Abstract
Signaling from collagen and G protein–coupled receptors leads to platelet adhesion and subsequent thrombus formation. Paracrine agonists such as ADP, thromboxane, and Gas6 are required for platelet aggregate formation. We hypothesized that thrombi are intrinsically unstable structures and that their stabilization requires persistent paracrine activity and continuous signaling, maintaining integrin αIIbβ3 activation. Here, we studied the disassembly of human and murine thrombi formed on collagen under high shear conditions. Platelet aggregates rapidly disintegrated (1) in the absence of fibrinogen-containing plasma; (2) by blocking or inhibiting αIIbβ3; (3) by blocking P2Y12 receptors; (4) by suppression of phosphoinositide 3-kinase (PI3K) β. In murine blood, absence of PI3Kγ led to formation of unstable thrombi, leading to dissociation of multiplatelet aggregates. In addition, blocking PI3Kβ delayed initial thrombus formation and reduced individual platelet-platelet contact. Similarly without flow, agonist-induced aggregation was reversed by late suppression of P2Y12 or PI3K isoforms, resulting in single platelets that had inactivated αIIbβ3 and no longer bound fibrinogen. Together, the data indicate that continuous outside-in signaling via P2Y12 and both PI3Kβ and PI3Kγ isoforms is required for perpetuated αIIbβ3 activation and maintenance of a platelet aggregate. This novel concept of intrinsic, dynamic thrombus instability gives possibilities for the use of antiplatelet therapy.
Introduction
Platelet plug formation at sites of vascular injury is considered to consist of 3 phases: initiation, propagation, and perpetuation.1,2 The initiation phase involves platelet adhesion to von Willebrand factor (VWF) and to collagen exposed in the vessel wall. In the propagation phase, activated platelets secrete mediators such as ADP, thromboxane A2 (TxA2), and Gas6, which activate other platelets to form aggregates. In the perpetuation phase, fibrin formation and less well-understood postaggregation events are assumed to accomplish stabilization of the thrombus.
At arterial shear rates, the thrombotic process is initiated by the tethering of platelets via glycoprotein (GP) Ib-IX-V to VWF, bound to collagen.3 In human and murine platelets, 2 interacting collagen receptors, GPVI and integrin α2β1, mediate stable adhesion to collagen.4-7 The signaling receptor GPVI triggers series of activation events, including integrin αIIbβ3 activation (providing binding sites, for example, for fibrinogen) and Ca2+ mobilization and secretion, which all function to recruit other platelets.8,9
During the propagation phase of thrombus formation, released ADP and TxA2 in a paracrine way pursue the activation process, mediated by P2Y1, P2Y12, and TPα receptors.8,10,11 The Gq-coupled P2Y1 and TPα receptors evoke Ca2+ mobilization and protein kinase C activity. The P2Y12 signaling pathway involves Gi-mediated inhibition of adenylyl cyclase, which lowers cyclic AMP and indirectly enhances Ca2+ signal generation.12-14 P2Y12 signaling via Gi also leads to activation of the phosphoinositide 3-kinase (PI3K) and protein kinase B signaling pathways. However, it is still unclear how all these various components combine to mediate inside-out αIIbβ3 signaling.15-17 Platelets express various PI3K isoforms, including the class Ia isoforms, p110α and p110β (PI3Kβ), and the class Ib isoform, p110γ (PI3Kγ; Jackson et al18 ). The literature is also unclear whether PI3Kβ18 or PI3Kγ19 regulates αIIbβ3 activation and, thus platelet aggregation.
Recent studies using knock-out mouse models have pointed to the existence of “late” mechanisms in the so-called “stabilization phase” of thrombus formation. Several molecular components appear to trigger the formation of close platelet-platelet contacts and hence consolidate thrombi to stable, nonembolizing structures. These include the P2Y1 and P2Y12 receptors,10,20 Gas6 and Gas6-receptors,21 ephrins and Eph kinases,22 CD40 ligand (CD40L),23 and signaling lymphocytic activation molecule (SLAM).24 Mice deficient in 1 of these components exhibit an intriguing similarity in phenotype (ie, all showing diminished thrombus formation and increased embolization). In essence, these findings raise the possibility that thrombi are intrinsically unstable, and may require multiple postaggregation events to become stabilized. This implies that a common platelet signaling mechanism may convoy the transition from unstable to stable aggregates.
In this paper, we hypothesized that thrombi are highly dynamic structures that require continuous paracrine activity and signaling and are needed for persistent integrin activation to keep platelets together and prevent their embolization. In contrast to the current static (3-phase) model of thrombus formation, the present results point to a dynamic model of thrombus buildup and stabilization, operative in human and murine blood and involving 2 different PI3K isoforms.
