Abstract
Erythropoiesis, the essential process of hematopoietic stem cell development into erythrocytes, is controlled by lineage-specific transcription factors that determine cell fate and differentiation and by the hormone erythropoietin that stimulates cell survival and proliferation. Here we identify the Sry-related high-mobility-group (HMG) box transcription factor Sox6 as an important enhancer of definitive erythropoiesis. Sox6 is highly expressed in proerythroblasts and erythroblasts in the fetal liver, neonatal spleen, and bone marrow. Mouse fetuses and pups lacking Sox6 develop erythroid cells slowly and feature misshapen, short-lived erythrocytes. They compensate for anemia by elevating the serum level of erythropoietin and progressively enlarging their erythropoietic tissues. Erythroid-specific inactivation of Sox6 causes the same phenotype, demonstrating cell-autonomous roles for Sox6 in erythroid cells. Sox6 potentiates the ability of erythropoietin signaling to promote proerythroblast survival and has an effect additive to that of erythropoietin in stimulating proerythroblast and erythroblast proliferation. Sox6 also critically facilitates erythroblast and reticulocyte maturation, including hemoglobinization, cell condensation, and enucleation, and ensures erythrocyte cytoskeleton long-term stability. It does not control adult globin and erythrocyte cytoskeleton genes but acts by stabilizing filamentous actin (F-actin) levels. Sox6 thus enhances erythroid cell development at multiple levels and thereby ensures adequate production and quality of red blood cells.
Introduction
Erythropoiesis starts in the mouse embryo around embryonic day 7.5 (E7.5), with the yolk sac producing so-called primitive red blood cells (RBCs), which are large and nucleated. Definitive erythropoiesis, producing small and enucleated RBCs, starts in the liver at approximately E10.5. The bone marrow takes over around birth and remains the main erythropoietic tissue throughout life. The spleen has erythropoietic activity in neonates and later on under erythropoietic stress conditions, as induced by anemia or hypoxia. Upon commitment to the definitive RBC lineage, hematopoietic stem cells evolve into progenitors of erythroid burst-forming units (BFU-Es) and then into progenitors of erythroid colony-forming units (CFU-Es). They subsequently differentiate into proerythroblasts and erythroblasts, which activate erythroid-specific markers, including globins and specific components of the RBC cytoskeleton. During terminal maturation, erythroblasts stop dividing, condense their nucleus and cytoplasm, and cease transcription of most genes. They accumulate hemoglobin, assemble the RBC cytoskeleton, and extrude the nucleus and most organelles. The resulting reticulocyte migrates into the blood stream and continues to synthesize protein for 2 to 3 days until it loses its ribosomes and acquires its definitive biconcave disc shape. RBCs circulate for approximately 50 days in the adult mouse until, senescent or deformed, they are taken up by macrophages.
Several transcription factors control erythroid commitment and differentiation.1-3 Best known are the Gata-1, Fog-1, and Gfi-1b zinc finger factors and the Eklf Krüppel-like factor. Mouse embryos lacking these factors die from failure of embryonic or definitive erythropoiesis, demonstrating their essential roles in erythropoiesis. Only a few transcription factors have been identified that control or may control erythroid terminal maturation. They include E2F4,4 whose inactivation in the mouse causes fetal anemia, and the ring finger Herf15 and forkhead FoxO3a6 factors. Erythropoietin (Epo) is the principal hormonal regulator of definitive erythropoiesis under physiologic and erythropoietic stress conditions.7,8 It acts primarily by rescuing erythroid precursors from apoptosis and thereby allows their further development. Mouse embryos lacking Epo or its receptor EpoR die from definitive erythropoiesis failure.9 The transducing pathways mediating Epo signaling are complex8 and lead to activation of transcription factors that include Stat1 and Stat5.10-13 Mice lacking these factors are, however, less affected than Epo–/– and EpoR–/– mice. From this overview of the transcriptional control of erythropoiesis, it is likely that additional yet-unknown transcription factors control erythroid differentiation and maturation and mediate or potentiate Epo signaling.
Twenty Sox transcription factors exist in mice and humans.14,15 They feature an Sry-related high-mobility-group (HMG) box DNA-binding domain. Many are known to determine cell fate and differentiation in specific lineages but none has yet been shown to control erythropoiesis. Sox6 is highly identical to Sox5 and Sox13, forming with them the Sox D subfamily. Sox6 is known to be highly expressed in neuronal cells, chondrocytes, notochord and spermatid cells, and weakly in muscle cells.16-19 Sox5–/–Sox6–/– fetuses develop severe skeletal dysplasia,18,20-22 as Sox5 and Sox6 redundantly promote chondrocyte proliferation, differentiation, and maturation and also promote notochord cell survival and maturation. Sox6–/– mice have mild skeletal defects. About half die at birth and the others fail to thrive after postnatal day 7 (P7) and die at approximately P14. The cause of death remains unclear. Mice with a chromosomal inversion (p100H) disrupting Sox6 have the same gross phenotype as Sox6–/– mice. They were shown to develop cardiac and skeletal myopathy, suggesting that Sox6 might promote myocyte maturation.19,23
This study demonstrates that Sox6 is an important enhancer of erythropoiesis. It was initiated upon noticing that Sox6–/– fetuses exhibit many nucleated RBCs and anemia. We show that Sox6 controls erythroid cells in a cell-autonomous manner. It enhances the cell survival function of Epo, stimulates cell proliferation, and promotes terminal maturation. It acts at least in part by stabilizing F-actin. This study complements a recent report that p100H/100H fetuses also feature nucleated RBCs and that Sox6 may silence embryonic globin genes.24
Materials and methods
Mice
Mice harboring Sox6 null,20 Sox6 conditional null,25 and ErGFPCre26 alleles were previously described. All experiments were performed with mice on the 129xB6 genetic background and were repeated with a minimum of 3 litters. Mouse livers were photographed using a Kodak EDAS 290 analysis system (Kodak, New Haven, CT).
Blood tests
Blood was harvested using heparinized microhematocrit capillary tubes. Blood smears were stained with Giemsa and photographed using an Olympus BX50 microscope (Melville, NY) and Uplanapo 10×/0.40 and Uplanapo 20×/0.70; ∞/0.17 lenses equipped with a Polaroid DMC2 digital camera (Waltham, MA). Transmission electron microscopy and scanning electron microscopy were done as described.27 Complete blood cell tests were performed using an ADVIA-120B blood analyzer (Bayer, Tarrytown, NY) with blood samples diluted 10 times in FBS, and data were analyzed with software for mice (ADVIA 120 Hematology System, version 2.2.06-MS). Serum erythropoietin levels were measured using the Quantikine mouse erythropoietin immunoassay kit (R&D Systems, Minneapolis, MN). Globin chains were visualized upon resolving blood cell lysates in polyacrylamide/urea/acetic acid electrophoresis gels and staining with Coomassie blue.28 Globin accumulation in cultured cells was assessed in Coomassie blue–stained sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) gels of cell lysates. Ghosts were prepared as described29 and analyzed in Coomassie blue–stained SDS-PAGE gels.
