Abstract
Self-renewal of hematopoietic stem cells (HSCs) is key to their reconstituting ability, but the signaling pathways that regulate this process remain poorly understood. Here we show that transduction of adult mouse bone marrow cells with a constitutively activated form of Stat3 (Stat3-C) increased their regenerative activity in lethally irradiated recipients. Conversely, transduction of these cells with a dominant-negative form of Stat3 suppressed their regenerative activity. Serial transplantation and clonal tracking of the HSC progeny regenerated in vivo from STAT3-C–transduced HSCs demonstrated that the major effect of forced expression of STAT3-C was to enhance HSC self-renewal during the initial phase of hematopoietic recovery. This acquired potential for enhanced self-renewal divisions then became latent, but was reactivated when the cells were transferred to new irradiated recipients. Increased levels of activated STAT3 were also found to be associated with greater preservation of primitive hematopoietic cells in short-term cultures. These results indicate a novel biphasic regulation of HSC self-renewal in vivo in which activated STAT3 promotes HSC self-renewal under stimulated, but not homeostatic, conditions. STAT3 may thus be an important regulator of hematopoietic regeneration and a novel target for HSC engineering.
Introduction
Hematopoietic stem cells (HSCs) constitute a rare subpopulation in hematopoietic tissues and are uniquely defined by their ability to divide many times with full retention of their pluripotentiality.1 This self-renewal function underlies the ability of HSCs to produce new blood cells throughout the life of the individual. It also allows them to regenerate the entire blood-forming system in vivo, including the HSC compartment, following their transplantation into irradiated recipients.2,3 In mice, the ability of individual HSCs to permanently reconstitute all blood-cell lineages provides a functional end point for the specific quantification of HSCs in limiting-dilution transplantation assays.4 The cells thus defined are operationally referred to as competitive repopulating units (CRUs), since these cells must compete with other HSCs whose presence is required for the survival of the host mice.
Expansion of the HSC (CRU) population normally occurs in vivo during fetal and early adulthood.5 Thereafter, the number of these cells remains stable with self-renewal divisions exactly balancing HSC losses by differentiation or death. However, a rapid and extensive net expansion of HSCs can be reinitiated in vivo following damage to the hematopoietic system.6 Accumulating evidence points to the involvement of both extrinsic cues (eg, Steel factor, factors that activate gp130,7 notch ligands,8 and Wnt9 ) as well as intrinsic regulators (eg, p21cip1/waf1,10 Bmi-1,11,12 and HoxB413-15 ) in controlling HSC self-renewal responses following their mitogenic activation in vitro or in vivo. Relatively little, however, is known about the signaling intermediates that direct or can modulate changes in HSC self-renewal when these cells are stimulated to proliferate in vivo.
Signal transducer and activator of transcription 3 (STAT3) is an intermediate signaling molecule that allows ligand-induced signals from gp130 and the granulocyte colony-stimulating factor (G-CSF) receptor, and from various endogenous nonreceptor kinases such as Src,16,17 to transactivate specific target genes. A key event in this process is the induced phosphorylation of the Tyr705 residue in the Src-homology 2 (SH2) domain of STAT3, which then leads to the formation of STAT3 dimers and their translocation into the nucleus. Distinct biologic roles of STAT3 in various types of tissues including hematopoietic cells have been described in several studies.18 In hematopoietic tissue, we showed that the forced expression of a dominant-negative (dn) form of Stat3 in fetal liver cells markedly reduced the competitive repopulating activity of the transduced HSCs in irradiated recipients of cotransplanted normal adult bone marrow cells. However, neither the proliferation nor the differentiation of slightly more mature progenitors in the original suspension of dnSTAT3-transduced fetal liver cells (detected in vivo as colony-forming units-spleen, CFU-Ss, or in vitro as colony-forming cells, CFCs) was affected.19 Subsequently, it was found that induced disruption of Stat3 also did not alter the short-term production of mature blood cells or their progenitor cells under homeostatic conditions in adult mice,20,21 and did not abrogate the radioprotecting ability of bone marrow cells tested under noncompetitive transplantation conditions.22
Based on these findings, we hypothesized that activated STAT3 might be an important and specific regulator of the initially enhanced HSC self-renewal that makes possible the rapid regeneration of hematopoiesis in lethally irradiated mice. The experiments described here provide evidence to support this concept. The early regenerative activity of transplanted HSCs could be either up- or down-regulated by genetically modulating the levels of activated STAT3 expressed. This effect was shown to be mediated by an autonomous effect on HSC self-renewal that appears to be temporally restricted to the early phase of hematopoietic recovery. Later, this effect of activated STAT3 becomes latent unless the HSCs are reactivated. Collectively, these findings suggest a novel role of STAT3 activity in modulating HSC self-renewal during regenerative, but not homeostatic, conditions.
Materials and methods
Animals
Mice, originally obtained from the Jackson Laboratories (Bar Harbor, ME), were housed in microisolator cages and provided with sterilized food and acidified water. Recipients were 8- to 12-week-old C57BL/6J-Ly5.2 (B6) mice and donors were C57BL/6J-Pep3b-Ly5.1 (Pep3b) mice. Experiments were undertaken with approval from the Animal Experiment Board of the Catholic University of Korea.
Vector construction and virus production
The parental murine stem cell virus–internal ribosomal entry site–green fluorescent protein (MSCV-IRES-GFP or MIG) and derivative dnStat3 vectors have been described previously.19 To produce an activated STAT3-C vector, an activated STAT3 (Stat3-C) cDNA was created by site-directed mutagenesis of the wild-type STAT3 cDNA as described.23 Briefly, overlapping polymerase chain reaction (PCR) was used to introduce base substitutions that converted Ala661 and Asn663 to Cys residues. A similar site-directed mutagenesis procedure was used to construct 2 mutant forms of STAT3-C, in which the Ser727 residue was replaced either with an Asp (CSD) residue or with an Ala (CSA) residue.24 All constructs were sequence verified and then cloned into the MIG vector using its EcoRI and XhoI restriction sites.
For production of high titer virus, a 2-step method was used.25 Briefly, 293T cells were first cotransfected with each retroviral vector plus plasmids containing gag-pol, vesicular stomatitis virus-glycoprotein (VSV-G), and gibbon ape leukemia virus (GALV) envelope. Supernatants were then collected, concentrated by ultracentrifugation, and used to infect GPE-86 cells 3 to 4 times. Supernatants of the GPE-86 cells titered on NIH3T3 target cells were found to contain 7 to 10 × 106 helper-free infectious units/mL.