Materials and methods
Materials
MRS2179, indomethacin, and wortmannin were from Sigma (St Louis, MO). The P2Y12 receptor antagonist AR-C69931MX was kindly provided by Astra-Zeneca (Charnwoord, United Kingdom). LY294002 was from Calbiochem (La Jolla, CA), aggrastat was from Merck-Sharp-Dohme (Haarlem, the Netherlands), iloprost (Ilomedine) was from Schering (Berlin, Germany) and Oregon green 488 (OG)–fibrinogen was from Molecular Probes (Eugene, OR). Isoform-selective PI3K inhibitors were a kind gift from the Baker Heart Research Institute (Melbourne, Victoria, Australia): TGX221, selective for the p110β isoform; AS252424, selective for p110γ; and IC87114, selective for PI3Kδ, synthesized as described.18,25 FITC-labeled monoclonal antibody (mAb) PAC1 was from Becton Dickinson (San Jose, CA) and nonblocking Alexa fluor 568 (AF)–labeled mAb Xia.B2 directed against mouse and human GPIb was a kind gift from Emfret (Würzburg, Germany). Other reagents were from sources as described before.26
Human blood and platelets
Human blood was drawn from healthy volunteers after full informed consent. Approval was obtained from the CARIM institutional review board for these studies. Blood was collected into citrate, acid citrate dextrose (ACD), or 40 μM Phe-Pro-Arg chloromethyl ketone (PPACK), as required.7 Platelet-rich plasma (PRP), platelet-free plasma (PFP), and washed platelets were obtained as described.27
Animal blood and platelets
Thrombus formation under flow
Flow experiments over collagen were performed using human blood collected in 40 μM PPACK, or mouse blood collected in 40 μM PPACK and 5 U/mL heparin. Coverslips were coated with fibrillar type I Horm collagen and blocked. Coverslips inserted into a transparent, parallel-plate perfusion chamber were subjected to fluorescence microscopy, as described.7 A Visitech digital imaging system (Visitech, Sunderland, United Kingdom) equipped with 2 intensified, charge-coupled device (CCD) cameras was used. The system was connected to an inverted microscope (Nikon Diaphot 200; Nikon, Tokyo, Japan). Blood was perfused through the flow chamber at shear rates of 150 to 1000 s–1 usually for 4 minutes. Secondary perfusion was performed with plasma or HEPES buffer (pH 7.45) for 10 minutes at the same shear rate. High-resolution phase-contrast and fluorescent images were recorded in parallel in real time (> 10 random microscopic fields with a 40×/1.3 NA oil objective). Where indicated, FITC-PAC1 (1:20) was added during secondary perfusion. Images were analyzed on surface area coverage and on distribution of areas of individual segmented features using ImagePro software, version 4.1 (ImagePro, Silver Spring, MD).7 Shear experiments under coagulant conditions were performed by infusion of 1/10 volume of 75 mM CaCl2 and 37.5 mM MgCl2 into the citrated blood during flow, basically as described elsewhere.29
High-speed confocal laser scanning microscopy
Using the same flow chamber, high-speed laser scanning microscopy was performed with blood containing anti-GPIb–labeled platelets to measure thrombus stability. A single-photon, LSM Live5 line-scanning confocal system from Carl Zeiss (Jena, Germany) was used to collect in real time 3D overviews of thrombi at an excitation wavelength of 532 nm and emission wavelength of 550 to 615 nm. Z-stacks of 8 slices (160 × 160 × 19.7 μm) were collected at 150 full frames/sec. Images were analyzed with minimal processing using LSM 3D deconvolution software (Carl Zeiss, Jena, Germany).
Flow cytometry
Platelets were stimulated with indicated agonist, after which inhibitors were added. After 10 minutes, label was added and samples were fixed in PBS with 1% paraformaldehyde. Integrin αIIbβ3 activation was detected from the binding of FITC-PAC1 antibody to the activated β3 chain of αIIbβ3, or by using OG-labeled fibrinogen.21
Platelet aggregation
Platelet shape change and aggregation were measured by aggregometry (Chronolog, Havertown, PA) under constant stirring (37°C). PPACK-anticoagulated PRP was adjusted to 250 × 109 platelets/L.
Statistics
Data are means ± SEM. Significance of differences was determined with the Mann-Whitney U test or the independent samples t test, as appropriate, using the statistical package for social sciences (SPSS 11.0; SPSS, Chicago, IL). Size distribution of platelet aggregates was evaluated by χ2 analysis.7
Results
Presence of fibrinogen and continuous platelet signaling is required to prevent disassembly of thrombi under flow
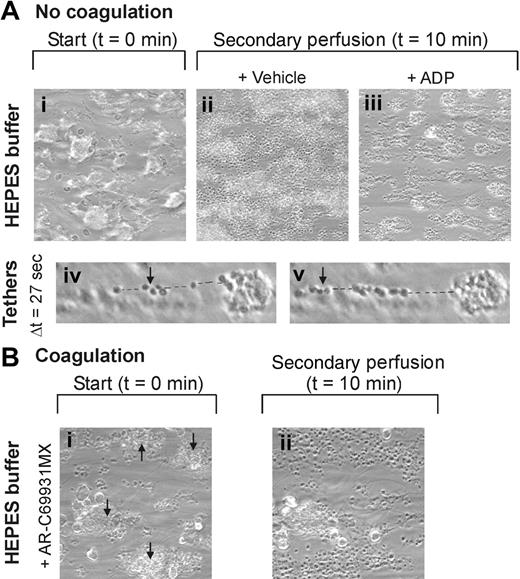
High-shear perfusion of PPACK-anticoagulated blood (containing physiologic millimolar concentrations of free Ca2+ and Mg2+) over VWF/collagen leads to rapid thrombus formation in a process that is similarly regulated for human and mouse platelets.4,8,18,26 With human thrombi that were formed via GPVI on a VWF/collagen surface at high shear rate (1000 s–1), we studied the factors contributing to thrombus disassembly by microscopic imaging. During secondary perfusion with plasma, platelet aggregates typically remained stable; only a few platelets left the microscopic field (Figure 1Ai-ii and Video S1, which is available on the Blood website; see the Supplemental Videos link at the top of the online article.). However, secondary perfusion with fibrinogen-depleted plasma provoked marked instability. Aggregates completely disintegrated, leaving only a single platelet layer at the VWF/collagen surface (Figure 1Aiii). Measurement of the size distribution of the remaining features showed that large and small aggregates were almost completely dissolved (Figure 1B). Essentially, the same results were obtained in perfusion experiments at a low shear rate of 150 s–1 (data not shown).