FACS analyses
Fluorescence-activated cell sorting (FACS) was carried out using a LSRII instrument (BD Biosciences, San Jose, CA) and a FACS Vantage cell sorter (BD Biosciences). Data were analyzed using FlowJo software (Ashland, OR). The cell markers TER119, CD71, c-kit, and Mac1 were measured on live cells by immunostaining with antibodies conjugated with phycoerythrin (PE), fluorescein isothiocyanate (FITC), or allophycocyanin (APC; BD Pharmingen, Heidelberg, Germany). Nucleic acids were measured using Thiazole orange (Retic-COUNT; BD Biosciences) after cell staining with surface markers. Dead cells were excluded from analysis using 7-aminoactinomycin D (7-AAD). Hemoglobin level was quantified using alpha-globin primary antibodies (Santa Cruz Biotechnology, Santa Cruz, CA) and AlexaFluor 488–labeled secondary antibodies (Molecular Probes, Eugene, OR). Cells were fixed in 3.7% formaldehyde in PBS overnight at 4°C, washed in PBS, blocked in 1% BSA in PBS for 30 minutes, and then incubated with primary antibody for 30 minutes in 0.1% Saponin, 1% BSA in PBS. Apoptosis was measured using 7-AAD and staining with APC-labeled annexin V (BD Pharmingen) following staining with cell surface markers. Cell proliferation was measured by incubating cells in culture for 4 hours with 100 μM BrdU reagent (Zymed Laboratories, South San Francisco, CA), followed by analysis using BrdU flow kit (BD Pharmingen).
RNA assays
RNA in situ hybridization of mouse embryo sections was performed using a Sox6-specific 35S-labeled antisense RNA probe.20 Pictures were taken as described for blood smears under dark field and using a red filter for RNA signals and blue fluorescence for nuclei stained with Hoechst 33258 dye. Northern blot was performed with RNA prepared using the TRIzol reagent (Invitrogen, Carlsbad, CA) and 32P-dCTP–labeled mouse Sox5, Sox6, and 18S cDNA probes.18 Mouse Sox13, α-globin, β-globin, and α1-spectrin probes corresponded to specific cDNA segments. Northern blot signals were quantified using a Storm 860 scanner (Molecular Dynamics, Piscataway, NJ) and ImageQuant software (GE Healthcare Life Sciences, Piscataway, NJ) and normalized with 18S rRNA signals. Reverse transcriptase–polymerase chain reaction (RT-PCR) was done using the ThermoScript System (Invitrogen) to synthesize cDNA. PCR amplification was performed for 40 cycles of 30 seconds at 94°C, 60 seconds at 60°C, and 60 seconds at 72°C using Sox6 primers (5′primer: ATCTGAGGTGATGGTGTGGTCGTT; 3′primer: TTGGGGAGTACAAGCAACTGATGC) and Hprt primers (5′primer: GCTGGTGAAAAGGACCTCT; 3′primer: CACAGGACTAGAACACCTGC). Products were analyzed by electrophoresis in ethidium bromide–containing agarose gels.
Immunofluorescence
Freshly isolated mouse cells were cytospun on microscope slides for 5 minutes at 500g (Thermo Shandon, Waltham, MA) and air dried. Cells were fixed in 3.7% formaldehyde in PBS, blocked in 10% FBS in PBS, and incubated overnight at 4°C with Sox6 rabbit polyclonal antibodies18 and then with AlexaFluor 594–conjugated anti–rabbit IgGs (Molecular Probes). Costaining for TER119 and c-kit was done using FITC-conjugated antibodies. Cell nuclei were stained with the Hoechst 33258 DNA dye. Immunofluorescence signals were visualized using a Leica TCS-SP spectral laser scanning confocal microscope and Leica confocal software 2.6.1 (Leica, Heidelberg, Germany).
Erythroid cell cultures
Cell cultures were started with TER119– cells purified from fetal liver cell suspensions using TER119-conjugated magnetic microbeads and LD columns (Miltenyi Biotec, Auburn, CA). Cells were seeded in methylcellulose media supplemented with Epo (M3334 medium; StemCell Technologies, Vancouver, BC, Canada). Erythroid colonies were stained for hemoglobin using benzidine (Sigma, St Louis, MO). CFU-Es contained 8 to 32 benzidine-positive cells and BFU-Es contained 3 or more clusters of CFU-Es. Cells were also seeded in 35-mm fibronectin-coated dishes (BD Labware) at a density of 5 × 105 in IMDM medium (StemCell Technologies) supplemented with 0 to 20 U/mL Epo (StemCell Technologies) as indicated in figure legends (starting concentration), detoxified BSA (Stem-Cell Technologies), insulin (Sigma), holo-transferrin (Sigma), and fetal bovine serum (Gibco, Carlsbad, CA), as described.30 After 16 hours, the medium was diluted 1:4 by addition of medium without Epo.
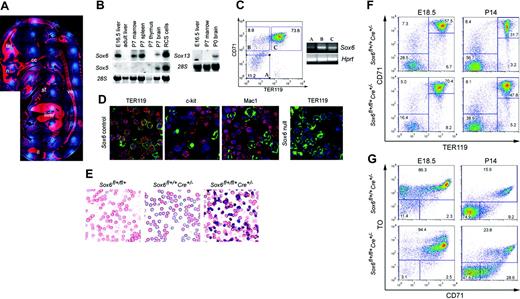
Sox6–/– mice feature abnormal RBCs and are anemic. (A) Blood smears of E14.5 and E16.5 Sox6–/– and control littermates. Arrowheads indicate large and nucleated primitive RBCs; arrows, small, enucleated, definitive RBCs; and double arrows, Sox6–/– nucleated and misshapen definitive RBCs. (B) Scanning electron microscopy (SEM) of E18.5 Sox6–/– and control RBCs. D indicates normal-looking discoid RBCs; *, SEM artifacts; and #, misshapen RBCs. (C) Transmission electron microscopy of RBCs in E18.5 Sox6–/– and control littermates. Nucleated and abnormally shaped RBCs are seen in the Sox6–/– fetus. (D) Globin chain analysis in E14.5 Sox6–/– and control fetuses. The migration level of the primitive Eγ globin chains, definitive β-globin haplotypes, and α-globin chains is indicated. The Sox6–/– mutant shows the same proportions of primitive and definitive globin chains as its control littermates. (E) TO/CD71 FACS profiles of blood cells in control and Sox6–/– mice from E18.5 to P13. Thiazole orange, which binds nucleic acids, distinguishes nucleated cells (TO++) from reticulocytes (TO+) and mature RBCs (TO–), and CD71 antibodies distinguish reticulocytes (CD71+) from mature RBCs (CD71–). Arrows indicate the trend of reticulocyte (CD71+/TO+) differentiation into mature RBCs (CD71–/TO–).
Sox6–/– mice feature abnormal RBCs and are anemic. (A) Blood smears of E14.5 and E16.5 Sox6–/– and control littermates. Arrowheads indicate large and nucleated primitive RBCs; arrows, small, enucleated, definitive RBCs; and double arrows, Sox6–/– nucleated and misshapen definitive RBCs. (B) Scanning electron microscopy (SEM) of E18.5 Sox6–/– and control RBCs. D indicates normal-looking discoid RBCs; *, SEM artifacts; and #, misshapen RBCs. (C) Transmission electron microscopy of RBCs in E18.5 Sox6–/– and control littermates. Nucleated and abnormally shaped RBCs are seen in the Sox6–/– fetus. (D) Globin chain analysis in E14.5 Sox6–/– and control fetuses. The migration level of the primitive Eγ globin chains, definitive β-globin haplotypes, and α-globin chains is indicated. The Sox6–/– mutant shows the same proportions of primitive and definitive globin chains as its control littermates. (E) TO/CD71 FACS profiles of blood cells in control and Sox6–/– mice from E18.5 to P13. Thiazole orange, which binds nucleic acids, distinguishes nucleated cells (TO++) from reticulocytes (TO+) and mature RBCs (TO–), and CD71 antibodies distinguish reticulocytes (CD71+) from mature RBCs (CD71–). Arrows indicate the trend of reticulocyte (CD71+/TO+) differentiation into mature RBCs (CD71–/TO–).
Results
Sox6–/– fetuses and pups have anemia associated with RBC maturation and survival defects
Sox6+/+ and Sox6+/– fetuses, referred to as controls, featured as many embryonic RBCs as definitive RBCs at E14.5 but mostly definitive RBCs by E16.5 (Figure 1A). At E14.5, Sox6–/– littermates had mostly nucleated RBCs, half of which were as large as primitive RBCs whereas the other half were as small as definitive RBCs. Normal-looking definitive RBCs appeared later on (Figure 1A), but approximately 10% RBCs were still nucleated at birth and approximately 1% at 2 weeks after birth (data not shown). Further, electron microscopy showed that most Sox6–/– RBCs were irregularly shaped at E18.5 (Figure 1B-C). The analysis of globin chains indicated normal proportions of adult and embryonic globin chains in E14.5 Sox6–/– fetus blood, strongly suggesting that the abnormal RBCs were definitive (Figure 1D).
Complete blood cell counts (CBCs) revealed that E18.5 Sox6–/– fetuses were anemic, with a RBC count and hematocrit value approximately three fourths the normal values and a mean cell hemoglobin concentration approximately two thirds the normal value (Table 1). The reticulocyte count was at least twice as high as normal and the RBC distribution width (RDW) approximately 160% of the normal value, indicating anisocytosis. The serum level of erythropoietin (sEpo) was increased 10-fold, further demonstrating anemia (Table 2). At P7, Sox6–/– pups had a normal RBC count, hematocrit, and hemoglobin content, but the RDW and sEpo were still high and the reticulocyte count was now half of normal. These pups thus had compensated anemia.
The increase in reticulocytes in Sox6–/– fetuses and decrease in Sox6–/– pups prompted us to develop a FACS assay to further characterize RBC maturation and survival. This assay used Thiazole orange (TO), which binds nucleic acids, and CD71 antibody. From E18.5 to P13, most control RBCs switched from a reticulocyte profile to a mature RBC profile, losing TO reactivity later than CD71 (Figure 1E). In contrast, fewer Sox6–/– RBCs reached the mature stage and most lost TO reactivity concomitantly with CD71. Because of their premature TO loss, P7 reticulocytes were thus underestimated in CBC tests, which measure RNA only. These data altogether demonstrated that Sox6–/– fetuses had anemia and Sox6–/– pups had compensated anemia, both associated with RBC abnormal maturation and decreased survival.
The development of erythropoietic tissues is delayed in Sox6–/– mice
We next used TER119/CD71 FACS to determine whether Sox6 controls erythropoietic tissue development. TER119 antibodies bind an RBC-specific cytoskeleton protein expressed from the erythroblast stage, whereas CD71 antibodies bind the transferrin receptor, which is highly expressed on proerythroblasts and early erythroblasts and decays in late erythroblasts and reticulocytes. The Sox6–/– fetal liver and neonatal spleen and bone marrow featured fewer erythroblasts than control tissues as they were developing and producing their first erythroid cells (ie, until E13.5 for the liver and P4 for the spleen and bone marrow; Figure 2A). In contrast, after reaching steady-state erythropoiesis, the Sox6–/– tissues featured more erythroblasts than control tissues (Figure 2B) and also became enlarged (Figure 2C; Table 3). These data strongly suggested that Sox6 promotes early as well as late erythroid cell development and that Sox6–/– mice are nevertheless able to increase their erythropoietic tissue volume to compensate for anemia.
Sox6 is highly expressed and has cell-autonomous roles in erythroid cells
To investigate the cellular basis of the Sox6–/– erythroid phenotype, we first tested whether Sox6 is expressed in erythropoietic tissues and erythroid cells. RNA in situ hybridization revealed high expression of Sox6 in the fetal liver (Figure 3A), and Northern blot showed similar expression in P7 spleen and bone marrow (Figure 3B). Neither Sox5 nor Sox13 were expressed in erythropoietic tissues, indicating that they do not share functions with their close relative in these tissues. Semiquantitative RT-PCR of RNA from E12.5 FACS-sorted liver cells (Figure 3C) and P7 bone marrow (data not shown) revealed that Sox6 expression was weak in nonerythroid cells and erythroid precursors, strong in proerythroblasts, and moderate in erythroblasts. Immunofluorescence of P7 bone marrow cytospins showed a strong nuclear signal for Sox6 protein in TER119+ erythroblasts and in most TER119– cells, which were mainly proerythroblasts, and a weak signal in c-kit+ hematopoietic precursors and Mac1+ macrophages (Figure 3D). Sox6 is thus highly expressed in proerythroblasts and erythroblasts.
Sox6–/– mice show abnormal development of erythropoietic tissues. (A) CD71/TER119 FACS profiles of control and Sox6–/– erythropoietic tissues during their first wave of erythropoiesis. Four subpopulations are distinguished: CD71neg/lowTER119– early erythroid and nonerythroid cells; CD71med/highTER119low proerythroblasts; CD71highTER119+ early erythroblasts; and CD71med-lowTER119+ late erythroblasts. The percentage of cells in each subpopulation is indicated. (B) CD71/TER119 FACS profiles of erythropoietic tissues beyond their first wave of erythropoiesis. (C) Pictures and weight of representative newborn control and mutant mouse livers. All mice had similar body weight.
Sox6–/– mice show abnormal development of erythropoietic tissues. (A) CD71/TER119 FACS profiles of control and Sox6–/– erythropoietic tissues during their first wave of erythropoiesis. Four subpopulations are distinguished: CD71neg/lowTER119– early erythroid and nonerythroid cells; CD71med/highTER119low proerythroblasts; CD71highTER119+ early erythroblasts; and CD71med-lowTER119+ late erythroblasts. The percentage of cells in each subpopulation is indicated. (B) CD71/TER119 FACS profiles of erythropoietic tissues beyond their first wave of erythropoiesis. (C) Pictures and weight of representative newborn control and mutant mouse livers. All mice had similar body weight.
To test whether Sox6 directly controls erythroid cells, we next inactivated it specifically in these cells by generating mice harboring Sox6 conditional null alleles25 (Sox6fl+/fl+) and an ErGFPCre allele.26 This latter allele, a knock-in of the green fluorescent protein and Cre recombinase genes in EpoR, leads to specific and efficient recombination of loxP-flanked genes in erythroid cells.25 Sox6fl+/fl+ErGFPCre fetuses, which lacked Sox6 in erythroid cells, featured many nucleated RBCs, anemia, RBC anisocytosis, and an enlarged liver (Figure 3E; Table 4), as do Sox6–/– fetuses. In contrast, Sox6fl+/fl+ and Sox6fl+/+ErGFPCre littermates, which had 2 or 1 Sox6 alleles intact, respectively, were normal. The erythropoietic tissue and blood profiles of Sox6fl+/fl+ErGFPCre fetuses and pups also matched those of Sox6–/– mice (Figure 3F-G). These data thus demonstrated that Sox6 controls erythroid cell development cell-autonomously.
Sox6 promotes early expansion and terminal maturation of erythroid cell populations
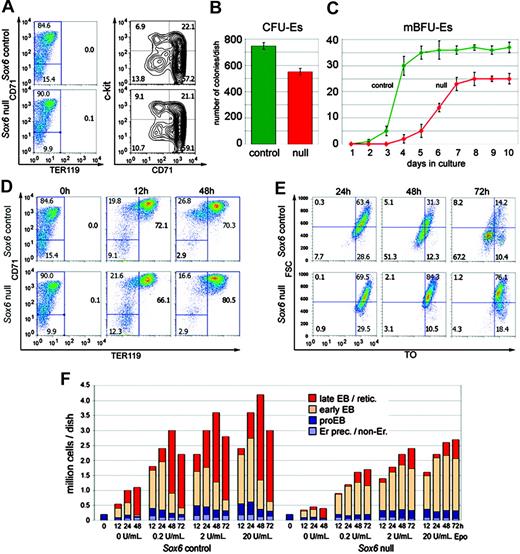
To define the roles of Sox6 in erythroid cells we used primary cultures rather than in vivo samples to avoid the mechanisms that Sox6–/– mice develop to compensate for anemia. We started these cultures with TER119– cells magnetically sorted from E14.5 fetal liver.30 Sox6–/– and control TER119– populations contained similar proportions of c-kit+ erythroid precursors (Figure 4A). In standard erythroid colony-formation assays, Sox6–/– cells formed fewer CFU-Es and mature BFU-Es (mBFU-Es) than control cells and formed mBFU-Es twice as slowly as control cells (Figure 4B-C). When cultured on fibronectin with Epo according to a procedure that supports development until terminal maturation,30 most control and Sox6–/– TER119– cells developed into TER119+ erythroblasts within 12 to 48 hours (Figure 4D), but Sox6–/– cultures reproducibly contained slightly fewer erythroblasts than control cultures during the first 12 to 24 hours and more by 48 hours, as seen in erythropoietic tissues before and after the first wave of erythropoiesis. As described previously,30 smears of control cells showed many condensed and enucleated cells at 48 and 72 hours (data not shown). However, virtually none were seen in Sox6–/– cell smears. To quantify this maturation defect of Sox6–/– cells we developed a FACS assay with TO and the cell forward scatter (FSC). Control and Sox6–/– nonerythroid and early erythroid cells (TER119–) were FSChigh/TOhigh at all times in culture (data not shown). Most control erythroblasts (TER119+) switched from a FSChigh/TOhigh profile to a FSClow/TOmed profile between 24 and 48 hours in culture and were thus condensing and enucleating, whereas most Sox6–/– erythroblasts were still FSChigh/TOhigh, thus immature, at 72 hours in culture (Figure 4E). Compilation of FACS data and cell counts revealed that Sox6–/– populations expanded half as much as controls and were blocked or delayed at the transition from early to late erythroblast (Figure 4F). They were able to increase their expansion rate in response to Epo but not as efficiently as control cells, and Epo did not affect the maturation rate of either control or Sox6–/– cells. Both in vivo and in vitro data thus demonstrated that Sox6 promotes early expansion and terminal maturation of erythroid cells.
Sox6 is highly expressed and has cell-autonomous roles in erythroid cells. (A) Sox6 RNA in situ hybridization of a wild-type E15.5 fetus midsagittal section. The RNA signal is seen in red and cell nuclei blue. Tel indicates telencephalon; n, nasal cartilage; cc, chondrocranium; st, sternum; ve, vertebrae; and il, ilium. (B) Northern blots of RNA from mouse tissues, hybridized with Sox D probes. Staining of 28S RNA is shown as loading control. (C) Sox6 expression in E12.5 fetal liver cells. Three subpopulations, A to C, were sorted from wild-type tissue by CD71/TER119 FACS. Semiquantitative RT-PCR was performed for Sox6 and Hprt, the latter serving as loading control. (D) Immunofluorescence of cytospins from control and Sox6–/– P7 bone marrow cells incubated with Sox6 antibodies (red signal) and FITC-labeled TER119, c-kit, or Mac-1 antibodies (green signal). Cell nuclei are blue. (E) Blood smears of E15.5 control and Sox6fl+/fl+ErGFPCre mutant littermates. (F) CD71/TER119 FACS profiles of E18.5 fetal liver and P14 bone marrow in control and Sox6fl+/fl+ErGFPCre littermates. (G) CD71/TO FACS profiles of blood from E18.5 and P14 control and Sox6fl+/fl+ErGFPCre littermates.
Sox6 is highly expressed and has cell-autonomous roles in erythroid cells. (A) Sox6 RNA in situ hybridization of a wild-type E15.5 fetus midsagittal section. The RNA signal is seen in red and cell nuclei blue. Tel indicates telencephalon; n, nasal cartilage; cc, chondrocranium; st, sternum; ve, vertebrae; and il, ilium. (B) Northern blots of RNA from mouse tissues, hybridized with Sox D probes. Staining of 28S RNA is shown as loading control. (C) Sox6 expression in E12.5 fetal liver cells. Three subpopulations, A to C, were sorted from wild-type tissue by CD71/TER119 FACS. Semiquantitative RT-PCR was performed for Sox6 and Hprt, the latter serving as loading control. (D) Immunofluorescence of cytospins from control and Sox6–/– P7 bone marrow cells incubated with Sox6 antibodies (red signal) and FITC-labeled TER119, c-kit, or Mac-1 antibodies (green signal). Cell nuclei are blue. (E) Blood smears of E15.5 control and Sox6fl+/fl+ErGFPCre mutant littermates. (F) CD71/TER119 FACS profiles of E18.5 fetal liver and P14 bone marrow in control and Sox6fl+/fl+ErGFPCre littermates. (G) CD71/TO FACS profiles of blood from E18.5 and P14 control and Sox6fl+/fl+ErGFPCre littermates.
Sox6 stimulates erythroid cell survival and proliferation
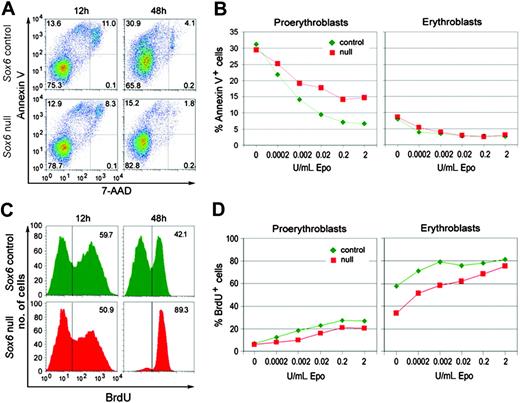
We assessed cell death and proliferation to determine how Sox6 controls expansion of erythroid populations. Cell death was tested by FACS using annexin V (AnnV), which binds apoptotic cells and enucleating erythroblasts, and 7-AAD, which labels necrotic cells. Sox6–/– and control populations had similar proportions of AnnV+/7-AAD– apoptotic/enucleating cells and AnnV+/7-AAD+ dead cells at 12 hours in fibronectin culture with 2 U/mL Epo (Figure 5A). By 48 hours, control populations had twice as many AnnV+/7-AAD+ dead cells, whereas Sox6–/– populations had the same amount as earlier. This result thus mainly reflected the fact that many control but few Sox6–/– cells were terminally maturing. Costaining of the cells with CD71 and TER119 antibodies allowed us to test erythroid cells at specific differentiation stages. As expected, control proerythroblasts were very sensitive to apoptosis in the absence of Epo and efficiently rescued by increasing doses of Epo (Figure 5B). Sox6–/– proerythroblasts died in the same proportion as control proerythroblasts without Epo. Interestingly, Epo did not protect them as well as control cells. Control and Sox6–/– erythroblasts were less sensitive to apoptosis than proerythroblasts and responded similarly to Epo. Sox6 thus potentiates the ability of Epo to promote proerythroblast survival.
Sox6–/– erythroid populations show impaired expansion and maturation in vitro. (A) CD71/TER119 and c-kit/CD71 FACS profiles of magnetically sorted TER119– liver cells from E14.5 control and Sox6–/– littermates. (B) CFU-E assay using control and Sox6–/– TER119– sorted cells. Data are presented as average with standard deviation of quadruplicates in a representative experiment. (C) Mature BFU-E assay in the same experiment as in panel B. (D) CD71/TER119 FACS profiles of control and Sox6–/– erythroid cells cultured on fibronectin with 2 U/mL Epo for 0, 12, and 48 hours. (E) Forward scatter (FSC)/TO FACS profiles of TER119+ cells from similar cultures as in panel D. (F) Graph combining cell counts and data from CD71/TER119 and FSC/TO FACS analysis for similar cultures as in panels E and F, maintained for 0 to 72 hours. Erythroid precursors/nonerythroid cells (Er prec/non-Er) are CD71–/TER119–; proerythroblasts (proEB) are CD71+/TER119–; early erythroblasts (EB) are TER119+and FSChigh and/or TOhigh; late erythroblasts/reticulocytes (retic) are TER119+/FSClow/TOlow.
Sox6–/– erythroid populations show impaired expansion and maturation in vitro. (A) CD71/TER119 and c-kit/CD71 FACS profiles of magnetically sorted TER119– liver cells from E14.5 control and Sox6–/– littermates. (B) CFU-E assay using control and Sox6–/– TER119– sorted cells. Data are presented as average with standard deviation of quadruplicates in a representative experiment. (C) Mature BFU-E assay in the same experiment as in panel B. (D) CD71/TER119 FACS profiles of control and Sox6–/– erythroid cells cultured on fibronectin with 2 U/mL Epo for 0, 12, and 48 hours. (E) Forward scatter (FSC)/TO FACS profiles of TER119+ cells from similar cultures as in panel D. (F) Graph combining cell counts and data from CD71/TER119 and FSC/TO FACS analysis for similar cultures as in panels E and F, maintained for 0 to 72 hours. Erythroid precursors/nonerythroid cells (Er prec/non-Er) are CD71–/TER119–; proerythroblasts (proEB) are CD71+/TER119–; early erythroblasts (EB) are TER119+and FSChigh and/or TOhigh; late erythroblasts/reticulocytes (retic) are TER119+/FSClow/TOlow.
Sox6–/– erythroid cells have decreased survival and proliferation rates in vitro. (A) AnnV/7-AAD FACS profiles of control and Sox6–/– erythroid cells cultured on fibronectin with 0.2 U/mL Epo for 12 and 48 hours. The quadrants in each plot correspond to live cells (AnnV–/7-AAD–), apoptotic cells (AnnV+/7-AAD–), dead cells (AnnV+/7-AAD+), and necrotic cells (AnnV–/7-AAD+). (B) Graphs showing the percentages of apoptotic (AnnV+/7-AAD–) proerythroblasts and erythroblasts in cell cultures on fibronectin for 12 hours with the indicated concentrations of Epo. (C) BrdU incorporation assay in control and Sox6–/– erythroid cells cultured on fibronectin with 0.2 U/mL Epo for 12 hours and 48 hours. The percentages of BrdU+ cells are indicated. (D) Graphs showing the percentages of BrdU+ proerythroblasts and erythroblasts in cell cultures on fibronectin for 12 hours with the indicated concentrations of Epo.
Sox6–/– erythroid cells have decreased survival and proliferation rates in vitro. (A) AnnV/7-AAD FACS profiles of control and Sox6–/– erythroid cells cultured on fibronectin with 0.2 U/mL Epo for 12 and 48 hours. The quadrants in each plot correspond to live cells (AnnV–/7-AAD–), apoptotic cells (AnnV+/7-AAD–), dead cells (AnnV+/7-AAD+), and necrotic cells (AnnV–/7-AAD+). (B) Graphs showing the percentages of apoptotic (AnnV+/7-AAD–) proerythroblasts and erythroblasts in cell cultures on fibronectin for 12 hours with the indicated concentrations of Epo. (C) BrdU incorporation assay in control and Sox6–/– erythroid cells cultured on fibronectin with 0.2 U/mL Epo for 12 hours and 48 hours. The percentages of BrdU+ cells are indicated. (D) Graphs showing the percentages of BrdU+ proerythroblasts and erythroblasts in cell cultures on fibronectin for 12 hours with the indicated concentrations of Epo.
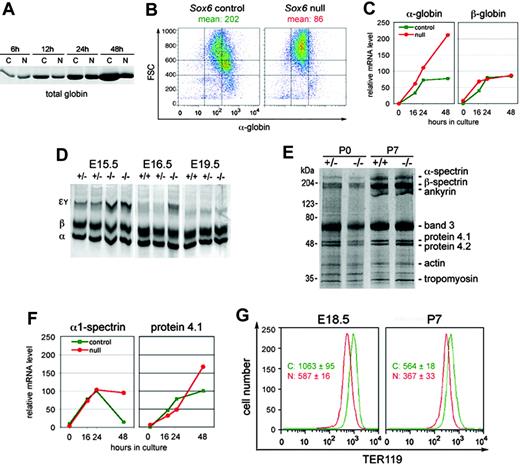
Sox6–/– erythroid cells show defects in hemoglobinization and cytoskeleton integrity. (A) Coomassie blue–stained SDS-PAGE of lysates from control (C) and Sox6–/– (N) fetal liver cells cultured on fibronectin with 2 U/mL Epo for 6 to 48 hours. All lanes were loaded with lysate amounts corresponding to equal cell numbers. The picture shows the section of the gel at the 15-kDa level, where the most intense protein band was seen, as expected for globin chains. (B) FACS analysis of hemoglobin level in control and Sox6–/– erythroid cells cultured on fibronectin with 2 U/mL Epo for 24 hours. The mean cell hemoglobin intensity is indicated. (C) Quantification of relative α-globin and β-globin RNA levels in control and Sox6–/– erythroid cells cultured on fibronectin with 2 U/mL Epo for 0 to 48 hours. (D) Globin chain analysis in E15.5, E16.5, and E19.5 Sox6–/– and control fetuses. The migration level of the primitive Eγ globin chains, definitive β-globin chains, and α-globin chains is indicated. (E) Ghost analysis of control and Sox6–/– RBCs at P0 and P7. Samples were loaded in amount corresponding to similar cell numbers. (F) Quantification of α1-spectrin and protein 4.1 RNA levels in control and Sox6–/– erythroid cells. The experiment was done and data are presented as described in panel C. (G) Histograms of TER119 FACS analysis of blood samples from E18.5 and P7 mice. The mean with standard deviation of TER119 fluorescence is indicated for 3 control (C) and 3 Sox6–/– (N) littermates. The differences in absolute values between E18.5 and P7 samples are due to experimental variability rather than differences between mouse ages.
Sox6–/– erythroid cells show defects in hemoglobinization and cytoskeleton integrity. (A) Coomassie blue–stained SDS-PAGE of lysates from control (C) and Sox6–/– (N) fetal liver cells cultured on fibronectin with 2 U/mL Epo for 6 to 48 hours. All lanes were loaded with lysate amounts corresponding to equal cell numbers. The picture shows the section of the gel at the 15-kDa level, where the most intense protein band was seen, as expected for globin chains. (B) FACS analysis of hemoglobin level in control and Sox6–/– erythroid cells cultured on fibronectin with 2 U/mL Epo for 24 hours. The mean cell hemoglobin intensity is indicated. (C) Quantification of relative α-globin and β-globin RNA levels in control and Sox6–/– erythroid cells cultured on fibronectin with 2 U/mL Epo for 0 to 48 hours. (D) Globin chain analysis in E15.5, E16.5, and E19.5 Sox6–/– and control fetuses. The migration level of the primitive Eγ globin chains, definitive β-globin chains, and α-globin chains is indicated. (E) Ghost analysis of control and Sox6–/– RBCs at P0 and P7. Samples were loaded in amount corresponding to similar cell numbers. (F) Quantification of α1-spectrin and protein 4.1 RNA levels in control and Sox6–/– erythroid cells. The experiment was done and data are presented as described in panel C. (G) Histograms of TER119 FACS analysis of blood samples from E18.5 and P7 mice. The mean with standard deviation of TER119 fluorescence is indicated for 3 control (C) and 3 Sox6–/– (N) littermates. The differences in absolute values between E18.5 and P7 samples are due to experimental variability rather than differences between mouse ages.
Cell proliferation was measured by BrdU incorporation. Sox6–/– populations incorporated almost as much BrdU as control populations at 12 hours in culture with 2 U/mL Epo (Figure 5C). By 48 hours, control populations significantly decreased their BrdU incorporation rate, whereas most Sox6–/– cells were still actively proliferating, again reflecting the fact that many control but few Sox6–/– cells were terminally maturing. Control proerythroblasts were proliferating less than erythroblasts, and Epo stimulated cell proliferation at both stages (Figure 5D). Sox6–/– proerythroblasts and erythroblasts were proliferating more slowly than control cells at all Epo concentrations but increased their proliferation rate in response to Epo as efficiently as control cells. Sox6 and Epo signaling thus have additive effects on stimulating erythroid cell proliferation.
Sox6 does not control adult globin and erythroid cytoskeleton genes
To further understand the maturation impairment of Sox6–/– cells we tested whether Sox6 controls hemoglobin production. Sox6–/– erythroid cells accumulated hemoglobin more slowly than control cells in primary culture (Figure 6A-B) but contained normal levels of RNA for both α- and β-globin RNA (Figure 6C). The level of these RNAs was not significantly altered in Sox6–/– erythropoietic tissues either (data not shown). The analysis of globin chains in RBCs showed similar proportions of embryonic and adult globins in control and Sox6–/– fetuses at E14.5 (Figure 1D). The proportion of embryonic globin chains decreased faster in control than mutant littermates later on but by E19.5, embryonic globin chains had virtually disappeared in both control and mutant fetuses (Figure 6D). Together with CBC results (Table 1), these data indicated that Sox6–/– erythroid cells were delayed in hemoglobinizing, as in other aspects of their maturation program, but could nevertheless accumulate an essentially normal amount of adult hemoglobin.
The abnormal morphology and survival of Sox6–/– RBCs (Figure 1) suggested that Sox6 might control assembly of the erythroid cytoskeleton. FACS analyses, however, demonstrated that Sox6–/– erythroid cells expressed the TER119 protein at a normal level in vivo and in vitro (Figures 2A and 4D) and preparations of RBC ghosts (insoluble cytoskeleton proteins) indicated that Sox6–/– RBCs contained normal proportions of all major cytoskeleton proteins (Figure 6E). Accordingly, the RNA levels of β-actin, α1- and β1-spectrin, protein 4.1, and glycophorin-A were essentially normal in vitro and in vivo (Figure 6F; data not shown). Interestingly, however, the TER119 staining intensity of circulating RBCs was reproducibly weaker in Sox6–/– mice than in controls (Figure 6G), suggesting a faster decay of the Sox6–/– RBC cytoskeleton.
Sox6 facilitates erythroid cell maturation at least in part by controlling F-actin assembly
F-actin is a major component of the cytoskeleton of every cell type and it also has specific functions in erythroid cells, forming the basis of the contractile ring that mediates erythroid cell enucleation31,32 and the basis of the mature RBC cytoskeleton. The enucleation and mature cytoskeleton defect of Sox6–/– erythroid cells therefore prompted us to test whether these cells were F-actin deficient. We used P7 bone marrow cells because, in contrast to primary cultures, they feature significant proportions of erythroid cells at all developmental stages, including enucleating cells. FACS analysis with phalloidin, which specifically labels F-actin, showed that control erythroid cells assembled twice as much F-actin at the proerythroblast stage as nonerythroid/early erythroid cells and reduced their F-actin content several-fold during erythroblast development (Figure 7A). Interestingly, Sox6–/– erythroid cells had significantly less F-actin than control cells from the proerythroblast stage, and the relative difference was most spectacular at the late erythroblast stage and clearly visible by confocal microscopy (Figure 7B). As indicated earlier, no significant difference, however, was seen in β-actin RNA level between control and Sox6–/– populations, suggesting that Sox6 regulates actin polymerization rather than monomer production.
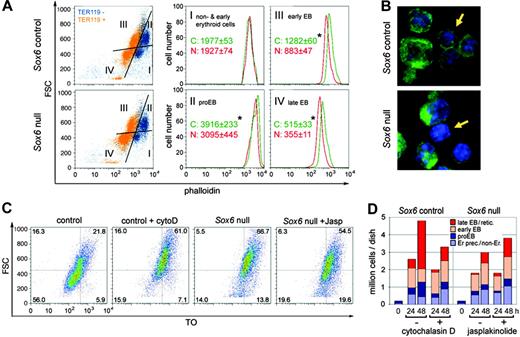
Sox6–/– erythroid cells have decreased F-actin levels that affect maturation. (A) FACS profiles of F-actin in bone marrow cells from P7 control and Sox6–/– littermates. Cells were stained with TER119 and phalloidin. (Left panels) FSC/phalloidin profiles, with TER119– cells shown in blue and TER119+cells in orange. Based on the FSC and TER119 staining, 4 subpopulations were identified: I, nonerythroid/early erythroid cells; II, proerythroblasts; III, early erythroblasts; and IV, late erythroblasts. (Middle and right panels) Distribution of F-actin in each subpopulation. The mean and standard deviation are indicated for 4 control and 3 mutant littermates. * indicates statistically significant differences between controls and mutants (Student t test, P < .05). (B) Confocal microscopy of representative P7 bone marrow cells stained with phalloidin (green) and the nucleic acid dye Hoechst 33258 (blue). The arrows point to a control cell with a thick F-actin cytoskeleton and a mutant cell with a thinner F-actin cytoskeleton. (C) FSC/TO profiles of control and Sox6–/– TER119+ erythroid cells from cultures on fibronectin for 48 hours with 0.2 U/mL Epo and with or without cytochalasin D (cytoD) and jasplakinolide (Jasp). (D) Graph combining cell counts and FACS profile data for the experiment described in panel C.
Sox6–/– erythroid cells have decreased F-actin levels that affect maturation. (A) FACS profiles of F-actin in bone marrow cells from P7 control and Sox6–/– littermates. Cells were stained with TER119 and phalloidin. (Left panels) FSC/phalloidin profiles, with TER119– cells shown in blue and TER119+cells in orange. Based on the FSC and TER119 staining, 4 subpopulations were identified: I, nonerythroid/early erythroid cells; II, proerythroblasts; III, early erythroblasts; and IV, late erythroblasts. (Middle and right panels) Distribution of F-actin in each subpopulation. The mean and standard deviation are indicated for 4 control and 3 mutant littermates. * indicates statistically significant differences between controls and mutants (Student t test, P < .05). (B) Confocal microscopy of representative P7 bone marrow cells stained with phalloidin (green) and the nucleic acid dye Hoechst 33258 (blue). The arrows point to a control cell with a thick F-actin cytoskeleton and a mutant cell with a thinner F-actin cytoskeleton. (C) FSC/TO profiles of control and Sox6–/– TER119+ erythroid cells from cultures on fibronectin for 48 hours with 0.2 U/mL Epo and with or without cytochalasin D (cytoD) and jasplakinolide (Jasp). (D) Graph combining cell counts and FACS profile data for the experiment described in panel C.
To determine whether the altered F-actin level of Sox6–/– erythroid cells was a cause or consequence of improper development, we treated cells with cytochalasin D to reduce actin polymerization or with jasplakinolide to increase actin polymerization.33 Control cells treated with cytochalasin D showed differentiation and maturation profiles that resembled those of untreated Sox6–/– cells, with reduction of the population expansion and erythroblast terminal maturation (Figure 7C-D). Tested at the same concentration, the drug resulted in massive death of Sox6–/– cells (data not shown), further suggesting critical weakness of their cytoskeleton. Jasplakinolide had no effect on control cell development (data not shown) but resulted in a slight but reproducible increase in Sox6–/– cell number and maturation, thus rescuing at least partially the Sox6–/– phenotype. F-actin assembly therefore appears as a key mechanism whereby Sox6 may facilitate erythroid cell development.
Discussion
This study has revealed that Sox6 enhances definitive erythropoiesis. Sox6–/– fetuses and pups have anemia, slow development of erythropoietic tissues, and RBC shape and survival defects. Sox6 cell-autonomously controls erythroid cells. It amplifies Epo's ability to rescue proerythroblasts from apoptosis, stimulates proerythroblast and erythroblast proliferation, and promotes terminal maturation through F-actin stabilization.
This study was initiated upon realizing that Sox6–/– fetuses had many nucleated RBCs and liver hyperplasia and that Sox6 likely controlled erythropoiesis directly. Nucleated RBCs indeed are often a sign of anemia or hypoxia, but Sox6–/– fetuses were not pale or cyanotic. Sox6–/– pups had a high RDW even though they compensated for anemia, suggesting an intrinsic RBC problem. Finding high expression of Sox6 in proerythroblasts and erythroblasts and weak or no expression in erythroid precursors and other erythropoietic tissue cells strengthened this notion. The erythroid-specific inactivation of Sox6 provided definitive demonstration that Sox6 has cell-autonomous roles in erythroid cells. Sox6 conditional null mice were crossed with ErGFPCre mice, in which the knock-in of Cre recombinase in EpoR leads to efficient recombination of loxP-flanked genes in erythroid cells but not in other cells, including macrophages and stromal cells.26 We confirmed this result25 and showed that Sox6fl+/fl+ErGFPCre mice developed the same erythroid phenotype as Sox6–/– mice but were otherwise normal. In addition to proving direct roles for Sox6 in erythroid cells, this experiment also demonstrated that Sox6 acts in erythroid cells after activation of EpoR, thus after commitment to the erythroid lineage. Thus, in contrast to such factors as Gata-1 and Fog-1, Sox6 does not control lineage commitment. Sox6 was found similarly expressed in fetal liver, neonatal spleen, and bone marrow, which is consistent with roles in definitive erythropoiesis throughout life.
Since inactivation of Sox6 did not block erythropoiesis, we asked whether Sox6 might share functions in erythroid cells with other proteins. Sox5, the closest relative of Sox6, is coexpressed with Sox6 in many cells but was not found expressed in erythropoietic tissues. Accordingly, Sox5–/– mice had normal RBCs and Sox5–/–Sox6–/– mice had the same erythroid phenotype as Sox6–/– mice (B.D. and V.L., unpublished data, December 2005). Sox13, the third Sox D gene, has an expression pattern largely distinct from that of Sox634 and its functions in vivo remain unknown. We showed that it is not expressed in erythropoietic tissues either. It is unknown whether other Sox genes are expressed in erythroid cells, but since they share with Sox6 only partial identity in the HMG box and none outside this domain, it is unlikely that they share redundancy with Sox6. The functions of Sox6 in erythroid cells thus resemble those of Sox5/Sox6 in chondrocytes and notochord cells,21,34 in that it is not required for lineage commitment but boosts development along the cell differentiation pathway.
We showed that Sox6 first acts along the erythroid pathway by promoting proerythroblast survival, which is one of Epo's primary functions, and found that Sox6 enhances this Epo function. Sox6–/– proerythroblasts indeed were dying at the same rate as controls without Epo, not at a higher rate as would be expected if Sox6 and Epo were acting independently. Moreover, Sox6–/– proerythroblasts responded less efficiently than control cells to Epo, thus demonstrating that Sox6 enhances Epo's function. These results suggest that Sox6 may modulate expression of genes involved in Epo signaling and that the Sox6 gene and/or protein may be targets of Epo signaling. We then showed that Sox6 stimulates proerythroblast and erythroblast proliferation, acting here in parallel with Epo. Sox6–/– cells were indeed proliferating more slowly than controls without Epo and increased their proliferation rate in parallel with controls in response to Epo. The roles of Sox6 in erythroid cell survival and proliferation explain the slow development of Sox6–/– erythropoietic tissues in vivo and Sox6–/– mBFU-Es in vitro and the reduced expansion of Sox6–/– erythroid cells cultured on fibronectin. The fact that Sox6 enhances but is not required for Epo's functions explains that the sEpo level of Sox6–/– mice, which is as high as in beta-thalassemia patients,36 must allow the mice to increase their erythropoietic tissue volume to compensate for anemia.
This study has also revealed a critical role for Sox6 in erythroid cell maturation. This finding is particularly welcome in view that the molecular mechanisms involved in this process remain largely unknown. This role was suggested in vivo by the abundance of nucleated RBCs in Sox6–/– fetuses, abnormal maturation and reduced survival of Sox6–/– RBCs, and high proportion of erythroblasts in Sox6–/– erythropoietic tissues. It was proven in vitro by showing that Sox6–/– erythroblasts started to differentiate as early as control cells but, in contrast to control cells, maintained a high proliferation rate and RNA level of erythroblast markers and failed to condense by 48 hours in culture. No evidence of increased cell death was found in mutant erythroblasts, confirming that the increased ratio of early/late erythroid cells in mutant cultures was due to a maturation delay or block. This defect did not involve adult globin and erythrocyte cytoskeleton genes. These genes were expressed at an expected level in vitro and in vivo. We previously showed that Sox5/Sox6 promote chondroblast proliferation and notochord cell survival and are also needed for maturation of both cell types.21,22 It is thus possible that Sox D proteins fulfill these roles by controlling the same genes in all 3 lineages.
We have shown that Sox6 controls erythroid maturation at least in part through F-actin. Sox6–/– cells indeed did not accumulate as much F-actin at the proerythroblast stage as controls and maintained less F-actin during erythroblast maturation. Moreover, destabilization of F-actin with cytochalasin D in control cells led to maturation defects as seen in untreated Sox6–/– cells and to cell death in Sox6–/– cells. Stabilization of F-actin in Sox6–/– cells improved their ability to mature while having no effect on control cells. These results are consistent with known roles for F-actin in important cellular processes, including proliferation, transcription, and chromatin remodeling37 ; erythroid cell enucleation31 ; and RBC cytoskeleton integrity. Similar levels of β-actin RNAwere found in control and Sox6–/– cells, suggesting that Sox6 may control genes involved in F-actin assembly or stability. It may thereby facilitate the development not only of erythroid cells but also of other cell types.
Despite expressing adult globin genes normally, we showed that Sox6–/– cultures were hemoglobinizing slowly. Since it is known, and was confirmed here, that erythroid cells continue to accumulate hemoglobin actively after condensation and enucleation, it is likely that the slow hemoglobinization of Sox6–/– erythroid cells primarily reflects their maturation defect. Efficient hemoglobinization of maturing cells is made possible by the particularly high stability of adult globin RNAs (> 24 hours)38 and retention of functional ribosomes for several days after enucleation. It was shown recently that E15.5 to E18.5 p100H/100H fetuses, which lack Sox6, expressed adult globin genes at a normal level and still actively expressed embryonic globin genes.24 Consistent with this result, we found a higher ratio of embryonic/adult globin chains in Sox6–/– RBCs than in controls. The increase, however, was small and seen only between E15.5 and birth. It has been demonstrated that the human embryonic ζ-globin RNA is much less stable than the adult α-globin RNA.38 It is thus possible that embryonic globin RNAs are highly transcribed in Sox6–/– fetal liver but not efficiently translated because they are largely decayed by the time erythroid cells reach maturation. The transient increase in embryonic globin RNA level may mainly reflect the developmental delay of the Sox6–/– fetal liver and/or its hyperplasia in response to anemia, as it has been shown that this tissue and embryonic stem cells in culture express embryonic globin genes during their first wave of erythropoiesis (hyperplasia may indeed involve de novo erythropoiesis) but not later on.39,40
The majority of definitive RBCs were nucleated in E14.5 Sox6–/– fetuses but most were enucleated by birth. As mentioned earlier, expression data strongly suggest that Sox6 maintains its roles in developing erythroid cells throughout life and does not share these roles with other proteins. The switch of erythropoiesis from the liver to the spleen and bone marrow also unlikely explains this change in proportion of nucleated RBCs, because the liver remains the main erythropoietic tissue until birth and because the Sox6–/– fetal liver, spleen, and bone marrow showed similar defects in erythroid cell developmental profiles. Rather, we propose that Sox6–/– fetuses had no alternative at E14.5 to E16.5 to avoid fatal anemia but to release very immature, still nucleated RBCs. However, as they progressively overcame anemia by increasing their erythropoietic tissue volume, they could afford to keep maturing cells longer in erythropoietic tissues, until enucleation. Moreover, since it was shown that primitive RBCs end up enucleating in the blood stream,41 it is likely that Sox6–/– nucleated definitive RBCs also did so, thereby contributing to lower the proportion of nucleated RBCs by birth.
In conclusion, this study has identified key roles for Sox6 in definitive erythropoiesis. Sox6 amplifies the function of erythropoietin in promoting erythroid cell survival and stimulating cell proliferation. It also facilitates terminal maturation. It thereby enhances the rate, quantity, and quality of red blood cell production. This leads us to suggest that Sox6 could be involved in some forms of erythroleukemias and inherited anemia diseases and that Sox6 could be used as a new tool to stimulate erythropoiesis in the treatment of various diseases with anemia.
Prepublished online as Blood First Edition Paper, April 20, 2006; DOI 10.1182/blood-2006-02-004184.
Supported by grants from the Lerner Research Institute of the Cleveland Clinic, the Roche Foundation for Anemia Research (V.L.), and a postdoctoral fellowship from the Cooley's Anemia Foundation (B.D.).
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Peter Dy, Harvey F. Lodish, Cathy Shemo, Patrick Smits, and Jing Zhang for precious scientific and technical advice. Acquisition of the Advia 120 instrument was made possible by a gift from Herbert and Judith Harvey.