Transduction of bone marrow cells
Pep3b mice were injected intravenously with 150 mg/kg body weight of 5-fluorouracil (5-FU; Sigma-Aldrich, St Louis, MO), and, 4 days later, the marrow cells were harvested from both femurs and tibia. In some experiments, more enriched populations of primitive hematopoietic cells were obtained by immunomagnetic isolation of lineage marker–negative (lin–) cells using a column (Stemsep; StemCell Technologies, Vancouver, BC, Canada). Prior to virus exposure, cells were cultured for 48 hours in a serum-free medium consisting of IMDM plus a serum substitute (BIT; StemCell Technologies), 10–4 M 2-mercaptoethanol (Sigma Chemicals, St Louis, MO), 40 μg/mL low-density lipoproteins (Sigma-Aldrich), 100 ng/mL murine Steel factor (R & D Systems, Minneapolis, MN), 100 ng/mL human flt3-ligand (R & D Systems), and 50 ng/mL human thrombopoietin (CytoLab/PeproTech, Rehovot, Israel). The cells were then harvested, resuspended in virus-containing medium plus the same growth factors, and cultured for approximately 16 hours with 2 repetitions of the latter step, as previously described.19 CRU and CFC assays19,26 were performed using cells obtained immediately after this transduction protocol was completed. For measurements of gene-transfer efficiency, the transduced cells were resuspended in a fresh aliquot of the same medium but without virus, and the frequency of GFP+ cells was then determined by flow cytometry 48 hours later. Additional aliquots of these cells were used for Western analyses.
In vivo repopulation and limiting-dilution CRU assays
Pep3b cells were injected immediately after transduction together with 105 unmanipulated B6 bone marrow cells into B6 mice that had been irradiated with 900 cGy within the previous 24 hours. Repopulation by the transduced cells was assessed using flow cytometry to measure the proportion of viable GFP+ Ly5.1+ white blood cells (WBCs) in serial peripheral-blood samples or bone marrow, spleen, or thymus cells at the time of death of the animals. Cells were stained with an anti-Ly5.1 antibody and propidium iodide (PI; Sigma Chemical) to identify viable (PI–) cells. In some experiments, bone marrow cells from primary mice were transplanted into secondary irradiated B6 recipients, and bone marrow cells from these secondary mice were transplanted into irradiated tertiary B6 recipients. To quantify CRUs, serial dilutions of test cells were injected intravenously with 105 B6 bone marrow cells into irradiated (900 cGy) B6 recipients, and the presence of regenerated test-cell–derived (GFP+ Ly5.1+) viable (PI–) B-lymphoid (B220+) and myeloid (Mac1/Gr1+) WBCs was determined by flow cytometric analysis of peripheral-blood samples obtained from the recipients 12 to 16 weeks later.19,26 Regenerated T cells derived from the transplanted transduced cells were identified either as the B220– cells in the GFP+ Ly-5.1+ lymphoid subpopulation (low forward and side light-scattering properties) of peripheral-blood cells or by positive staining of the GFP+ Ly-5.1+ cells with an anti-CD3 antibody. Mice whose WBCs contained at least 1% GFP+ Ly5.1+ B-lymphoid and myeloid cells were scored as positive. All other mice were scored as negative. The cell dose calculated to result in 37% of mice tested being negative after 12 to 16 weeks was defined as containing one CRU, as previously described.26 CRU frequencies were calculated by applying Poisson statistics to the proportion of negative mice in groups of recipients given different numbers of cells, using L-Calc software (StemCell Technologies). Morphology of bone marrow in recipients was examined by touch smear of the bone marrow and subsequent staining with Wright-Giemsa (Sigma-Aldrich). The samples were visualized under an Olympus BX50 microscope equipped with a 20×/0.5 uPlan semi-apochromat objective lens (Olympus, Center Valley, PA), and images were acquired with Olympus DP70 software at original magnification, ×200.
Short-term suspension cultures
Transduced bone marrow cells, infected as described in “Transduction of bone marrow cells” were cultured in IMDM with 10% fetal bovine serum (FBS) plus 100 ng/mL murine Steel factor, 100 ng/mL human flt3-ligand, and 50 ng/mL human thrombopoietin for either another 2 days for phenotype analyses (a total of 6 days in culture) or for another 3 days for assessing effects on CRUs (a total of 7 days in culture).
Proviral integration site analysis
Genomic DNA was extracted from bone marrow, spleen, thymus, and peripheral-blood cells, and 5- to 10-μg aliquots were then digested with HindIII, which cuts once within each of the proviruses. After electrophoresis and transfer onto nylon membranes, the digested DNA was hybridized with a GFP probe isolated from the pEGFP-Nl plasmid (Clontech, Palo Alto, CA) by EcoRI/NotI digestion.
Western blot analysis
Cells were lysed in 2 × Laemlli buffer, and the lysates were then electrophoresed, subjected to immunoblotting using an anti–mouse STAT3 antibody (K-15; Santa Cruz Biotechnology, Santa Cruz, CA), and visualized with ECL (Amersham, Buckinghamshire, United Kingdom).
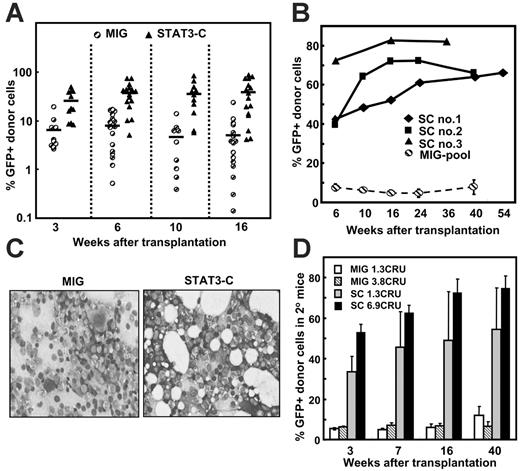
Effect of manipulating STAT3 activities of mouse bone marrow cells on their hematopoietic repopulating ability. (A) Schematic drawings of the first 3 retroviral vectors used: MSCV-IRES-GFP (MIG, the control vector) and vectors encoding a dnStat3 cDNA or a Stat3-C cDNA. (B) Protein expression of STAT3-C or dnSTAT3 in transduced bone marrow cells analyzed 2 days after transduction. Arrows show the position of the proteins indicated. (C) Effect of altering the STAT3 activity of transplanted bone marrow cells on their in vivo repopulating ability. Bone marrow cells from 5-FU–pretreated Pep3b mice were transduced and then transplanted immediately into irradiated B6 recipients (1 to 2 × 105 cells plus 105 normal B6 bone marrow cells per recipient). The values shown are the mean ± SEM of the percent transduced (GFP+) cells detected in the peripheral-blood WBCs in 3 independent experiments (MIG, 16 mice; dnSTAT3, 11 mice; STAT3-C, 13 mice). (D) Lineage distribution of WBCs regenerated from transplanted Stat3-C–or dnStat3-transduced bone marrow cells analyzed 16 weeks after transplantation. GFP+ cells were analyzed for expression of myeloid (Mac1/Gr1) and B-lymphoid (B220) markers. The number of GFP+ T cells present was estimated as the number of B220– cells within the total lymphoid population (cells with low side and forward light-scattering properties). Values shown are the mean ± SEM of values from 5 individual mice in a representative experiment. (E) Multilineage reconstitution of mice by Stat3-C–transduced cells. Single-cell suspensions were prepared from the bone marrow (BM), thymus (Thy), spleen (Spl), and peripheral blood (PB) of mice that received a transplant of MIG- or Stat3-C–transduced bone marrow cells 36 weeks earlier, and the percentage of GFP+ cells was then determined. Values shown are the mean ± SEM (4 mice in each group). (F) Southern blots showing common proviral integrants in different tissues repopulated with Stat3-C–transduced cells. Genomic DNA was extracted, digested with Hindlll, and then probed with a GFP probe.
Effect of manipulating STAT3 activities of mouse bone marrow cells on their hematopoietic repopulating ability. (A) Schematic drawings of the first 3 retroviral vectors used: MSCV-IRES-GFP (MIG, the control vector) and vectors encoding a dnStat3 cDNA or a Stat3-C cDNA. (B) Protein expression of STAT3-C or dnSTAT3 in transduced bone marrow cells analyzed 2 days after transduction. Arrows show the position of the proteins indicated. (C) Effect of altering the STAT3 activity of transplanted bone marrow cells on their in vivo repopulating ability. Bone marrow cells from 5-FU–pretreated Pep3b mice were transduced and then transplanted immediately into irradiated B6 recipients (1 to 2 × 105 cells plus 105 normal B6 bone marrow cells per recipient). The values shown are the mean ± SEM of the percent transduced (GFP+) cells detected in the peripheral-blood WBCs in 3 independent experiments (MIG, 16 mice; dnSTAT3, 11 mice; STAT3-C, 13 mice). (D) Lineage distribution of WBCs regenerated from transplanted Stat3-C–or dnStat3-transduced bone marrow cells analyzed 16 weeks after transplantation. GFP+ cells were analyzed for expression of myeloid (Mac1/Gr1) and B-lymphoid (B220) markers. The number of GFP+ T cells present was estimated as the number of B220– cells within the total lymphoid population (cells with low side and forward light-scattering properties). Values shown are the mean ± SEM of values from 5 individual mice in a representative experiment. (E) Multilineage reconstitution of mice by Stat3-C–transduced cells. Single-cell suspensions were prepared from the bone marrow (BM), thymus (Thy), spleen (Spl), and peripheral blood (PB) of mice that received a transplant of MIG- or Stat3-C–transduced bone marrow cells 36 weeks earlier, and the percentage of GFP+ cells was then determined. Values shown are the mean ± SEM (4 mice in each group). (F) Southern blots showing common proviral integrants in different tissues repopulated with Stat3-C–transduced cells. Genomic DNA was extracted, digested with Hindlll, and then probed with a GFP probe.
Results
The regenerative activity of transplanted adult mouse bone marrow cells can be modulated by genetically engineering their STAT3 activity
Bone marrow cells from 5-FU–pretreated mice were infected with a control retroviral vector (MIG), or the same vector encoding either a dnStat327 or a constitutively activated form of Stat3 (Stat3-C)23 (Figure 1A), and the cells were then transplanted into lethally irradiated Ly5-congenic recipients. The 2-step method used to obtain virus preparations of high titer25 allowed high gene-transfer efficiencies to be obtained (80%-97% total GFP+ cells 48 hours after transduction and 65%-72% GFP+ CFCs assessed immediately after transduction; see Figure S1A-D, available on the Blood website by clicking on the Supplemental Materials link at the top of the online article) with expression of the expected protein products (Figure 1B).
Six weeks after transplantation of the transduced cells into mice, 37% of the WBCs in the recipients of control cells (cells exposed to the MIG vector) were GFP+. GFP+ WBCs were then maintained at this level in these mice until termination of the experiment another 18 weeks later (Figure 1C). In contrast, transplantation of the progeny of the same numbers of cells exposed to either the dnStat3 or the Stat3-C viruses resulted in a 4-fold reduction and a 2-fold increase, respectively, in the output of WBCs 16 weeks after transplantation (< 10% and 73% GFP+ WBCs, respectively, P < .05, Figure 1C and Figure S1A). Reverse transcriptase–PCR analysis of WBCs from the recipients of Stat3-C–transduced cells demonstrated that the enhanced repopulating activity exhibited by the STAT3-C–transduced cells correlated with persistent expression of an intact STAT3-C gene, suggesting a causal relationship (Figure S2).
Of importance, however, neither the modulating effect of dnSTAT3 nor that of STAT3-C on total WBC production was found to be accompanied by any change in the types of WBCs produced (Figure 1D), suggesting that the effects of altered levels of STAT3 activity had influenced the self-renewal of the parental transduced HSCs rather than their differentiation into particular WBC lineages. Consistent with this interpretation was the finding that the number and differentiation of GFP+ CFCs detected immediately after transduction were also the same for all transduced populations (ie, neither dnSTAT3 nor STAT3-C affected the differentiation of CFCs in vitro [Figure S3]). As expected, the output or lineages of WBCs from the small fraction (2%-8%) of cotransplanted, nontransduced (Ly5.1+ GFP–) cells was also not altered (Figure S1E).
Evidence of enhanced repopulating activity by the Stat3-C–transduced cells was also seen in the bone marrow, thymus, and spleen of the mice shown in Figure 1C when they were killed 36 weeks after transplantation (Figure 1E and Figure S4). Proviral integration site analysis demonstrated the presence of common clones of STAT3-C–transduced cells in these different repopulated tissues (Figure 1F).
In another experiment, mice received a transplant of a limiting number of control (MIG) or Stat3-C–transduced CRUs (< 1/recipient, 16 recipients per group) to compare the size of individual clones produced by single transduced CRUs. Within 3 weeks, the number of STAT3-C–transduced WBCs present in mice that received a transplant of these cells was, on average, 4-fold higher than in the recipients of the control cells. By 10 weeks, this difference in output of WBCs had almost doubled (to > 7-fold, Figure 2A) after which it was sustained but did not increase further (Figure 2A-B). At no time was there any evidence of leukemic transformation or myeloproliferative disease detected in any of the mice, as assessed by the number and morphology of the cells in the bone marrow (Figure 2C and Table S1).