The role of ADP release and signaling was investigated by secondary perfusion with plasma containing the P2Y12 receptor antagonist AR-C69931MX.10,13 Application of this compound resulted in immediate disintegration of the thrombi, with a 50% reduction in aggregate size after 4 to 6 minutes (Figure 1Aiv). Quantitative analysis again indicated complete disappearance of large aggregates after 10 minutes (Figure 1B). Secondary perfusion with the αIIbβ3 antagonist aggrastat also gave rapid, complete disaggregation (data not shown). Further, secondary perfusion with the PI3K inhibitors wortmannin (Figure 1Av) or LY294002 (data not shown) had a strong but less complete effect, leaving only small aggregates adhered to the collagen.
Fibrinogen binding and ADP receptor function are needed for thrombus stability under flow. (A) Human blood was flowed over VWF/collagen at 1000 s–1 for 4 minutes. Thrombi were formed on coverslips (i). These were then perfused again for 10 minutes with PPACK-anticoagulated plasma containing indicated antagonists: vehicle (ii), fibrinogen-depleted plasma (iii); AR-C69931MX (30 μM) (iv), wortmannin (1 μM) (v), MRS2179 (100 μM) (vi), and AR-C69931MX plus MRS2179 (vii). Representative phase-contrast images (180 × 180 μm) are given after secondary perfusion (n = 3-5). (B) Histograms of features obtained by image analysis; estimated numbers of platelets per feature were 1 to 2, 2 to 8, 8 to 40, 40 to 130, and more than 130, as indicated. Secondary perfusion was with control plasma, fibrinogen-depleted plasma, or plasma plus AR-C69931MX (χ2 analysis). Data are shown as mean ± SEM (n = 3–5).
Fibrinogen binding and ADP receptor function are needed for thrombus stability under flow. (A) Human blood was flowed over VWF/collagen at 1000 s–1 for 4 minutes. Thrombi were formed on coverslips (i). These were then perfused again for 10 minutes with PPACK-anticoagulated plasma containing indicated antagonists: vehicle (ii), fibrinogen-depleted plasma (iii); AR-C69931MX (30 μM) (iv), wortmannin (1 μM) (v), MRS2179 (100 μM) (vi), and AR-C69931MX plus MRS2179 (vii). Representative phase-contrast images (180 × 180 μm) are given after secondary perfusion (n = 3-5). (B) Histograms of features obtained by image analysis; estimated numbers of platelets per feature were 1 to 2, 2 to 8, 8 to 40, 40 to 130, and more than 130, as indicated. Secondary perfusion was with control plasma, fibrinogen-depleted plasma, or plasma plus AR-C69931MX (χ2 analysis). Data are shown as mean ± SEM (n = 3–5).
Secondary perfusion of the thrombi with plasma containing the P2Y1 receptor blocker, MRS2179, led to a delayed and partial disappearance of platelets, which was about 3-fold slower than that observed with AR-C69931MX (Figure 1Avi). Simultaneous blocking of P2Y12 and P2Y1 did not further increase the AR-C69931MX effect (Figure 1Avii). Apparently, P2Y12 plays a more important role in stabilization of the thrombi than P2Y1.
In a next set of experiments, aggregates on VWF/collagen were perfused again with HEPES buffer instead of plasma. This resulted in rapid dissolution, in which platelets detached from the aggregates and sometimes readhered to uncovered collagen surface (Figure 2Ai-ii; Video S2). Real-time confocal microscopic recording using platelets stained with AF-labeled mAb against GPIb indicated that both height and volume of the thrombi rapidly decreased upon secondary perfusion with HEPES buffer but not plasma (not shown). In marked contrast, addition of ADP to the HEPES buffer prevented the disaggregation and resulted in compact and stable thrombi that remained resistant to disassembly as long as ADP was present (Figure 2Aiii). Without ADP, platelets typically detached as strings connected by membrane tethers (Figure 2Aiv-v). Together, these results indicate that the presence of plasma fibrinogen and continuous platelet signaling via ADP, P2Y12, and PI3K is required to prevent disintegration of the thrombi.
Since these experiments were performed in the absence of coagulation, we examined whether continuous ADP signaling also controls thrombus stability under conditions of thrombin and fibrin formation. In perfusion experiments where citrated blood was recalcified before reaching the flow chamber, mixed thrombi of platelets and fibrin were formed on the VWF/collagen surface (Figure 2Bi). Secondary perfusion with AR-C69931MX in buffer resulted in disaggregation of the fibrin-containing thrombi, except for those which contained large amounts of visible fibrin and had a central core with rigid appearance (Figure 2Bi-ii; Video S3). Again, this disaggregation was completely prevented by secondary perfusion with ADP (Video S4). These results demonstrate the importance of persistent ADP receptor function in thrombus stability under conditions of coagulation.
Key role of ADP receptor in thrombus stability in the absence and presence of coagulation. (A) Human PPACK-anticoagulated blood was flowed over VWF/collagen at 1000 s–1 for 4 minutes. Thrombi were formed on coverslips (i). These were perfused again for 10 minutes with HEPES buffer (pH 7.45) containing 2 mM CaCl2 and 2 mM MgCl2. Vehicle (ii) or ADP (20 μM) (iii) was added during secondary perfusion. Representative phase-contrast images (180 × 180 μm) are given after 10 minutes. Strings of platelets connected by tethers (dotted line) detaching during 27 seconds of perfusion (iv-v). (B) Citrated blood was recalcified with CaCl2/MgCl2 before flow over VWF/collagen at 1000 s–1. Fibrin-containing thrombi on coverslips were perfused again for 10 minutes with the same HEPES buffer supplemented with AR-C69931MX (30 μM) (Video S3). Representative phase-contrast images are given of 1 field before and after secondary perfusion (i-ii). Note dissolution of all but one of the fibrin-containing thrombi. Arrows indicate fibrin-containing thrombi. Shown are representative images (n = 3-5 experiments).
Key role of ADP receptor in thrombus stability in the absence and presence of coagulation. (A) Human PPACK-anticoagulated blood was flowed over VWF/collagen at 1000 s–1 for 4 minutes. Thrombi were formed on coverslips (i). These were perfused again for 10 minutes with HEPES buffer (pH 7.45) containing 2 mM CaCl2 and 2 mM MgCl2. Vehicle (ii) or ADP (20 μM) (iii) was added during secondary perfusion. Representative phase-contrast images (180 × 180 μm) are given after 10 minutes. Strings of platelets connected by tethers (dotted line) detaching during 27 seconds of perfusion (iv-v). (B) Citrated blood was recalcified with CaCl2/MgCl2 before flow over VWF/collagen at 1000 s–1. Fibrin-containing thrombi on coverslips were perfused again for 10 minutes with the same HEPES buffer supplemented with AR-C69931MX (30 μM) (Video S3). Representative phase-contrast images are given of 1 field before and after secondary perfusion (i-ii). Note dissolution of all but one of the fibrin-containing thrombi. Arrows indicate fibrin-containing thrombi. Shown are representative images (n = 3-5 experiments).
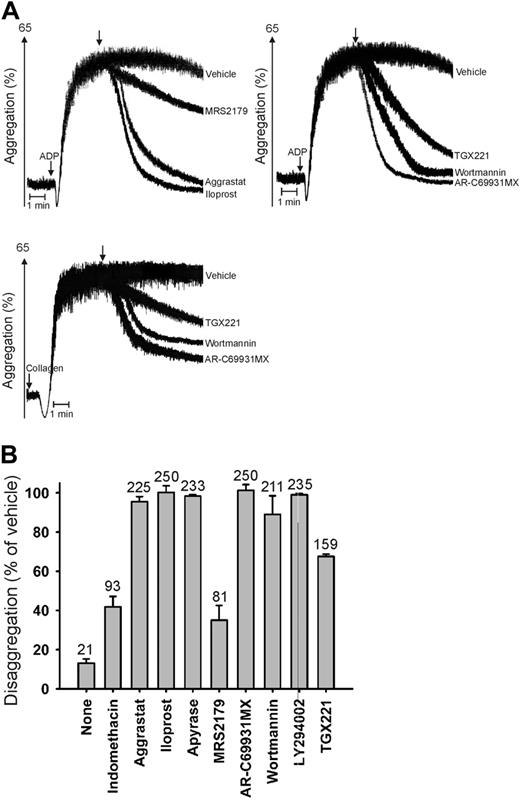
Continuous ADP-induced signaling via P2Y12 and PI3Kβ is needed for irreversible platelet aggregation. (A) Aggregation of PPACK-anticoagulated PRP was triggered while stirring with 20 μM ADP or 1 μg/mL collagen (n = 3-5). Inhibitors were added after 2 minutes, when aggregation was maximal: indomethacin (55 μM), aggrastat (2 μg/mL), iloprost (20 nM), apyrase (0.2 U/mL), MRS2179 (100 μM), AR-C69931MX (30 μM), wortmannin (1 μM), LY294002 (50 μM), or TGX221 (1 μM). Shown are representative aggregation traces. Arrows indicate addition of inhibitors. (B) Percentages of disaggregation with ADP after 12 minutes; numbers above bars indicate platelet counts (× 109/L). Data are mean ± SEM.
Continuous ADP-induced signaling via P2Y12 and PI3Kβ is needed for irreversible platelet aggregation. (A) Aggregation of PPACK-anticoagulated PRP was triggered while stirring with 20 μM ADP or 1 μg/mL collagen (n = 3-5). Inhibitors were added after 2 minutes, when aggregation was maximal: indomethacin (55 μM), aggrastat (2 μg/mL), iloprost (20 nM), apyrase (0.2 U/mL), MRS2179 (100 μM), AR-C69931MX (30 μM), wortmannin (1 μM), LY294002 (50 μM), or TGX221 (1 μM). Shown are representative aggregation traces. Arrows indicate addition of inhibitors. (B) Percentages of disaggregation with ADP after 12 minutes; numbers above bars indicate platelet counts (× 109/L). Data are mean ± SEM.
Continuous signaling via P2Y12 and PI3K isoforms is required for irreversible platelet aggregation and persistent αIIbβ3 activation
Aggregation of platelets in suspension in response to weak agonists like ADP or thrombin-receptor–activating peptide can be reversible.19 In particular, this is true at physiologic millimolar concentrations of free Ca2+ and Mg2+. However, the mechanism underlying this reversibility is still obscure. We examined the disaggregation of platelets at conditions mimicking those of the flow experiments (ie, in PPACK-anticoagulated plasma). Platelets were first activated with ADP, after which interventions were applied at the time of maximal aggregation (Figure 3A). Secondary platelet inhibition with cyclic AMP-elevating iloprost or αIIbβ3 blockade with aggrastat caused rapid and complete disassembly of the aggregates, but cyclooxygenase blocking with indomethacin was only partly effective (Figure 3B). A similar rapid reversion of aggregation was seen when ADP-degrading apyrase or the P2Y12 receptor antagonist AR-C69931MX was added. In contrast, the P2Y1 receptor blocker MRS2179 was only partly effective. Secondary addition of AR-C69931MX regained as much as 98% of the original platelet concentration, illustrating complete reversibility of the aggregation process. After 2 to 3 minutes, the disaggregation was partially complete, which is in a similar time interval as seen in the flow experiments. AC-C69931MX also reverted most of the platelet aggregation induced with collagen (72%; Figure 3A) or the thrombin-receptor peptide SFFLRN (63%).
To identify the involvement of signaling pathways downstream of the P2Y12 receptor, the PI3K inhibitors wortmannin and LY294002 were added 2 minutes after ADP or collagen stimulation (Figure 3A-B). Either compound completely (ADP) or nearly completely (collagen or SFFLRN) reversed the aggregate formation. In the case of ADP stimulation, secondary PI3K inhibition almost fully regained the original platelet count. Together, these results suggest that persistent ADP- or collagen-induced signaling via PI3K is required to keep platelet aggregates together. This was confirmed by triggering aggregation with the Gz-stimulating agonist epinephrine. A substantial part of the aggregation with epinephrine became reversible by secondary inhibition of P2Y12 or PI3K (data not shown). Typically, the specific PI3Kβ inhibitor TGX221 (Figure 3A) had a marked but less complete disaggregating effect, when given after ADP, collagen, or SFFLRN.
There is earlier and new evidence that αIIbβ3, once activated, can be switched off by late platelet inhibition.30,31 We used flow cytometry to determine how secondary P2Y12 and PI3K blockade affected the active conformation of αIIbβ3 in human platelets that were stimulated with ADP or other agonists. Secondary addition of AR-C69931MX to ADP-stimulated platelets rapidly and nearly completely reversed binding of fluorescent PAC1 mAb, directed against the activated β3 chain (Figure 4A). AR-C69931MX also abolished the binding of fluorescent fibrinogen to αIIbβ3 on preactivated platelets; apyrase and prostaglandin E1 were similarly effective. PI3K contributed to this perpetuation of integrin activation, since later addition of wortmannin (Figure 4B) or TGX221 reversed the binding of PAC1 and fibrinogen. Together, this indicates that ADP-stimulated αIIbβ3 activation requires continuous inside-out stimulation via P2Y12 and PI3K to remain in an active state.
Integrin αIIbβ3 activation was also investigated under flow conditions. Thrombi formed by perfusion of PPACK-anticoagulated blood were perfused again with plasma containing vehicle or antagonist. In the presence of vehicle, large PAC1-staining aggregates were seen (Figure 5Ai, 5B). In agreement with the flow cytometry results, secondary perfusion with AR-C69931MX (Figure 5Aiii), wortmannin (Figure 5Aiv), or iloprost (Figure 5B) led to almost complete reversion of the PAC1 staining of platelets on coverslip. To demonstrate that the absence of staining was not due to limited detection sensitivity, single platelets on collagen were allowed to spread for 20 minutes, and thereafter were stained with fluorescent PAC1 mAb (Figure 5Aii). These results indicate that under shear, P2Y12 and PI3K are also important for the persistent activation status of αIIbβ3. These novel findings indicate that platelet aggregation and αIIbβ3 activation are in fact intrinsically dynamic processes which require continuous (paracrine) signaling via especially P2Y12 and PI3K to become irreversible.
Continuous P2Y12 signaling is required for persistent αIIbβ3 activation. (A) Platelets in PPACK-anticoagulated PRP were activated with ADP (no stirring), and 2 minutes later inhibitors or antagonists were added (Figure 3). At indicated time points, activated αIIbβ3 was detected with FITC-PAC1, and integrin function was analyzed with OG-labeled fibrinogen. Histograms are given of FITC-PAC1 and OG-fibrinogen fluorescence distribution before and after AR-C69931MX addition. (B) Mean FITC-PAC1 fluorescence intensities ± SEM after incubation with inhibitor/antagonist (n = 3-4).
Continuous P2Y12 signaling is required for persistent αIIbβ3 activation. (A) Platelets in PPACK-anticoagulated PRP were activated with ADP (no stirring), and 2 minutes later inhibitors or antagonists were added (Figure 3). At indicated time points, activated αIIbβ3 was detected with FITC-PAC1, and integrin function was analyzed with OG-labeled fibrinogen. Histograms are given of FITC-PAC1 and OG-fibrinogen fluorescence distribution before and after AR-C69931MX addition. (B) Mean FITC-PAC1 fluorescence intensities ± SEM after incubation with inhibitor/antagonist (n = 3-4).
Dual contribution of murine p110β and p110γ PI3K isoforms to thrombus stability on collagen under flow
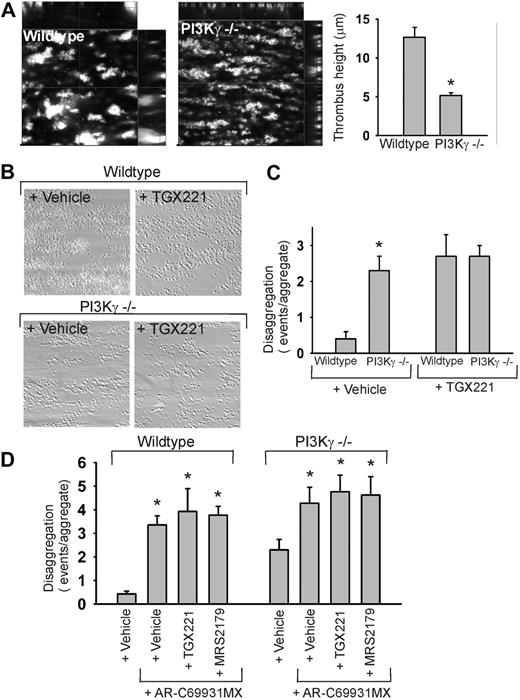
In mouse platelets, PI3Kγ (p110γ) has been implicated in ADP-dependent thrombus formation in vivo.19 We studied the contribution of this PI3K isoform to thrombus stabilization using blood from PI3Kγ–/– and wild-type control mice. Upon flow of blood over VWF/collagen, PI3Kγ-deficient platelets were normally activated via GPVI (not shown). However, they formed loose and very mobile thrombi, which often disintegrated shortly after the formation (Figure 6A; Video S5). This sharply contrasted to the quite stable thrombi that were formed with PI3Kγ+/+ blood (Figure 6A; Video S6). As a consequence, the average thrombus height reached after 4 minutes of perfusion was 60% lower in the case of PI3Kγ–/– blood (Figure 6A). Total thrombus volume per microscopic field was reduced similarly. While in wild-type blood, platelets only incidentally left from the aggregates, the knock-out platelets detached at a much higher frequency (Figure 6B-C). These platelets typically disaggregated as strings connected by membrane tethers, similar to that seen upon buffer perfusion of human blood.
Using newly designed isoform-selective PI3K inhibitors, it has appeared that PI3Kβ is involved in shear-induced platelet adhesion to VWF.18 Here we used these inhibitors applied at selective doses,18 to study the contribution to thrombus stability of the isoforms, PI3Kβ or p110β (TGX221), PI3Kγ or p110γ (AS252424), and PI3Kδ (IC87114). In murine thrombus formation, inhibition of PKI3β with TGX221 caused detachment of platelets in the case of wild-type blood, but did not further enhance the high detachment in PI3Kγ–/– blood (Figure 6C). Secondary perfusion with TGX221 resulted in nearly complete disintegration of aggregates of both PI3Kγ+/+ and PI3Kγ–/– blood, whereas wild-type aggregates remained largely intact after secondary perfusion with vehicle. Together, this indicates that PI3Kβ and PI3Kγ are both necessary for the formation of stable murine aggregates.
P2Y12 and PI3K signaling is needed for persistent αIIbβ3 activation under flow. (A) Human blood was flowed over VWF/collagen at 1000 s–1 for 4 minutes. Thrombi on coverslips were perfused again for 10 minutes with PPACK-anticoagulated plasma, and stained with FITC-PAC1 (1:20). Indicated antagonists were present during secondary perfusion: vehicle (i), AR-C69931MX (30 μM) (ii-iii), and wortmannin (1 μM) (iv). As a positive control, single platelets on coverslips were allowed to spread on collagen for 20 minutes before staining (ii). Shown are representative fluorescent images (180 × 180 μm) after secondary perfusion. (B) Mean surface area coverage of FITC-PAC1 fluorescence after secondary perfusion (n = 3); iloprost (20 nM) was present as indicated. Mean ± SEM; *P = .05 vs vehicle.
P2Y12 and PI3K signaling is needed for persistent αIIbβ3 activation under flow. (A) Human blood was flowed over VWF/collagen at 1000 s–1 for 4 minutes. Thrombi on coverslips were perfused again for 10 minutes with PPACK-anticoagulated plasma, and stained with FITC-PAC1 (1:20). Indicated antagonists were present during secondary perfusion: vehicle (i), AR-C69931MX (30 μM) (ii-iii), and wortmannin (1 μM) (iv). As a positive control, single platelets on coverslips were allowed to spread on collagen for 20 minutes before staining (ii). Shown are representative fluorescent images (180 × 180 μm) after secondary perfusion. (B) Mean surface area coverage of FITC-PAC1 fluorescence after secondary perfusion (n = 3); iloprost (20 nM) was present as indicated. Mean ± SEM; *P = .05 vs vehicle.
Yet, despite the similar number of disaggregation events of TGX221-treated wild-type blood and PI3Kγ–/– blood, there were marked differences in the type of detachment. Whereas TGX221 provoked detachment of (strings of) single platelets from a thrombus, in PI3Kγ–/– blood many multiplatelet complexes were separating. Addition of TGX221 to the PI3Kγ–/– blood greatly increased the time to initial thrombus formation, from 30 seconds to 51 seconds. This suggests that PI3Kβ and PI3Kγ have complementary but different roles in thrombus build-up and stability, in which PI3Kβ functions to keep individual platelets together on the collagen surface while PI3Kγ is essential for maintaining thrombus integrity.
To determine the importance of P2Y12 activity, the stability of murine thrombi under flow was measured in the presence of AR-C69931MX. The blockage of P2Y12 in PI3Kγ+/+ and also in PI3Kγ–/– blood led to a significant increase in number of disaggregation events (Figure 6D). Combined blockage of P2Y12 and PI3Kβ (TGX221) did not further increase these disaggregation events in either wild-type or PI3Kγ-deficient blood. Also, combined blockage of P2Y12 and P2Y1 with MRS2179 did not further increase the number of disaggregating platelets (Figure 6D). Together, this suggests that the P2Y12 signaling pathway controls murine thrombus stability in part via PI3Kγ and, in a compensatory way, via PI3Kβ.
Complementary roles of PI3Kβ and PI3Kγ in stabilization of murine thrombi. (A) Formation of thrombi during flow over VWF/collagen at 1000 s–1 for 4 minutes, recorded by high-speed fluorescence imaging. Blood from wild-type and PI3Kγ–/– mice was labeled with nonblocking AF5-568–anti-GPIb mAb Xia.B2. Left panel shows representative x-y images; partitions represent x-z and y-z cross-sections. Right panel shows height of thrombi after 4 minutes. (B-C) Murine blood containing vehicle or TGX221 (1 μM) was flowed over VWF/collagen. (B) Representative phase-contrast images (120 × 120 μm) after secondary perfusion. (C) Disaggregation events from murine thrombi during 4 minutes of flow. (D) Disaggregation events from murine thrombi in the presence of AR-C69931MX (30 μM) with/without TGX221 (1 μM) or MRS2179 (100 μM). Indicated are numbers of embolizing events (single or clustered) platelets per aggregate. Data are means ± SEM (n = 3-5 experiments; *P < .05 vs vehicle).
Complementary roles of PI3Kβ and PI3Kγ in stabilization of murine thrombi. (A) Formation of thrombi during flow over VWF/collagen at 1000 s–1 for 4 minutes, recorded by high-speed fluorescence imaging. Blood from wild-type and PI3Kγ–/– mice was labeled with nonblocking AF5-568–anti-GPIb mAb Xia.B2. Left panel shows representative x-y images; partitions represent x-z and y-z cross-sections. Right panel shows height of thrombi after 4 minutes. (B-C) Murine blood containing vehicle or TGX221 (1 μM) was flowed over VWF/collagen. (B) Representative phase-contrast images (120 × 120 μm) after secondary perfusion. (C) Disaggregation events from murine thrombi during 4 minutes of flow. (D) Disaggregation events from murine thrombi in the presence of AR-C69931MX (30 μM) with/without TGX221 (1 μM) or MRS2179 (100 μM). Indicated are numbers of embolizing events (single or clustered) platelets per aggregate. Data are means ± SEM (n = 3-5 experiments; *P < .05 vs vehicle).
Roles of human p110β and p110γ PI3K isoforms in thrombus stability on collagen under flow
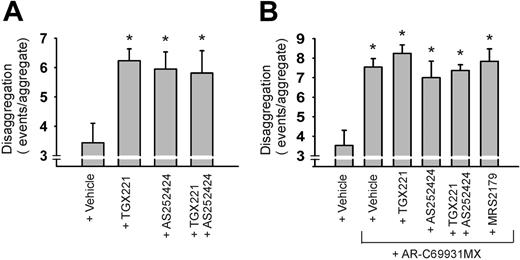
To study the function of the PI3K isoforms, human thrombi were formed on VWF/collagen under flow. When added to plasma after perfusion, either TGX221 or AS252424 greatly promoted the detachment of platelets (Figure 7A). In either case, strings of platelets continuously detached from the preformed aggregates, resulting in thrombus disintegration. Simultaneous application of both compounds to block both P3Kβ and PI3Kγ did not have an extra effect. Inhibition of PI3Kδ with IC87114 was completely ineffective.
To determine whether the contribution of PI3Kβ and PI3Kγ indeed involves P2Y12 receptor function, human thrombi were perfused again with human plasma supplemented with AR-C69931MX, alone or in combination with PI3K inhibitors. As shown in Figure 7B, additional inhibition of P3Kβ (TGX221) and/or PI3Kγ (AS252424) did not further increase the aggregate destabilizing effect of AR-C69931MX (P > .87). Since P2Y1 receptor blockage with MRS2179 could partly reverse the ADP-induced aggregation in platelet suspensions (Figure 3A, 3B), we re-examined the effect of this blocker under flow. In second perfusion with plasma, MRS2179 led to a 156% ± 32% increase in disaggregation events (ie, about one-third of the effect seen with AR-C69931MX). Importantly, MRS2179 was unable to further enlarge the effect of P2Y12 blockage with AR-C69931MX (Figure 7B). On the other hand, MRS2179 increased the combined effect of PI3Kβ and PI3Kγ inhibition on disaggregation events up to 158% ± 26% (n = 4; P = .05). Similar results were obtained with blood from wild-type and PI3Kγ–/– mice (data not shown). These results strongly suggest that the PI3K isoforms operate via a different pathway than P2Y1 receptors, but via the same pathway as used by P2Y12 receptors. Together with the earlier results, this indicates that the PI3K isoforms play a major role in thrombus stabilization mostly or exclusively downstream of P2Y12.
Discussion
The present results indicate that at physiologic millimolar concentrations of Ca2+ and Mg2+, continuous paracrine release of ADP and persistent signaling via PI3K is required to prevent dissociation of thrombi formed on VWF/collagen. This leads to the novel insight that thrombi are intrinsically dynamic structures, which is in contrast to the hitherto “static” model of thrombus initiation, propagation, and consolidation. Accordingly, platelet aggregation appears to be a reversible process, which can be reverted by suppressing paracrine processes, absence of fibrinogen, blocking of P2Y12 or αIIbβ3, or late platelet inhibition with cyclic AMP-elevating or PI3K-inhibiting agents. Together, this strongly suggests that, during the thrombotic process, integrins need to be continuously activated to prevent disassembly of platelet aggregates. Video microscopic imaging indicated that this disaggregation was an ongoing process, with platelets detaching as strings connected by membrane tethers. This suggests a gradual closing down of integrins, perhaps leaving patches of activated αIIbβ3 at contact sites in the tethered ends.
The current data explain why in animal thrombosis models10,32 and in human flow studies,8,18 absence or blocking of ADP receptors (P2Y12) is accompanied by increased embolization and reduced thrombus stability. Moreover, our data indicate that paracrine ADP and P2Y12 receptors play a crucial role in the dynamic stabilization of aggregates, both under static and flow conditions. Other signaling pathways (eg, by thromboxane receptors) also seemed to play moderate roles. Importantly, we also found that the thrombus-stabilizing effect of P2Y12 persists at conditions of thrombin generation and (limited) fibrin formation, which is relevant for the in vivo situation, where coagulation usually accompanies platelet aggregation.
Roles of P2Y12, PI3Kβ, and PI3Kγ in stabilization of human thrombi. Human blood was flowed over VWF/collagen at 1000 s–1 for 4 minutes. Disaggregation events were measured from thrombi during 10 minutes of secondary perfusion with plasma containing indicated inhibitors. (A) Effect of PI3K inhibitors TGX221 (1 μM) and AS252424 (1 μM). (B) Effect of P2Y12 inhibitor AR-C69931MX (30 μM) alone or combined with TGX221 (1 μM), AS252424 (1 μM), or MRS2179 (100 μM). Data are means ± SEM (n = 4 experiments; *P < .05 vs vehicle).
Roles of P2Y12, PI3Kβ, and PI3Kγ in stabilization of human thrombi. Human blood was flowed over VWF/collagen at 1000 s–1 for 4 minutes. Disaggregation events were measured from thrombi during 10 minutes of secondary perfusion with plasma containing indicated inhibitors. (A) Effect of PI3K inhibitors TGX221 (1 μM) and AS252424 (1 μM). (B) Effect of P2Y12 inhibitor AR-C69931MX (30 μM) alone or combined with TGX221 (1 μM), AS252424 (1 μM), or MRS2179 (100 μM). Data are means ± SEM (n = 4 experiments; *P < .05 vs vehicle).
Our findings are in contrast to the recent observation that P2Y1 and Ca2+ signaling are major factors contributing to late integrin activation.31 On the other hand, others have noted that P2Y1-, thromboxane-, and PAR1-dependent activation events, in turn, rely on feed-forward loops mediated by P2Y12.12,33 In all current sets of experiments (ie, disaggregation of platelets in suspension and in disaggregation of human and murine thrombi under flow), blocking of P2Y1 receptors with MRS2179 had a small but significant effect in causing aggregate instability. However, in these experiments, blocking of P2Y12 had a much larger effect and, importantly, MRS2179 did not increase the effect of P2Y12 blockage. Furthermore, the disaggregation effect of P2Y1 blockage, but not of P2Y12 blockage, was surpassed by additional inhibition of PI3Kβ (TGX221) and PI3Kγ (AS252424). We thus can conclude that P2Y12 or PI3K neutralization has a much larger effect on thrombus stability than P2Y1 inhibition, but also that it overlaps with the effect of P2Y1 inhibition. Together, this indicates that the contribution of P2Y1 to thrombus stabilization is either downstream from, or bypassed by, P2Y12 signaling.
Our findings lead to the intriguing concept that integrin αIIbβ3 needs continuous inside-out stimulatory signals (eg, by P2Y12 signaling) to prevent its inactivation. Phosphorylation of the β3 chain is supposed to be required for irreversible αIIbβ3 activation.34 However, because this phosphorylation can be reversible as well, the relation between the integrin phosphorylation state and the presently studied delayed platelet disaggregation still needs to be established. This also holds for the cAMP/protein kinase A–mediated mechanism of integrin closure, which is likely to be distinct from the cAMP effect on Ca2+ signal generation,35 but is still unidentified.
Others have proposed that P2Y12 plays a common role in the regulation of thrombus stabilization.10 The current findings significantly extend this by showing that the role of P2Y12 (1) is a continuous and long-term one; and (2) involves 2 effector pathways that are nonexclusively downstream of P2Y12 (ie, those of PI3Kβ and PI3Kγ). Although both PI3K isoforms are activated by ADP and have been implicated in integrin activation, likely via Gi, the downstream signaling effects are still debated (discussed in Jackson et al18 ). PI3K has been linked to Akt and Rap1b activation.17 Also, protein kinase C and the GDP-exchange proteins, Vav and CalDAG-GEFI, have been implicated in Rap1b and subsequent integrin activation.36,37 The literature thus points to various signaling pathways that can lead to conformational changes of αIIbβ3. It remains to be determined how PI3K isoforms are involved in these signaling pathways.
In vivo, knock-out mouse models lacking Gas6 (receptors), CD40L, or SLAM have in common with P2Y12–/– mice a delayed and impaired thrombus growth and an increased embolization.21,23,24 Platelets from these knock-out mice share an impaired ability to aggregate with ADP, suggesting that a common mechanism is responsible for the decreased thrombus stability. One possibility is that P2Y12 activity is a prerequisite for late contact-dependent signaling via these proteins or, alternatively, that diminished platelet-platelet contact may lead to reduced ADP secretion and paracrine activation via P2Y12. Recent data indicate that occupied Gas6 receptors of platelets signal via class Ia PI3K forms, although the precise isoform is unknown.21 Considering our current findings, it is not unlikely that ADP secretion and the P2Y12 pathway is de facto upstream of Gas6 signaling via PI3K, but this remains to be determined.
The finding that PI3Kα has a distinct function in the stabilization of aggregates is not unexpected. This corresponds with published evidence that PI3Kγ-deficient mice show impaired platelet aggregation together with a reduced thrombotic tendency.19,38 Interestingly, this isoform accomplishes kinase-dependent and -independent effects in a variety of cells.39 Whether (lipid) kinase activity is involved in the thrombus-stabilizing effect is currently unknown. Using the isoform-specific inhibitor TGX221, we also find that PI3Kβ contributes to the dynamic consolidation of thrombi in both human and murine (PI3Kγ knock-out) blood. This compound has a highly specific inhibitory activity toward PI3Kβ (inhibitory concentration at 50% [IC50], 5 nM with 1000-fold selectivity over a broad range of protein kinases), and has previously been shown to affect αIIbβ3-mediated stable adhesion under high shear.18 PI3Kβ is likely to be an important effector, since TGX221 is similarly active in provoking disaggregation as the general PI3K inhibitor wortmannin. In the prevention of platelet disaggregation, each isoform PI3Kβ and PI3Kγ seems to have a distinct function. Active PI3Kβ appears to mediate individual platelet-platelet contact, whereas PI3Kγ is essential for keeping the thrombus together (ie, to maintain the integrity of the entire thrombus). In sum, in contrast to the static (3-phase) model of thrombus formation, we propose a dynamic model of thrombus buildup and stabilization. Herein, continuous signaling, involving paracrine P2Y12 and both isoforms PI3Kβ and PI3Kγ, are needed to stabilize growing thrombi and prevent their dissolution. This signaling then leads to perpetuated αIIbβ3 activity and fibrinogen binding, both in human and mouse platelets. This novel concept of intrinsic, dynamic thrombus instability gives new possibilities for the use of antiplatelet therapy (eg, with PI3Kβ and PI3Kγ as new targets). Also, we infer that P2Y12-inhibitory medication such as clopidogrel, although this drug is only partly effective in blocking P2Y12 signaling,27 may not only prevent thrombus formation, but also act to dissolve preformed thrombotic aggregates.
Prepublished online as Blood First Edition Paper, July 13, 2006; DOI 10.1182/blood-2006-03-006338.
Supported by grants from the Netherlands Heart Foundation (2002B014) and the Netherlands Organization for Scientific Research (902-16-276).
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Carl Zeiss Jena for access to confocal equipment and D. van Meensel for assistance.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal