiovert 200 M; Carl Zeiss, Oberkochen, Germany) equipped with a CoolSnap HQ cooled charge-coupled device camera (Roeper Scientifique, Evry, France). Fluorescence and phase contrast images were acquired using a 63×/1.4 NA oil objective and a 10×/0.25 NA phase objective, respectively. Image acquisition and analysis were performed with the MetaMorph Imaging System version 6.2 (Universal Imaging, Sunnyvale, CA). Microsoft Powerpoint 2003 (Microsoft, Redmond, WA) was used to prepare figures.
Apoptosis assays
For VN- or FN-coated plates, S 36578-2 or cyclic RGD was added 3 hours after seeding, when the cells were well spread. For PH-coated plates, the indicated compound was added when cells were seeded. Briefly, after treatment, floating and attached cells were collected, incubated in experimental medium enriched in FCS for 1 hour at 37°C, and subjected to annexin V/propidium iodide (PI) staining using annexin V–Alexa Fluor 488 (Molecular Probes) and PI (Sigma). The resultant fluorescence was measured by flow cytometry.
Staining cells with a combination of annexin V and PI revealed nonapoptotic cells (annexin V–/PI–), early apoptotic cells (annexin V+/PI–), late apoptotic cells (annexin V+/PI+), and necrotic cells (annexin V–/PI+).
SDS-PAGE and Western blotting
After the indicated treatment, floating and attached cells were lysed in Laemmli buffer containing 4% SDS, 20% glycerol, and 200 mM Tris, pH 6.8. Protein concentrations were determined using the BCA assay (Sigma). Equal amounts of total protein were fractionated under reducing conditions by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and then were blotted onto PVDF membranes. Membranes were blocked with 5% skimmed milk in PBS hybridized with the primary antibody of interest. Membranes were washed in PBS and then hybridized with secondary antibody. Anti–mouse and anti–rabbit peroxidase- or alkaline phosphatase–conjugated secondary antibodies were diluted in PBS containing 5% skimmed milk. After washing with PBS, immune complexes on membranes were detected by enhanced chemiluminescence (Pierce, Rockford, IL).
Results
S 36578-2, a small molecule highly selective for αv integrins
To characterize the specificity and potency of the S 36578-2 compound, binding affinities were evaluated on human integrins. IC50 values were 0.4 nM for αvβ3 and 0.3 nM for αvβ5 (vitronectin displacement), 18 nM for α5β1 (fibronectin displacement), and 1987 nM for αIIbβ3 (fibrinogen displacement). In addition, S 36578-2 efficiently inhibited the adhesion of endothelial cells to vitronectin with IC50 values for HUVECs and CDC.HMEC-1 microvascular endothelial cells of 389 nM and 455 nM, respectively. By contrast, the adhesion of HUVECs and CDC.HMEC-1 cells to fibronectin was poorly affected, with IC50 values superior to 10 μM for both cell types. Adhesion of both cell types to vitronectin was mediated primarily by αvβ3 and αvβ5 integrins, whereas adhesion to fibronectin occurred largely through α5β1 integrin, as revealed by the use of specific blocking antibodies (data not shown). These results demonstrate that S 36578-2 specifically inhibited the adhesion of endothelial cells to vitronectin by binding to αvβ3 and αvβ5 integrins; no significant effect was observed on the adhesion of cells to fibronectin at submicromolar concentrations.
Evaluation of endothelial cell surface integrin expression and extracellular matrix composition before the addition of S 36578-2
In the experimental set-up designed to evaluate the effects of S 36578-2 in this study, HUVECs and CDC.HMEC-1 cells were allowed to fully spread on vitronectin- or fibronectin-coated surfaces for 3 hours in experimental medium before treatment. Under these conditions, chosen to limit the presence of competing serum- or cell-derived extracellular matrix proteins, cells attached and spread equally well on both substrates, and no major difference in organization of the actin cytoskeleton was observed. We verified by immunostaining that the deposit of vitronectin and fibronectin matrix by these endothelial cells was very low under our experimental conditions (data not shown). The expression of αvβ3, αvβ5 (targets of S 36578-2), and α5β1 integrins on the cell surfaces was examined by fluorescence-activated cell sorting (FACS). As shown in Table 1, cells displayed similar surface integrin expression profiles on both matrix components. However, consistent with the differential integrin engagement and cell-matrix adhesion formation on these 2 substrates, the distribution of these integrins on spread cells was different (Figure 1). In HUVECs, αvβ3 integrin was recruited to adhesive structures when cells were plated on vitronectin, whereas α5β1 integrin staining was diffuse, suggesting that this integrin did not mediate attachment to vitronectin. In contrast, α5β1 integrin was enriched in functional cell-matrix adhesions in cells plated on fibronectin-coated coverslips, whereas αvβ3 integrin remained dispersed. In CDC.HMEC-1 cells, integrin localization was similar, except for αvβ3 integrin for which some staining was detected in adhesive structures when cells were plated on fibronectin, suggesting that binding of fibronectin by this integrin might occur (not shown).
Localization/organization of endothelial integrins αvβ3 and α5β1. HUVECs were grown for 3 hours on vitronectin- or fibronectin-coated plates before fixing and staining. β3 and α5 integrin subunits were detected by indirect immunofluorescence and F-actin with fluorescently-conjugated phalloidin. Scale bar represents 20 μm. Results from a representative experiment (of at least 3) are shown.
Localization/organization of endothelial integrins αvβ3 and α5β1. HUVECs were grown for 3 hours on vitronectin- or fibronectin-coated plates before fixing and staining. β3 and α5 integrin subunits were detected by indirect immunofluorescence and F-actin with fluorescently-conjugated phalloidin. Scale bar represents 20 μm. Results from a representative experiment (of at least 3) are shown.
Effects of S 36578-2 on endothelial cells spread on vitronectin
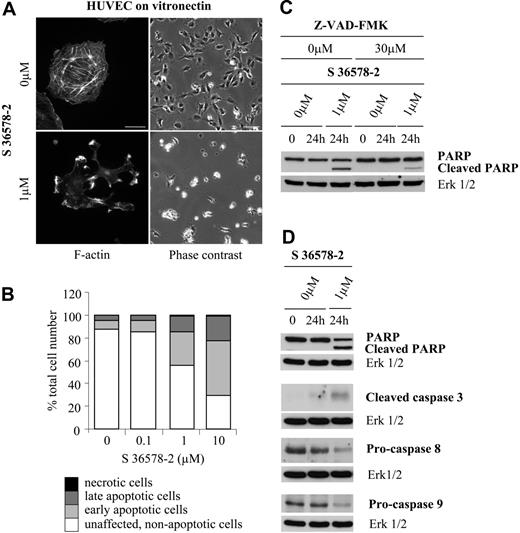
We next examined the ability of S 36578-2 to detach adherent cells from their substratum. In the case of endothelial cells plated on vitronectin, adhesion is dependent on the engagement of αvβ3 and αvβ5 integrins and their localization in functional cell-matrix adhesions. As shown in Figure 2A, S 36578-2 induced rapid disruption of actin microfilaments in HUVECs followed by cell detachment from vitronectin-coated surfaces. Similar effects were obtained in CDC.HMEC-1 cells, with cell detachment less pronounced (Figure 3A). Although essentially all HUVECs were in suspension by 24 hours, most CDC.HMEC-1 cells remained adherent at this time (compare Figures 2A and 3A). Because detachment has been shown to promote anoikis in endothelial cells,18 cell death was evaluated by flow cytometry after treatment of HUVECs with S 36578-2. As shown in Figure 2B, S 36578-2 promoted a dose-dependent induction of apoptosis, as determined by annexin V/propidium iodide staining 24 hours after treatment. Whereas the presence of early and late apoptotic cells was minimal in control conditions (dose 0, 13%) and in cells treated with 0.1 μM S 36578-2 (15%), 44% of cells treated with a dose of 1 μM and up to 70% of cells treated with a dose of 10 μM underwent apoptosis. In subsequent experiments, only results obtained with S 36578-2 at a concentration of 1 μM are shown. At this concentration approximately 50% of the cells were affected 24 hours after treatment.
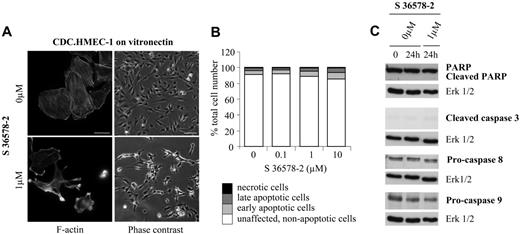
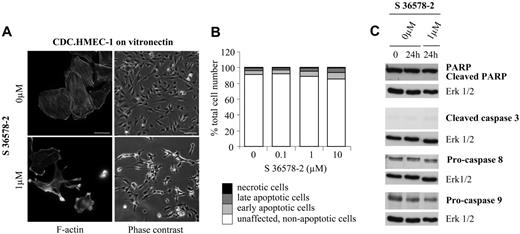
To determine the mechanism by which S 36578-2–induced apoptosis proceeds, we examined the activation of caspases by Western blotting of cell lysates prepared 0 to 24 hours after treatment. Caspases, the principal biochemical effectors of apoptosis, are present in cells as inactive zymogens (procaspases). Upstream apoptotic signals convert these precursors to mature proteases that cleave their substrates after aspartate residues.42 Treatment of HUVECs plated on vitronectin with S 36578-2 led to a time- and dose-dependent cleavage of poly(ADP-ribose) polymerase (PARP), a well-known caspase substrate whose cleavage is considered a hallmark of the execution stage of apoptosis43 (Figure 2C and data not shown). This effect was attenuated in the presence of the specific pan-caspase inhibitor Z-VAD-FMK, which did not prevent the effects of S 36578-2 on cell morphology or adhesion (data not shown). Cleavage of PARP in S 36578-2-treated cells was accompanied by the activation of caspase-3, one of the key effector caspases, as determined by the appearance of the cleaved/active form of the enzyme (Figure 2D). Treatment of cells with S 36578-2 also induced a decrease in the pro-form of caspase-8, an initiator caspase of the extrinsic pathway, indicating that cell death occurred by the activation of this pathway. In some experiments a decrease of procaspase-9, an initiator caspase of the intrinsic pathway, was also detected (Figure 2D), most notably when cell death was more extensive. Cell death induced by S 36578-2 was also evaluated in CDC.HMEC-1 cells plated on vitronectin-coated surfaces (Figure 3B). Interestingly, in this microvascular line, only a low percentage of early and late apoptotic cells was detected by flow cytometry 24 hours after treatment at doses 1 or 10 μM (Figure 3B). Western blotting revealed no evidence of PARP cleavage or caspase activation (Figure 3C). Treatment of a second immortalized human microvascular endothelial cell line (HMVEC)44 yielded similar results, with little or no effect of S 36578-2 observed on survival or detachment of cells from vitronectin-coated surfaces (not shown). Thus, the extent of cell death after S 36578-2 treatment correlates with the antiadhesive effect of the compound and involves caspase-8 activation.
Effects of S 36578-2 on endothelial cell spread on fibronectin
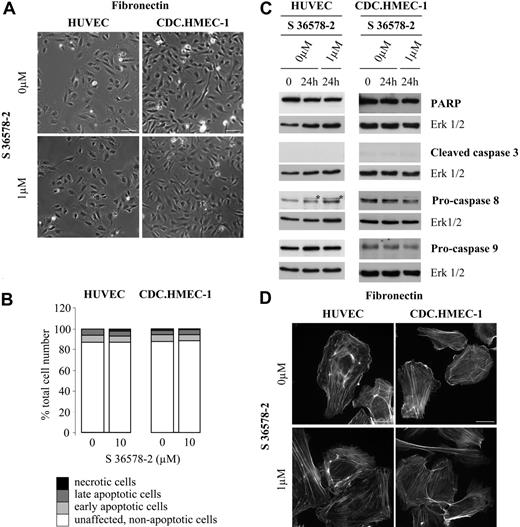
To test the effect of S 36578-2 on unligated integrins, cells were plated on fibronectin. On this substrate, the adhesion of HUVECs and CDC.HMEC-1 cells was largely mediated by α5β1 integrin, and targets of S 36578-2 (αvβ3 and αvβ5 integrins) were not bound. Morphologic analyses (Figure 4A) and apoptosis assays (Figures 4B-C) revealed that S 36578-2 had no significant effect on HUVECs and CDC.HMEC-1 cells seeded on fibronectin. No changes were observed in the diffuse staining of αvβ3 integrin after S 36578 treatment of HUVECs (data not shown), though we did observe a modest effect on lamellipodia formation (Figure 4D). This suggested that αv targeting might have affected cortical actin dynamics in spreading cells without compromising adhesion. The compound did not alter the morphology or survival of cells plated on type I collagen, another constituent of the provisional interstitial matrix surrounding endothelial cells in vivo that is not a recognized ligand for αvβ3 and αvβ5 integrins (Figure S1, which is available on the Blood website; see the Supplemental Figures link at the top of the online article). These results reinforce our findings that the action of S 36578-2 is substrate specific and related to cell detachment. Further, they demonstrate that the compound does not induce off-target cytotoxic effects, including direct caspase activation, which has been described for certain integrin antagonists.23,24,26
Effects of S 36578-2 on HUVECs spread on vitronectin. The compound S 36578-2 was added to cells 3 hours after seeding in experimental medium. (A) Cells were processed for F-actin detection 30 minutes after the addition of S 36578-2. Scale bar represents 20 μm. Phase contrast micrographs were taken 24 hours after treatment. Scale bar represents 100 μm. Results from a representative experiment, repeated at least 3 times, are shown. (B) Floating and attached cells were collected 24 hours after treatment. Apoptosis was assessed by flow cytometry after annexin V and propidium iodide staining. Results of 1 of 2 experiments with similar results are shown. (C) PARP cleavage was assessed by Western blotting 24 hours after treatment of cells with S 36578-2. Where indicated, cells were treated for 1 hour with the caspase inhibitor Z-VAD-FMK before S 36578-2 was added. Erk1/2 was used as loading control. Results of 1 of 3 experiments with similar results are shown. (D) Procaspase-8, procaspase-9, cleaved caspase-3, and PARP were detected by Western blotting. Erk1/2 was used as loading control. Results of 1 of 12 experiments with similar results are shown.
Effects of S 36578-2 on HUVECs spread on vitronectin. The compound S 36578-2 was added to cells 3 hours after seeding in experimental medium. (A) Cells were processed for F-actin detection 30 minutes after the addition of S 36578-2. Scale bar represents 20 μm. Phase contrast micrographs were taken 24 hours after treatment. Scale bar represents 100 μm. Results from a representative experiment, repeated at least 3 times, are shown. (B) Floating and attached cells were collected 24 hours after treatment. Apoptosis was assessed by flow cytometry after annexin V and propidium iodide staining. Results of 1 of 2 experiments with similar results are shown. (C) PARP cleavage was assessed by Western blotting 24 hours after treatment of cells with S 36578-2. Where indicated, cells were treated for 1 hour with the caspase inhibitor Z-VAD-FMK before S 36578-2 was added. Erk1/2 was used as loading control. Results of 1 of 3 experiments with similar results are shown. (D) Procaspase-8, procaspase-9, cleaved caspase-3, and PARP were detected by Western blotting. Erk1/2 was used as loading control. Results of 1 of 12 experiments with similar results are shown.
Effects of S 36578-2 on endothelial cells maintained in suspension
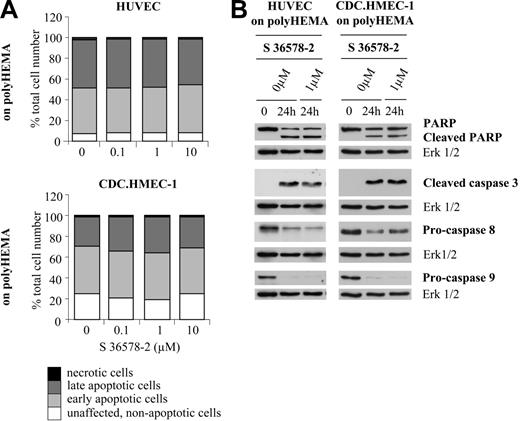
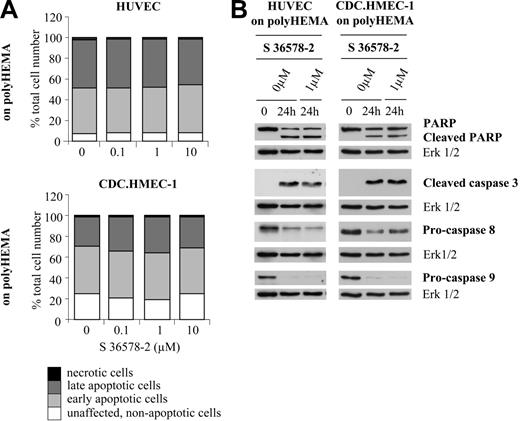
To determine whether anoikis could be accelerated or potentiated by S 36578-2, HUVECs and CDC.HMEC-1 cells were seeded on poly-HEMA-coated surfaces that totally prevented cell adhesion and spreading. Under these conditions, annexin V/propidium iodide staining revealed the presence of 91% apoptotic HUVECs and 74% apoptotic CDC.HMEC-1 cells 24 hours after plating (Figure 5A). Cell death was characterized by the cleavage of PARP, the appearance of cleaved caspase-3, the decrease of procaspase-8, and, less often, the decrease of procaspase-9 (Figure 5B). Importantly, no differences in the rate or extent of apoptosis were detected in the absence or presence of S 36578-2 for HUVECs or CDC.HMEC-1 cells (Figures 5 and S2) for less than 24 hours, suggesting no specific effect of the compound under these experimental conditions.
Effects of S 36578-2 on CDC.HMEC-1 cells spread on vitronectin. S 36578-2 was added to cells 3 hours after seeding in experimental medium. (A) Cells were processed for F-actin detection 30 minutes after the addition of S 36578-2. Scale bar represents 20 μm. Phase contrast micrographs were taken 24 hours after treatment. Scale bar represents 100 μm. Results from a representative experiment, repeated at least 3 times, are shown. (B) Floating and attached cells were collected 24 hours after treatment. Apoptosis was assessed by flow cytometry after annexin V and propidium iodide staining. Results of 1 of 2 experiments with similar results are shown. (C) Procaspase-8, procaspase-9, cleaved caspase-3, and PARP were detected by Western blotting. Erk1/2 was used as loading control. Results from 1 of 4 representative experiments are shown.
Effects of S 36578-2 on CDC.HMEC-1 cells spread on vitronectin. S 36578-2 was added to cells 3 hours after seeding in experimental medium. (A) Cells were processed for F-actin detection 30 minutes after the addition of S 36578-2. Scale bar represents 20 μm. Phase contrast micrographs were taken 24 hours after treatment. Scale bar represents 100 μm. Results from a representative experiment, repeated at least 3 times, are shown. (B) Floating and attached cells were collected 24 hours after treatment. Apoptosis was assessed by flow cytometry after annexin V and propidium iodide staining. Results of 1 of 2 experiments with similar results are shown. (C) Procaspase-8, procaspase-9, cleaved caspase-3, and PARP were detected by Western blotting. Erk1/2 was used as loading control. Results from 1 of 4 representative experiments are shown.
Effects of S 36578-2 on HUVECs and CDC.HMEC-1 cells spread on fibronectin. S 36578-2 was added to cells 3 hours after seeding in experimental medium. (A) Phase contrast micrographs were taken 24 hours after treatment. Scale bar represents 100 μm. Results from a representative experiment, repeated at least 3 times, are shown. (B) Floating and attached cells were collected 24 hours after treatment. Apoptosis was assessed by flow cytometry after annexin V and propidium iodide staining. Results of 1 of 2 experiments with similar results are shown. (C) Procaspase-8, procaspase-9, cleaved caspase-3, and PARP were detected by Western blotting. Erk1/2 was used as loading control. *Nonspecific detection of BSA. Results from 1 of 5 representative experiments for HUVECs and 1 of 4 representative experiments for CDC.HMEC-1 cells are shown. (D) Cells were processed for F-actin detection 30 minutes after the addition of S 36578-2. Scale bar represents 20 μm.
Effects of S 36578-2 on HUVECs and CDC.HMEC-1 cells spread on fibronectin. S 36578-2 was added to cells 3 hours after seeding in experimental medium. (A) Phase contrast micrographs were taken 24 hours after treatment. Scale bar represents 100 μm. Results from a representative experiment, repeated at least 3 times, are shown. (B) Floating and attached cells were collected 24 hours after treatment. Apoptosis was assessed by flow cytometry after annexin V and propidium iodide staining. Results of 1 of 2 experiments with similar results are shown. (C) Procaspase-8, procaspase-9, cleaved caspase-3, and PARP were detected by Western blotting. Erk1/2 was used as loading control. *Nonspecific detection of BSA. Results from 1 of 5 representative experiments for HUVECs and 1 of 4 representative experiments for CDC.HMEC-1 cells are shown. (D) Cells were processed for F-actin detection 30 minutes after the addition of S 36578-2. Scale bar represents 20 μm.
Effects of a small cyclic RGD peptide on endothelial cells
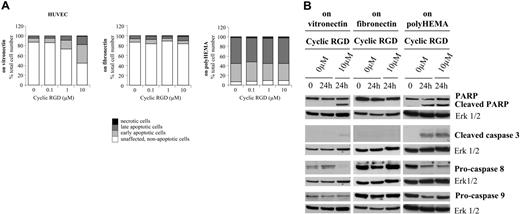
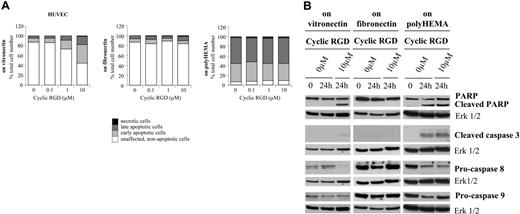
In light of findings described in the literature with other RGD mimetics, we compared the effects of S 36578-2, a small heterocyclic non–peptide RGD mimetic, with those induced by a small cyclic RGD peptide also highly selective for αvβ3 and αvβ5 integrins (IC50, 0.4 nM and 0.8 nM, respectively). Similar to S 36578-2, this compound inhibited the adhesion of HUVECs and CDC.HMEC-1 cells to vitronectin (IC50, 550 nM and 665 nM, respectively) and not to fibronectin (IC50, greater than 10 μM for each cell type). In HUVECs plated on vitronectin, the cyclic RGD peptide induced rapid morphologic changes followed by cell detachment (data not shown) and cell death (Figure 6A). As evaluated by flow cytometry, the induction of apoptosis was dose dependent with approximately 50% of affected cells after 24 hours of treatment at a concentration of 10 μM. Under these conditions, apoptosis occurred predominantly through the caspase-8–dependent extrinsic pathway (Figure 6B). No specific effect of the cyclic RGD peptide on cell morphology (data not shown) or cell death pathways was detected in cells plated on fibronectin or in cells maintained in suspension (Figures 6A-B). The effects of the cyclic RGD peptide were also evaluated on CDC.HMEC-1 cells and were found to be similar to those observed with S 36578-2 (data not shown). Results demonstrate that these 2 RGD mimetics with different molecular structures (non–peptide and peptide) and displaying similar target specificity exert similar biologic effects on endothelial cells.
Effects of S 36578-2 on HUVECs and CDC.HMEC-1 cells maintained in suspension. S 36578-2 was added to HUVECs or CDC.HMEC-1 cells, as indicated, at the time of seeding on polyHEMA. (A) Floating cells were collected 24 hours after treatment. Apoptosis was assessed by flow cytometry after annexin V and propidium iodide staining. Results of 1 of 2 experiments with similar results are shown. (B) Procaspase-8, procaspase-9, cleaved caspase-3, and PARP were detected by Western blotting. Erk1/2 was used as loading control. Results of 1 of 4 representative experiments are shown.
Effects of S 36578-2 on HUVECs and CDC.HMEC-1 cells maintained in suspension. S 36578-2 was added to HUVECs or CDC.HMEC-1 cells, as indicated, at the time of seeding on polyHEMA. (A) Floating cells were collected 24 hours after treatment. Apoptosis was assessed by flow cytometry after annexin V and propidium iodide staining. Results of 1 of 2 experiments with similar results are shown. (B) Procaspase-8, procaspase-9, cleaved caspase-3, and PARP were detected by Western blotting. Erk1/2 was used as loading control. Results of 1 of 4 representative experiments are shown.
Effects of S 36578-2 on nonimmortalized microvascular-derived endothelial cells
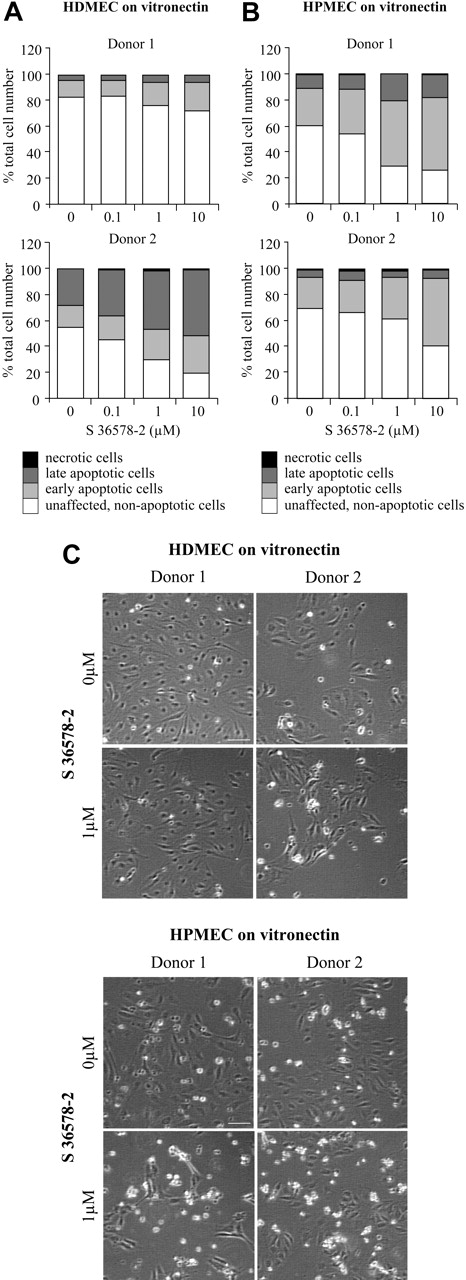
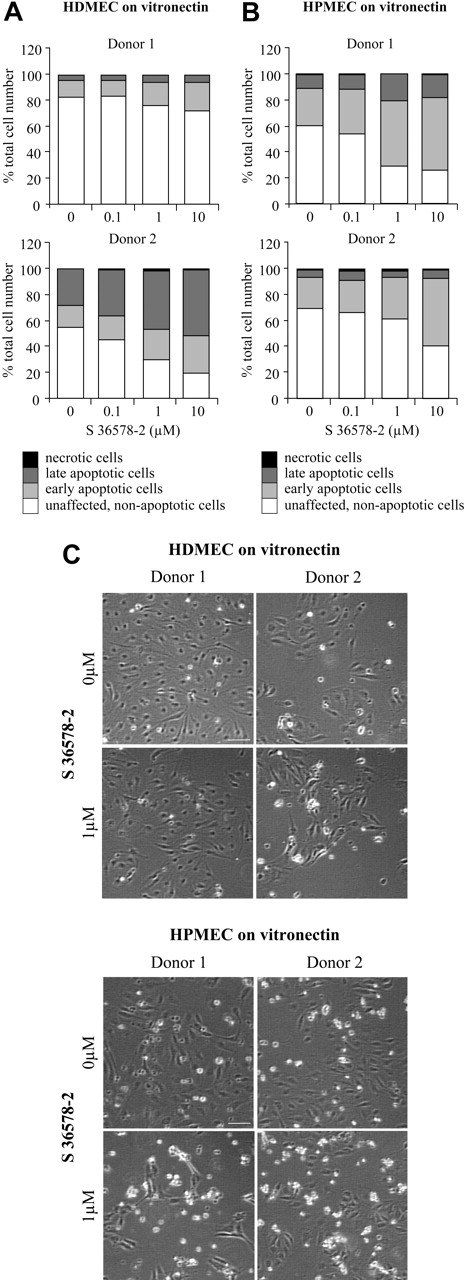
Finally, to determine whether observed differences in the response to S 36578-2 between vitronectin-attached HUVECs and microvascular lines (CDC.HMEC-1 cells and HMVECs) could be attributed to their different origins (large vs small blood vessels) or to establishment of the latter cell lines in culture, we examined the effect of the αv targeting agent on primary cultured microvascular endothelial cells. In addition to their not having been subjected to an immortalizing protocol that might have altered adhesive or antiapoptotic properties, these cells represent a more physiologically relevant in vitro angiogenesis model. Results from our analyses using cells isolated from 2 distinct sites (skin and lung) and 4 different donors are shown in Figure 7. In each case, S 36578-2 induced a dose-dependent increase of apoptosis (Figure 7A). Once again, cell death was linked to detachment (Figure 7B). In these primary cultures, apoptosis was triggered to various extent by merely switching the cells from growth medium to experimental medium (with reduced serum), and adding S 36578-2 enhanced cell death. These findings are consistent with our previous observations in fibroblasts that removing serum survival factors can potentiate anoikis.45
Effects of a small cyclic RGD peptide (cyclic RGD) on HUVECs spread on vitronectin or fibronectin or plated on polyHEMA-coated surfaces. Peptide was added 3 hours after seeding of cells on matrix components or when cells were seeded on polyHEMA-coated surfaces. (A) Apoptosis was assessed by flow cytometry after annexin V and propidium iodide staining. Results of 1 of 2 experiments with similar results are shown. (B) Procaspase-8, procaspase-9, cleaved caspase-3, and PARP were detected by Western blotting. Erk1/2 was used as loading control. *Nonspecific detection of BSA. Results of 1 of at least 2 experiments with similar results are shown.
Effects of a small cyclic RGD peptide (cyclic RGD) on HUVECs spread on vitronectin or fibronectin or plated on polyHEMA-coated surfaces. Peptide was added 3 hours after seeding of cells on matrix components or when cells were seeded on polyHEMA-coated surfaces. (A) Apoptosis was assessed by flow cytometry after annexin V and propidium iodide staining. Results of 1 of 2 experiments with similar results are shown. (B) Procaspase-8, procaspase-9, cleaved caspase-3, and PARP were detected by Western blotting. Erk1/2 was used as loading control. *Nonspecific detection of BSA. Results of 1 of at least 2 experiments with similar results are shown.
Discussion
Since the realization that endothelial cells are exquisitely sensitive to their ECM environment and that antagonism of integrin-mediated signals can induce apoptosis of endothelial cells in vivo,8 several antiangiogenic strategies to target integrin-based adhesion have been devised.2,46-48 Consequently, a number of models of antagonist-induced cell death have been proposed based on results from in vitro and in vivo studies carried out in different systems. Here we have analyzed the direct effects on cultured endothelial cells of a novel heterocyclic, non–peptide RGD mimetic, S 36578-2, which targets αvβ3 and αvβ5 integrins. The effects observed with this compound compared with those obtained using a cyclic RGD peptide displaying similar target specificity. Although our findings corroborate certain results in the literature, they are at variance with others.
Induction of cell death by S 36578-2 was found to closely correlate with its antiadhesive effect. In HUVECs and CDC.HMEC-1 cells, αvβ3 and αvβ5 integrins mediate interaction with vitronectin. As expected, S 36578-2 efficiently prevented adhesion and spreading of cells on this substrate. Further, it exerted a potent antiadhesive effect in HUVECs, preceded by the disruption of cell-matrix adhesion complexes and depolymerization of actin microfilaments, which led to the activation of the cellular apoptotic machinery. The findings that caspase-3 and its substrate, PARP, were cleaved in response to treatment demonstrated that HUVECs undergo a caspase-dependent apoptotic program. Two principal caspase-mediated death pathways under the influence of integrin ligation have been described in endothelial cells, an extrinsic pathway initiated by the activation of caspase-8 and an intrinsic pathway mediated by the release of mitochondrial components that activate caspase-9.49 In our experimental conditions, the extrinsic pathway appeared to predominate over the intrinsic pathway because the cleavage of caspase-8 was always observed whereas the cleavage of caspase-9 was detected only in the event of advanced cell death. Marconi et al22 obtained similar results after the blockade of β1 integrins in human keratinocytes. In the CDC.HMEC-1 cell line, most cells remained attached and partially spread on vitronectin despite the presence of S 36578-2, and this proved to be sufficient to suppress apoptosis. From these observations, we conclude that the antiadhesive effect exerted by the compound S 36578-2 is responsible for cell death. Accordingly, we observed a similar pattern of caspase activation and time course of PARP cleavage when cells were placed in suspension on poly-HEMA-coated plates. Under similar conditions, Aoudjit and Vuori21 reported that detachment-induced cell death in HUVECs resulted from activation of the Fas pathway by its ligand Fas-L, Fas-L/Fas interaction, Fas-FADD complex formation, and caspase-8 activation. Previous reports on epithelial cells also documented the involvement of FADD and caspase-8 in detachment-induced apoptosis.19,20 Although we do not know the mechanism by which caspase-8 becomes activated on the disruption of endothelial cell adhesion, we were unable to demonstrate an increased surface expression of Fas in HUVECs, as reported by Fawzi Aoudjit (unpublished observations, December 2000).
When cells were allowed to spread on fibronectin-coated surfaces, adding S 36578-2 had no effect on cell morphology or survival. In addition, in cells maintained in suspension (on polyHEMA-coated surfaces), the presence of the compound did not accelerate the onset of apoptosis or potentiate cell death. These findings led us to propose that S 36578-2 does not directly activate intracellular caspases in an integrin-independent manner, as reported for peptidic compounds containing an RGD motif. Indeed, previous studies have demonstrated that linear RGD-based peptides can penetrate cells and directly activate procaspase-3 in lymphocytes and cardiomyocytes23,24 or procaspases 8 and 9 in endothelial cells.26 In these studies, linear-derivatized RGD peptides were detected within cells, and it was proposed that caspase activation occurred after interaction of the RGD motif with a specific binding site present on proteases. Concentrations of RGD peptides in these experiments were considerably higher than those of the non–peptide RGD mimetic S 36578-2 used in our study. Interestingly, cyclic RGD peptides were also found to enter cells by an integrin-independent, fluid-phase endocytosis pathway, without altering the number of functional integrins at the cell surface, whereas a blocking anti–αv integrin monoclonal antibody entered cells through an integrin-dependent endocytic pathway and resulted in the down-regulation of surface integrins.25 Thus far, we have no data suggesting that S 36578-2 escapes integrin-independent or -dependent internalization and remains outside of cells. However, the cellular consequences of treatment with different classes of targeting agents may have important biologic consequences in vivo that limit certain applications, such as function blockers, while being advantageous for others, such as imaging agents or vice versa.
Effects of S 36578-2 on HDMECs and HPMECs spread on vitronectin. The compound S 36578-2 was added to cells 3 hours after seeding cells in experimental medium. Floating and attached cells were collected 24 hours after treatment. Apoptosis was assessed by flow cytometry after annexin V and propidium iodide staining in HDMECs (A) and HPMECs (B). Results of 2 experiments (1 per donor) are shown. (C) Phase contrast micrographs were taken 24 hours after treatment. Scale bar represents 100 μm. Results of 2 experiments (1 per donor) are shown.
Effects of S 36578-2 on HDMECs and HPMECs spread on vitronectin. The compound S 36578-2 was added to cells 3 hours after seeding cells in experimental medium. Floating and attached cells were collected 24 hours after treatment. Apoptosis was assessed by flow cytometry after annexin V and propidium iodide staining in HDMECs (A) and HPMECs (B). Results of 2 experiments (1 per donor) are shown. (C) Phase contrast micrographs were taken 24 hours after treatment. Scale bar represents 100 μm. Results of 2 experiments (1 per donor) are shown.
Our finding that endothelial cells plated on immobilized fibronectin, or type I collagen, were totally resistant to apoptosis-inducing effects of S 36578-2 shows that this compound does not promote IMD. According to the IMD model, unligated or antagonized integrins directly recruit and activate caspase-8 in adherent cells.27 We verified that levels of αvβ3 and αvβ5 integrins were not significantly down-regulated in cells plated on fibronectin, in contrast to cells plated on vitronectin, to exclude the possible loss of targeted receptors and thereby the loss of a potential proapoptotic stimulus. Thus, in cultured endothelial cells, S 36578-2 does not induce apoptosis when αvβ3 and αvβ5 integrins are empty or antagonized, whereas it promotes cell death only when cell adhesion is dependent on αvβ3 and αvβ5 integrins. These conditions were artificially established in our experiments by plating cells on vitronectin-coated surfaces in reduced serum medium and by treating them with S 36578-2 before significant amounts of αvβ3/αvβ5 integrin binding components were secreted and incorporated into the ECM. However, as has been pointed out by others,4 it is unlikely that integrins remain unligated in vivo. In addition to vitronectin, αvβ3 integrin can bind multiple ECM and matrix-associated proteins, including fibronectin, proteolyzed collagen, thrombospondin, osteopontin, DEL1 (developmentally regulated endothelial locus-1), MMP-2 (matrix metalloproteinase), thrombin, von Willebrand factor, and bFGF. Furthermore, αvβ3 integrin associates with transmembrane molecules such as growth factor receptors (insulin and PDGFβ receptors, VEGF-R2),50 and it is a target for many antiangiogenic proteins and fragments.3 All these interactions are likely to influence the proangiogenic or antiangiogenic actions of αvβ3 in given sites and in different pathologic situations, thus accounting for difficulties encountered in assessing the role of this integrin in angiogenesis.
Our results obtained with S 36578-2 could be extended to the cyclic RGD peptide used in our study, which displays similar specificity for αvβ3 and the related αvβ5 integrin. However, 10-fold higher concentrations of the cyclic peptide were required to induce cell detachment from vitronectin and death. A cyclic RGDfV peptide was also recently found to induce detachment and apoptosis in human brain microvascular endothelial cells plated on vitronectin by a mechanism involving ceramide metabolic pathways.51 In this study, the authors claimed that cell detachment was not required for RGDfV-induced apoptosis based on their observation that peptide treatment of cells plated on poly-L-lysine–coated surfaces led to cell death without detachment. Cell rounding, however, did occur. In our hands, HUVECs plated on poly-L-lysine did not spread either, yet rounded cells eventually detached and underwent cell death in the absence or presence of an RDG mimetic (data not shown). Finally, the cyclic RGD peptide did not affect cell morphology or induce apoptosis in cells spread on fibronectin (or type I collagen; data not shown). Similarly, Nisato et al52 reported that the same peptide failed to exhibit morphologic changes or cytotoxic effects in bovine endothelial cells plated on fibrin or type I collagen gels at doses up to 50 μM. This cyclic RGD peptide did inhibit in vitro capillary morphogenesis of the bovine endothelial cells.
CDC-HMEC-1 cells displayed decreased sensitivity to S 36578-2 and the cyclic RGD peptide compared with HUVECs. In addition, the HMVEC line, established by expression of the human telomerase catalytic enzyme (hTERT), rather than SV40 large T antigen expression, as in CDC.HMEC-1 cells, was totally resistant to S 36578-2–induced detachment and death. Whereas endothelial cells from small vessels have been shown to display decreased sensitivity to a cyclic RGD mimetic compared with large-vessel cells,52 our data showing the sensitivity of primary microvascular endothelial cells to S 36578-2 suggest that the immortalized nature of the 2 microvascular cell lines, rather than their origin, may confer this resistance. The failure of CDC.HMEC-1 cells and HMVECs to detach from vitronectin-coated plates after treatment may be attributed to the presence of additional vitronectin-binding components on their surfaces, such as urokinase-type plasminogen activator receptor (uPAR),53 yet this remains to be examined.
It should be noted that endothelial cells may not be the sole or most relevant target of αv integrin targeting agents for antiangiogenic therapy. An anti–human αvβ3 antibody, which is strictly species specific, was found to slow the neovascularization and growth of human tumors implanted in mice, indicating that the host vasculature was negatively regulated by an undefined mechanism.8 Indeed, in addition to endothelial cells, αvβ3 can be found on tumor cells and on perivascular cells such as smooth muscle cells.54 In this regard, the present study has unequivocally shown that targeting of αvβ3 and αvβ5 integrins with a non–peptide RGD mimetic does not provoke a direct apoptotic response in endothelial cells plated on provisional matrix components, such as fibronectin. Clearly, further studies are warranted to understand the antiangiogenic action of these compounds in vivo and the role of their targets, αv integrins, to guide the optimization of therapeutic intervention and possibly develop new functions for these molecules, such as tumor site–directed delivery or imaging agents.
Prepublished online as Blood First Edition Paper, July 11, 2006; DOI 10.1182/blood-2006-05-023580.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Stéphane Léonce and Stéphanie Giraudet for the flow cytometric analyses and Annie Genton for the binding data. We also thank Dominique Grall for her valuable contributions during the initial stages of this work.