Long-term maintenance of the ability of STAT3-C to enhance the regenerative activity of transplanted bone marrow cells. (A) Regenerative activity of transduced bone marrow cells in mice that received a transplant of limiting numbers of transduced CRUs. Cells (4-5 × 104) from 5-FU–pretreated Pep3b mice were transduced with MIG or Stat3-C and then transplanted into each B6 recipient in 3 independent experiments (5-6 mice per group per experiment). Assays for the frequency of GFP+ CRUs in the cells injected showed that the primary recipients were given either 0.3 MIG or 0.5 to 0.8 Stat3-C–transduced CRUs each. Shown are the percent GFP+ WBCs present in the blood of individual mice at the times shown. In the third experiment, the mice were analyzed after 6 and 16 weeks only, and mean values, indicated by the bars, are based on values from both positively and negatively engrafted mice. (B) Long-term follow-up of mice repopulated with Stat3-C–transduced cells. Individual mice repopulated with Stat3-C–transduced cells from the experiments shown in panel A were monitored for up to 54 weeks for the continued presence of GFP+ WBCs. Also shown for comparison is the mean ± SEM of values obtained for the 5 positively engrafted mice that received a transplant of MIG-transduced cells. (C) Normal appearance of bone marrow of mice that received a transplant of each type of transduced cells. Shown are representative touch smears of pelvic bone marrow 40 weeks after transplantation of mice with transduced cells. (D) Reactivation of the enhancing effect of STAT3-C following the transfer of Stat3-C–transduced cells from primary recipients into secondary mice. Marrow cells from the primary recipients shown in panel B were pooled, and aliquots containing the indicated numbers of GFP+ CRUs (determined in independent CRU assays) were transplanted into secondary irradiated B6 recipients. Values shown are the mean ± SEM of the percentage of GFP+ WBCs detected in the secondary mice at the times shown (a total of 12 secondary mice for each source of primary recipient cells assayed).
Long-term maintenance of the ability of STAT3-C to enhance the regenerative activity of transplanted bone marrow cells. (A) Regenerative activity of transduced bone marrow cells in mice that received a transplant of limiting numbers of transduced CRUs. Cells (4-5 × 104) from 5-FU–pretreated Pep3b mice were transduced with MIG or Stat3-C and then transplanted into each B6 recipient in 3 independent experiments (5-6 mice per group per experiment). Assays for the frequency of GFP+ CRUs in the cells injected showed that the primary recipients were given either 0.3 MIG or 0.5 to 0.8 Stat3-C–transduced CRUs each. Shown are the percent GFP+ WBCs present in the blood of individual mice at the times shown. In the third experiment, the mice were analyzed after 6 and 16 weeks only, and mean values, indicated by the bars, are based on values from both positively and negatively engrafted mice. (B) Long-term follow-up of mice repopulated with Stat3-C–transduced cells. Individual mice repopulated with Stat3-C–transduced cells from the experiments shown in panel A were monitored for up to 54 weeks for the continued presence of GFP+ WBCs. Also shown for comparison is the mean ± SEM of values obtained for the 5 positively engrafted mice that received a transplant of MIG-transduced cells. (C) Normal appearance of bone marrow of mice that received a transplant of each type of transduced cells. Shown are representative touch smears of pelvic bone marrow 40 weeks after transplantation of mice with transduced cells. (D) Reactivation of the enhancing effect of STAT3-C following the transfer of Stat3-C–transduced cells from primary recipients into secondary mice. Marrow cells from the primary recipients shown in panel B were pooled, and aliquots containing the indicated numbers of GFP+ CRUs (determined in independent CRU assays) were transplanted into secondary irradiated B6 recipients. Values shown are the mean ± SEM of the percentage of GFP+ WBCs detected in the secondary mice at the times shown (a total of 12 secondary mice for each source of primary recipient cells assayed).
Taken together, these findings suggest that altered STAT3 activity in primitive bone marrow cells with multilineage differentiation potential can affect their initial regenerative activity in irradiated transplant recipients without apparently perturbing the processes of lineage choice or subsequent cell maturation.
The ability of STAT3-C to enhance the initial regenerating activity of transplanted bone marrow cells is reactivated upon their transfer to secondary recipients
We next asked whether the early but transiently displayed ability of activated STAT3 to enhance the regenerative activity of transplanted bone marrow cells was caused by a permanent shutdown of this ability, or whether it might be regulated by conditions in the host. If the latter were the case, we hypothesized that an enhanced regeneration response might be seen when cells from stabilized mice that underwent a primary transplantation were transferred into secondary irradiated mice. To test this possibility, low numbers of bone marrow cells from primary recipients that received a transplant 36 to 54 weeks earlier were injected into secondary irradiated recipients. Subsequent analysis of the secondary mice that had received approximately 1 to 7 GFP+ CRUs showed that the initial outputs of GFP+ WBCs in the secondary recipients of the regenerated STAT3-C–transduced CRUs were larger than those seen in secondary recipients of comparable numbers of regenerated MIG-transduced CRUs (P < .05, Figure 2D). Thus, the forced overexpression of STAT3-C in HSCs (CRUs) does not promote the continuing expansion of these cells after hematopoiesis has stabilized, but can be reactivated by conditions elicited in mice given a lethal dose of irradiation.
Elevated levels of activated STAT3 enhance the self-renewal of CRUs regenerating in vivo
We next sought to investigate the cellular mechanisms responsible for the increased regenerative activity obtained with transplants of Stat3-C–transduced bone marrow cells. As a first step, we compared the bone marrow seeding efficiency of control and STAT3-C–transduced CRUs. For this assessment, a large number of transduced cells was injected into a group of primary mice with the actual input number of GFP+ CRUs in these transplants determined from a set of simultaneously performed limiting-dilution assays. The number of GFP+ CRUs present 20 hours later in the bone marrow of the primary recipients of the large transplants was then determined in a second set of limiting-dilution assays. From the results of these experiments, there was no evidence that the seeding efficiency of the Stat3-C–transduced CRUs was altered (ie, ∼ 10% of the number of GFP+ CRUs initially injected could be recovered from the recipients' bone marrow 20 hours later, regardless of the vector used to transduce them; Table S2).
Transplantation of Stat3-C– or MIG-transduced bone marrow cells together with nontransduced cells also did not affect the repopulating activity of the cotransplanted, nontransduced cells (Figure S5), reinforcing the cell-autonomous nature of the effect of STAT3-C on HSCs. Taken together, these findings show that the initially enhanced repopulating activity exhibited by Stat3-C–transduced bone marrow cells was not attributable to an alteration of their marrow seeding efficiency or the activation of an autocrine or paracrine mechanism.
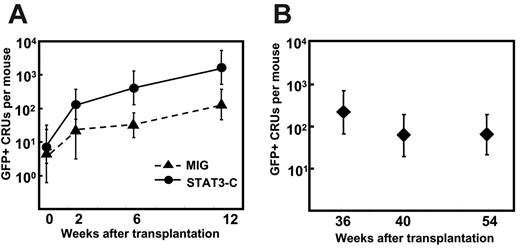
We next sought to determine whether activated STAT3-C directly promotes the self-renewal of regenerating CRUs. To test this possibility, we measured the number of progeny (GFP+) CRUs produced in primary recipients of transduced cells by performing standard limiting-dilution CRU assays of their bone marrow cells transplanted into secondary recipients (see experimental design in Figure 3A). The data obtained from these assays are provided in Table S3, and the derived GFP+ CRU values are shown in Figure 3B-C. It can be seen that, over a period of 40 weeks in the primary mice, the number of STAT3-C–derived CRUs increased 200 ± 36-fold from an initial transplant containing, on average, 0.8 or 4 CRUs per primary recipient. In contrast, the corresponding expansion of the MIG-transduced control CRUs was 10-fold lower (28 ± 2-fold). We then also measured the subsequent expansion of both types of CRUs after a similar period of time (48 weeks) in the secondary recipients by performing CRU assays in tertiary recipients (Figure 3A). The results of these latter analyses showed that the Stat3-C–transduced CRUs had amplified a further 55 ± 20-fold, in contrast to an 11 ± 9-fold expansion of the MIG-transduced CRUs (Figure 3B-C, with detailed data in Table S3). Thus, the accumulated expansion of the Stat3-C–transduced CRUs through 2 successive transplants was 40-fold higher than for the control CRUs (accumulated expansion of STAT3-C CRUs = 11 700-fold vs 300-fold for the MIG-transduced CRUs, Figure 3C). Proviral integration analysis of WBC DNA from the primary, secondary, and tertiary recipients confirmed that the same clones had expanded during each transplant generation (Figure 3D). These experiments demonstrate that forced expression of activated STAT3 confers on transduced CRUs a sustained potential to increase their self-renewal in response to conditions produced in irradiated recipients.
The expansion of Stat3-C–transduced CRUs in recipients that underwent transplantation is markedly enhanced. (A) Experimental design for assessing the expansion of HSCs in mice that underwent serial transplantation. From the CRU frequencies obtained before and after the primary and secondary recipients underwent transplantation, input and output GFP+ CRU numbers were determined and then used to calculate the extent of CRU expansion obtained in each group. (Detailed results from which the CRU frequencies shown were determined are provided in Table S3). (B) Expansion of GFP+ CRU numbers in primary and secondary recipients of MIG-transduced (▨) and Stat3-C–transduced (▪) bone marrow cells from the experiments shown in panel C. Values shown are the mean ± SEM for each group. (*P < .05 in a Student t test ANOVA by comparison with the value for MIG-transduced cells). (C) Cumulative expansion of transduced (GFP+) CRUs during the course of 2 serial transplantations. The number of Stat3-C– or MIG-transduced CRUs injected into primary recipients (Input) was determined from a separate analysis of their frequency. The primary (1°) output indicates the total number of GFP+ CRUs determined to be present in the marrow of the primary mice (mean ± 2 SEM) 40 weeks later. Marrow cells from the primary recipients of STAT3-C–transduced cells (ie, SC no. 21, SC no. 22, and SC no. 23) were assessed individually. For the 4 primary recipients of MIG-transduced cells, the marrow cells were pooled and then assayed. The further expansion of GFP+ CRUs attained after 48 weeks in secondary recipients was similarly determined from assays in tertiary recipients. Cumulative values (2° outputs) were calculated by multiplying the numbers obtained from the primary mice (1° output) by the further expansion calculated to have occurred in the secondary mice (for details, see Table S3). (D) Southern blot analysis of proviral integrations in the cells regenerated from Stat3-C–transduced HSCs. Analyses of the bone marrow from a recipient of a limiting number of Stat3-C–transduced CRUs (SC no. 23: 0.3-2.2 CRUs, 95% CI; and SC nos. 21-22: 1.6-11 CRUs, 95% CI [from panel C]) and from their secondary and tertiary recipients of these cells are shown. Genomic DNA was digested with Hin dlll and electrophoresed, and blots were hybridized with a GFP probe. M denotes molecular weight markers, and vertical lines designate noncontiguous lanes in the same autoradiography. Each lane contained DNA from an individual mouse.
The expansion of Stat3-C–transduced CRUs in recipients that underwent transplantation is markedly enhanced. (A) Experimental design for assessing the expansion of HSCs in mice that underwent serial transplantation. From the CRU frequencies obtained before and after the primary and secondary recipients underwent transplantation, input and output GFP+ CRU numbers were determined and then used to calculate the extent of CRU expansion obtained in each group. (Detailed results from which the CRU frequencies shown were determined are provided in Table S3). (B) Expansion of GFP+ CRU numbers in primary and secondary recipients of MIG-transduced (▨) and Stat3-C–transduced (▪) bone marrow cells from the experiments shown in panel C. Values shown are the mean ± SEM for each group. (*P < .05 in a Student t test ANOVA by comparison with the value for MIG-transduced cells). (C) Cumulative expansion of transduced (GFP+) CRUs during the course of 2 serial transplantations. The number of Stat3-C– or MIG-transduced CRUs injected into primary recipients (Input) was determined from a separate analysis of their frequency. The primary (1°) output indicates the total number of GFP+ CRUs determined to be present in the marrow of the primary mice (mean ± 2 SEM) 40 weeks later. Marrow cells from the primary recipients of STAT3-C–transduced cells (ie, SC no. 21, SC no. 22, and SC no. 23) were assessed individually. For the 4 primary recipients of MIG-transduced cells, the marrow cells were pooled and then assayed. The further expansion of GFP+ CRUs attained after 48 weeks in secondary recipients was similarly determined from assays in tertiary recipients. Cumulative values (2° outputs) were calculated by multiplying the numbers obtained from the primary mice (1° output) by the further expansion calculated to have occurred in the secondary mice (for details, see Table S3). (D) Southern blot analysis of proviral integrations in the cells regenerated from Stat3-C–transduced HSCs. Analyses of the bone marrow from a recipient of a limiting number of Stat3-C–transduced CRUs (SC no. 23: 0.3-2.2 CRUs, 95% CI; and SC nos. 21-22: 1.6-11 CRUs, 95% CI [from panel C]) and from their secondary and tertiary recipients of these cells are shown. Genomic DNA was digested with Hin dlll and electrophoresed, and blots were hybridized with a GFP probe. M denotes molecular weight markers, and vertical lines designate noncontiguous lanes in the same autoradiography. Each lane contained DNA from an individual mouse.
Enhanced self-renewal of STAT3-C–transduced CRUs is dependent on stimuli that promote hematopoietic regeneration
The transient but reversible nature of the enhancing effect of STAT3-C in bone marrow cells transplanted into irradiated recipients (Figure 2) suggested that this might be caused by changes in the conditions present at early and late times after transplantation. To investigate this possibility, we examined the kinetics of Stat3-C– and MIG-transduced (GFP+) CRU expansion in the primary recipients by measuring the numbers of GFP+ CRUs present in their bone marrow at different time points after the initial transplantation was performed. As shown in Figure 4A, the expansion of the Stat3-C–transduced CRUs was faster than the expansion of MIG-transduced CRUs during the first 12 weeks (200-fold vs 27-fold during this period). However, the enhancing effect of STAT3-C on CRU expansion, like the effect on mature WBC production, was again restricted to the initial phase of hematopoietic recovery. Thus, when a similar analysis was performed on cells from mice that had been repopulated with transduced cells for 36 to 54 weeks (SC nos. 1-3, Figure 2B), no further increases in Stat3-C–transduced CRU numbers were noted (Figure 4B).
Kinetics of STAT3-C–transduced CRU expansion in mice that underwent transplantation. (A) The numbers of GFP+ CRUs present in primary recipients of 4 × 105 transduced bone marrow cells from 5-FU–pretreated Pep3b mice was assessed at the indicated time points. Transduced bone marrow cells were transplanted into primary mice (9 mice per group), and then at each time point indicated, bone marrow cells from 3 primary mice in each group were pooled and transplanted into secondary recipients to determine the number of transduced (GFP+) CRU numbers present. Values shown are the mean ± 2 SEM (95% CI). The number of transduced CRUs initially injected was determined in a separate limiting-dilution experiment. (B) Number of GFP+ CRUs regenerated over time in 3 individually assessed primary recipients of single STAT3-C–transduced CRUs (SC nos. 1-3, Figure 2B). Values shown are the mean ± 2 SEM.
Kinetics of STAT3-C–transduced CRU expansion in mice that underwent transplantation. (A) The numbers of GFP+ CRUs present in primary recipients of 4 × 105 transduced bone marrow cells from 5-FU–pretreated Pep3b mice was assessed at the indicated time points. Transduced bone marrow cells were transplanted into primary mice (9 mice per group), and then at each time point indicated, bone marrow cells from 3 primary mice in each group were pooled and transplanted into secondary recipients to determine the number of transduced (GFP+) CRU numbers present. Values shown are the mean ± 2 SEM (95% CI). The number of transduced CRUs initially injected was determined in a separate limiting-dilution experiment. (B) Number of GFP+ CRUs regenerated over time in 3 individually assessed primary recipients of single STAT3-C–transduced CRUs (SC nos. 1-3, Figure 2B). Values shown are the mean ± 2 SEM.
Consistent with the eventual suppression of the effect of STAT3-C on the early amplification of small numbers of transplanted CRUs was the finding that this effect was also abrogated by the transplantation of very large numbers of CRUs. Thus, increasing the number of STAT3-C–transduced CRUs transplanted from approximately 1 CRU to approximately 5 CRUs per mouse caused an equivalent increase in the number of daughter CRUs generated after 8 weeks (Figure 5A), but when 50 Stat3-C–transduced CRUs were transplanted the number of progeny CRUs regenerated at this early time was not increased further. In the same experiments, the relative output of GFP+ CFCs (per Stat3-C–transduced CRU injected) was also compromised in recipients of very large numbers of these cells (1.3 × 105 GFP+ CFCs per GFP+ CRU in recipients of ∼ 1 Stat3-C–transduced CRU vs 5.3 × 103 GFP+ CFCs per GFP+ CRU in the recipients of ∼ 50 Stat3-C–transduced CRUs; for details of the data, see Table S4). Thus, the output of GFP+ CFCs mirrored the expansion of the GFP+ CRUs as expected. These results suggest that the ability of increased levels of activated STAT3 in transplanted CRUs to enhance their self-renewal is subject to conditions in the recipient that can vary with their hematologic status.
The finding that forced expression of STAT3-C could have different effects on CRU self-renewal under different conditions in the host was unexpected in view of the fact that STAT3-C can undergo spontaneous dimerization, independent of prior Tyr phosphorylation.23 However, because phosphorylation of Ser727 has also been reported to influence the transactivating activity of STAT3,24 it was of interest to determine whether mutation of this residue would influence the effect of STAT3-C on initially regenerating CRUs. To test this possibility, vectors encoding 2 mutants of Stat3-C were constructed. One was designed to substitute an Ala (CSA) residue for Ser727. The other was designed to substitute an Asp (CSD) residue at the same site to mimic constitutively dephosphorylated and phosphorylated Ser727 residues, respectively.28 Figure 5B shows the patterns of GFP+ WBC regeneration obtained in recipients of equivalent numbers of bone marrow cells transduced with these modified Stat3-C constructs by comparison with wild-type Stat3-C or control (MIG) vectors. It can be seen that the Asp mutation (CSD) suppressed the ability of STAT3-C to enhance the regenerative capacity of the transduced cells, whereas the substitution of an Ala residue (CSA) did not significantly alter the effect of STAT3-C. In one experiment, the number of GFP+ CRUs that had been regenerated in the bone marrow of the primary recipients after 26 weeks was also measured. The result showed that the effects obtained on the output of mature WBCs were accompanied by similar changes in the self-renewal responses of the CSD- and CSA-transduced CRUs (17 vs 360 CRUs regenerated after 26 weeks, respectively). These results suggest that phosphorylation of Ser727 may contribute to the ability of STAT3-C to alter HSC self-renewal in response to varying environmental cues delivered in vivo.
Regulation of the ability of STAT3-C to enhance CRU expansion in mice that underwent transplantation. (A) Transplant dose-response effect of the ability of STAT3-C to enhance the in vivo expansion of transplanted HSCs. Different numbers of Stat3-C–transduced bone marrow cells from 5-FU–pretreated Pep3b mice were transplanted into groups of B6 recipients, and then 8 weeks later the marrows of these mice were assayed in secondary mice to determine the numbers of GFP+ CRUs that had been regenerated in the primary mice. Primary mice were estimated to have received 1 (1x), 5 (5x), or 50 (50x) GFP+ CRUs based on results of other experiments. Values shown are the mean ± 2 SEM for the total number of regenerated Stat3-C–transduced CRUs per primary mouse. The hatched area indicates the total number of CRUs present in a normal B6 mouse. (B) Effects of different mutations of the Ser727 residue of STAT3-C on the repopulating activity of transduced CRUs. 5-FU–enriched bone marrow cells (105) were transduced with MIG, Stat3-C, or a mutant STAT3-C containing either an Asp (CSD) or Ala (CSA) residue instead of Ser727. The cells were then transplanted into irradiated recipients, and the proportion of GFP+ WBCs was determined at the times shown. Values shown are the mean ± SEM from 2 independent experiments (5 mice per group for each experiment). The average gene-transfer efficiencies in these experiments were 80%, 73%, 73%, and 76%, respectively, for MIG, Stat3-C, CSA, and CSD.
Regulation of the ability of STAT3-C to enhance CRU expansion in mice that underwent transplantation. (A) Transplant dose-response effect of the ability of STAT3-C to enhance the in vivo expansion of transplanted HSCs. Different numbers of Stat3-C–transduced bone marrow cells from 5-FU–pretreated Pep3b mice were transplanted into groups of B6 recipients, and then 8 weeks later the marrows of these mice were assayed in secondary mice to determine the numbers of GFP+ CRUs that had been regenerated in the primary mice. Primary mice were estimated to have received 1 (1x), 5 (5x), or 50 (50x) GFP+ CRUs based on results of other experiments. Values shown are the mean ± 2 SEM for the total number of regenerated Stat3-C–transduced CRUs per primary mouse. The hatched area indicates the total number of CRUs present in a normal B6 mouse. (B) Effects of different mutations of the Ser727 residue of STAT3-C on the repopulating activity of transduced CRUs. 5-FU–enriched bone marrow cells (105) were transduced with MIG, Stat3-C, or a mutant STAT3-C containing either an Asp (CSD) or Ala (CSA) residue instead of Ser727. The cells were then transplanted into irradiated recipients, and the proportion of GFP+ WBCs was determined at the times shown. Values shown are the mean ± SEM from 2 independent experiments (5 mice per group for each experiment). The average gene-transfer efficiencies in these experiments were 80%, 73%, 73%, and 76%, respectively, for MIG, Stat3-C, CSA, and CSD.
STAT3-C promotes the maintenance of phenotypically as well as functionally defined primitive hematopoietic cells in vitro
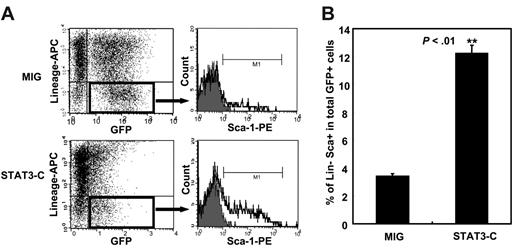
To determine whether an effect of elevated levels of activated STAT3 on primitive hematopoietic cells could also be discerned in vitro, we first measured the number of GFP+ Sca-1+lin– hematopoietic cells present 2 days after the transduction procedure was completed. The results showed that there were significantly more of these cells in the cultures of Stat3-C–transduced bone marrow cells than in the cultures of MIG-transduced cells (12% vs 3%, P < .01; Figure 6). Assessment of the number of transduced CRUs present 3 days later showed that these were present at 3-fold higher levels in the cultures of Stat3-C–transduced bone marrow cells than in the cultures of MIG-transduced cells (Table 1). Thus, evidence of an enhancing effect of STAT3-C on the maintenance of primitive hematopoietic cells in vitro was also obtained.
Effect of forced expression of STAT3-C on CRU maintenance in vitro
Transduction/no. of GFP+ cells injected* . | % positively engrafted mice . | CRU frequency*(95% CI) . |
|---|---|---|
| MIG | 1/1 290 000 (1/5 300 000-1/310 000) | |
| 4.8 × 105 | 20† | |
| 9.6 × 104 | 20† | |
| STAT3-C | 1/410 000 (1/1 340 000-1/130 000) | |
| 3.8 × 105 | 60† | |
| 3.8 × 104 | 0‡ | |
| 7.6 × 103 | 0† |
Transduction/no. of GFP+ cells injected* . | % positively engrafted mice . | CRU frequency*(95% CI) . |
|---|---|---|
| MIG | 1/1 290 000 (1/5 300 000-1/310 000) | |
| 4.8 × 105 | 20† | |
| 9.6 × 104 | 20† | |
| STAT3-C | 1/410 000 (1/1 340 000-1/130 000) | |
| 3.8 × 105 | 60† | |
| 3.8 × 104 | 0‡ | |
| 7.6 × 103 | 0† |
5-FU—pretreated bone marrow cells were transduced with retroviral vectors encoding MIG or Stat3-C. After infection, the cells were cultured for an additional 3 days in medium containing cytokines and 10% FBS. CI indicates confidence interval.
Cell dose representing the day-7 progeny of the number of indicated input cells. Thus, the frequencies shown are directly comparable as they are expressed per starting cell equivalents.
n = 5.
n = 4.
Forced expression of STAT3-C enhances the output of Sca-1+lin– bone marrow cells in vitro. Purified lin– bone marrow cells were transduced with MIG or Stat3-C and the cells were then maintained for an additional 48 hours under the same conditions (but without virus). The proportion of lin–Sca-1+ cells within the transduced (GFP+) population was then measured. (A) Representative FACS plots. (B) Mean percent ± SEM of results from 3 experiments. **P <.01.
Forced expression of STAT3-C enhances the output of Sca-1+lin– bone marrow cells in vitro. Purified lin– bone marrow cells were transduced with MIG or Stat3-C and the cells were then maintained for an additional 48 hours under the same conditions (but without virus). The proportion of lin–Sca-1+ cells within the transduced (GFP+) population was then measured. (A) Representative FACS plots. (B) Mean percent ± SEM of results from 3 experiments. **P <.01.
Discussion
The control of HSC self-renewal is of great interest because of its central role in the developmental process that allows these rare cells to sustain the lifelong integrity of hematopoietic tissues. Improved understanding of HSC self-renewal is also important for dissecting the pathogenesis of multiple diseases of the hematopoietic system and for generating new therapeutic approaches requiring amplified HSC populations. Here we provide evidence that the self-renewal of bone marrow HSCs transplanted in vivo can be profoundly and specifically influenced, both positively and negatively, by the level of activated STAT3 they contain. Down-regulation of STAT3 activity in HSCs achieved by forced expression of a dnSTAT3 markedly reduced the competitive reconstituting ability of transduced HSCs when these were transplanted together with unmanipulated cells into lethally irradiated recipients. Conversely, up-regulation of STAT3 activity by transduction of HSCs with a constitutively activated form of Stat3 (Stat3-C) could enhance the regenerative activity of small transplants of transduced bone marrow cells by enhancing the expansion of the transduced HSCs during the initial phase of hematologic recovery. Thus, during the course of 2 successive transplantations that spanned a period of 2 years, small numbers of Stat3-C–transduced HSCs could be stimulated to expand more than 10 000-fold, in contrast to the approximately 300-fold expansion of control HSCs measured in parallel. Nevertheless, the recipients of Stat3-C–transduced cells showed no progressive increases in WBC numbers nor any other signs of leukemia or dysregulated myelopoiesis despite the evidence of continued STAT3-C activity and reports that STAT3-C can act as an oncogene.23
Overexpression of STAT3-C also promoted the maintenance of CRU activity and cells with a primitive lin–Sca-1+ phenotype in vitro. This result appears to be different from that recently reported by Kato et al,21 although we note that the 2 studies also used different culture conditions. For example, we have found that the enhancing effects of STAT3-C are strongly serum dependent (data not shown), which might have contributed to the differences in the results.
An important observation from the present studies was the finding that the enhancing effect of STAT3-C on HSC self-renewal in mice that underwent transplantation was limited to the early phase of hematopoietic regeneration and was attenuated when the production of mature blood cells had recovered. Indeed, the effect of STAT3-C expression was greatest and most prolonged when the HSC content of the transplant was low, and the converse was seen with very large transplants. However, this activity remained latent and could be consistently reactivated when the transduced cells were transferred to new irradiated recipients. Thus, the ability of elevated levels of activated STAT3 to promote the self-renewal of proliferating HSCs appears subject to additional regulatory mechanisms that are determined by the physiologic status of the host, becoming only transiently active during the early postirradiation recovery period. In this context, it is interesting to note that the induced inactivation of STAT3 in hematopoietic cells under homeostatic conditions in adult mice also did not affect the cellularity of the bone marrow or maintenance of more primitive cells for up to 5 weeks.21
While these findings support the view that the ability of activated STAT3 to alter HSC self-renewal is, in turn, dependent on conditions prevailing in the host, the molecular mechanisms that regulate the effects of activated STAT3 in proliferating HSCs remains unclear. Our finding that mutation of the Ser727 residue of STAT3-C could negate its effect suggests that interactions with unknown external factors may be able to regulate the potential of STAT3-C to enhance HSC self-renewal. While it was previously shown that phosphorylation of Ser727 could have a negative effect on the activity of STAT3 in other systems,29,30 further studies will clearly be needed to establish the specific role of Ser727 phosphorylation in HSCs.
STAT3 activity has also been found to be a regulator of the undifferentiated state in murine embryonic stem (ES) cells31,32 and has been implicated in the control of other regenerative processes including hepatic-cell regeneration,33,34 hypertrophy of cardiac muscle cells,35 and wound repair of the gastrointestinal mucosa.36 It is tempting to speculate that STAT3 activation might serve as a widely conserved mechanism regulating the rate of regeneration of a variety of stem cells, as inferred from recent indications of multiple common genes expressed in such populations.37-39 Identification of the key molecules involved in mediating the consequences of STAT3 activation should facilitate further investigation of this concept of shared mechanisms operative in different stem-cell types.
Prepublished online as Blood First Edition Paper, April 13, 2006; DOI 10.1182/blood-2006-01-010199.
Supported by grants from the Korea Health 21 R&D project, Ministry of Health & Welfare, Republic of Korea (0405-DB01-0104-0006). C.J.E. was supported by grants from the National Cancer Institute of Canada with funds from the Terry Fox Run.
Y.-J.C., B.-B.P., Y.-J.K., and T.-m.K. performed laboratory experiments; C.J.E. and I.-H.O. supervised and prepared the article.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
The authors thank Dr Hal Broxmeyer for critical review of the paper, Dr Keith Humphries (Terry Fox Laboratory, BC Cancer Agency, Vancouver, BC, Canada) for the MSCV-IRES-GFP plasmid, and Dr Gyeongsin Park (Department of Pathology, Catholic University of Korea, Seoul, South Korea) for pathologic analysis of bone marrow and peripheral-blood smears. We also thank Ms Nuri Moon, Mr Jae-Seung Shim, and Mr Seung-Jip Yang for their excellent assistance with the mouse transplantation experiments.


![Figure 3. The expansion of Stat3-C–transduced CRUs in recipients that underwent transplantation is markedly enhanced. (A) Experimental design for assessing the expansion of HSCs in mice that underwent serial transplantation. From the CRU frequencies obtained before and after the primary and secondary recipients underwent transplantation, input and output GFP+ CRU numbers were determined and then used to calculate the extent of CRU expansion obtained in each group. (Detailed results from which the CRU frequencies shown were determined are provided in Table S3). (B) Expansion of GFP+ CRU numbers in primary and secondary recipients of MIG-transduced (▨) and Stat3-C–transduced (▪) bone marrow cells from the experiments shown in panel C. Values shown are the mean ± SEM for each group. (*P < .05 in a Student t test ANOVA by comparison with the value for MIG-transduced cells). (C) Cumulative expansion of transduced (GFP+) CRUs during the course of 2 serial transplantations. The number of Stat3-C– or MIG-transduced CRUs injected into primary recipients (Input) was determined from a separate analysis of their frequency. The primary (1°) output indicates the total number of GFP+ CRUs determined to be present in the marrow of the primary mice (mean ± 2 SEM) 40 weeks later. Marrow cells from the primary recipients of STAT3-C–transduced cells (ie, SC no. 21, SC no. 22, and SC no. 23) were assessed individually. For the 4 primary recipients of MIG-transduced cells, the marrow cells were pooled and then assayed. The further expansion of GFP+ CRUs attained after 48 weeks in secondary recipients was similarly determined from assays in tertiary recipients. Cumulative values (2° outputs) were calculated by multiplying the numbers obtained from the primary mice (1° output) by the further expansion calculated to have occurred in the secondary mice (for details, see Table S3). (D) Southern blot analysis of proviral integrations in the cells regenerated from Stat3-C–transduced HSCs. Analyses of the bone marrow from a recipient of a limiting number of Stat3-C–transduced CRUs (SC no. 23: 0.3-2.2 CRUs, 95% CI; and SC nos. 21-22: 1.6-11 CRUs, 95% CI [from panel C]) and from their secondary and tertiary recipients of these cells are shown. Genomic DNA was digested with Hin dlll and electrophoresed, and blots were hybridized with a GFP probe. M denotes molecular weight markers, and vertical lines designate noncontiguous lanes in the same autoradiography. Each lane contained DNA from an individual mouse.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/108/4/10.1182_blood-2006-01-010199/3/m_zh80160699790003.jpeg?Expires=1767710749&Signature=W4cruGts0r5NY1hiNV19c0iSrSurJ~6jVlZ57B5LIqAwilQFYh3BRucOnhCY8CempNwdSz1v694uHt9XamU230dyDOpisRa-cZLO3JJ1LHjjYDAUMgBCdYPUMm9XMKeuSd2dG1d6Di8EriVp1qshIyZhQNyCffaaWDrzjqIRlZxk8CHL7jtIHAYTqBGE9DTTQyDtiT5ZYMLXpbBtOEyJzhcf6AYGts48krIPwg0P49xJY4ncRc-WwrEmrRBNupGGEcSvdqaRYZx7fjU6X0AlmV9EsT-NNeBnCRrMLWMPRQwgYSKqEUTBywNwL51jc-RnJWIG~quWEXLHvqIP5tMJHw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal