Abstract
In follicular lymphoma (FL) and mantle cell lymphoma (MCL) the monoclonal antibody rituximab (R) improves the prognosis when combined with chemotherapy. The present study investigated R-maintenance after R-chemotherapy. Patients with recurring or refractory FL and MCL were randomized to 4 courses of fludarabine, cyclophosphamide, and mitoxantrone (FCM) alone or combined with R (R-FCM). Responding patients underwent a second randomization for R-maintenance comprising 2 further courses of 4-times-weekly doses of R after 3 and 9 months. The first randomization was stopped after 147 patients, when R-FCM revealed a significantly better outcome. All subsequent patients received R-FCM. Of the 176 patients who are currently evaluable (as of October 2005), 138 received R-FCM for remission induction. Response duration was significantly prolonged by R-maintenance after R-FCM, with the median not being reached in this evaluation versus an estimated median of 16 months (P = .001). This beneficial effect was also observed when analyzing FL (P = .035) and MCL (P = .049) separately. Hence, R-maintenance is effective after salvage with R-chemotherapy and significantly prolongs response duration in patients with recurring or refractory FL or MCL.
Introduction
The introduction of rituximab (R) into the treatment of malignant lymphomas of the B-cell lineage has had a major impact on the management of these diseases. In the 2 most frequent lymphoma subtypes, the diffuse large B-cell lymphomas (DLBCLs) and the follicular lymphomas (FLs), several multicenter prospective randomized trials consistently demonstrated an improved outcome when R was added to chemotherapy. In DLBCL, R-chemotherapy combinations resulted in higher response rates and an increase in long-term remissions and potential cures.1-5 Significant increases in response rates, response duration, and even in overall survival by R-chemotherapy combinations were also observed in FL in first- and second-line therapy.6-12 A significantly higher response rate and a moderate effect on response duration was also seen by R-chemotherapy combinations in mantle cell lymphomas (MCLs).13
In FL and DLBCL it was attempted to further prolong response duration and survival by administering R during remission as maintenance therapy. In DLBCL, Habermann et al4 performed a study in patients 60 years of age and older who were initially randomized for chemotherapy with cyclophosphamide, hydroxydaunorubicin, vincristine, and prednisone (CHOP) versus CHOP plus R (R-CHOP). Responding patients underwent a second randomization for R maintenance versus observation only.4 In patients receiving R maintenance, a significant prolongation of the time to treatment failure was observed. This beneficial effect was restricted, however, to patients who had received CHOP alone for initial therapy, while in R-CHOP–treated cases no differences in response duration were found. It therefore appears that at least in DLBCLs, an initial combination of R and CHOP chemotherapy may abolish the effect of a subsequent R maintenance. Similar to these findings, Hochster et al14,15 reported a significant prolongation of response duration and even survival by R maintenance in patients with previously untreated advanced-stage FL responding to initial therapy without R, comprising chemotherapy by cyclophosphamide, vincristine, and prednisone (CVP) alone. In preceding investigations, Ghielmini et al16 and Hainsworth et al17,18 already found that R maintenance was beneficial when given to patients with FL who achieved a partial remission (PR) or complete remission (CR) after initial single-agent first-line R therapy.
So far, the question remains unanswered, however, of whether R maintenance is also effective when remission is induced by a R-containing chemotherapeutic protocol. To address this question, the German Low Grade Lymphoma Study Group (GLSG) embarked on a prospective randomized comparison of R maintenance versus no further treatment in patients with recurring and refractory FL or MCL responding to salvage therapy with R in combination with fludarabine, cyclophosphamide, and mitoxantrone (R-FCM). This study initially included a preceding randomization for R-FCM versus FCM alone, which was stopped after 147 patients when R-FCM treatment revealed a significant improvement in response rates, response duration, and survival over that of FCM alone, as recently reported by Forstpointner et al.6 The present report describes the results of the second randomization and is particularly focused on the effect of R maintenance compared with no further treatment in remission after response to initial R-FCM therapy.
Patients, materials, and methods
Patients and entry criteria
This study was performed as a prospective, randomized, open-label multicenter phase 3 trial. It was started in 1998 and included patients of ages 18 years and older with recurring or refractory FL or MCL according to the World Health Organization (WHO) classification.19 Entry criteria comprised a nonresponse or relapse after at least one preceding chemotherapy as well as disease recurrence after autologous stem cell transplantation. Prior treatment with an R-chemotherapy combination was also allowed. The histologic specimens underwent a central review at one of 6 designated pathology reference centers.
Pregnant or lactating women and patients of childbearing potential not using a reliable contraceptive method were not allowed to enroll.
The initial diagnostic work-up comprised the assessment of the extent of the disease, including bone marrow biopsy, ultrasound examination of the abdomen, and computed tomography (CT) scans of chest and abdomen. Normal organ function was assured by the respective laboratory tests, as well as by echo- and electrocardiograms.
Randomization and treatment protocol
Patients were enrolled in the study by the responsible physician after giving their written informed consent. All patients underwent a central randomization procedure at the study center by telephone. Randomization was done by a computer program stratified for histology; response to the preceding chemotherapy or R chemotherapy, respectively; and the number of previous therapies using the method of random permutated blocks.
The R-FCM combination comprised R at a dose of of 375 mg/m2/day on day 0, fludarabine at a dose of 25 mg/m2/day over 30 minutes intravenously on days 1 to 3, cyclophosphamide at a dose of 200 mg/m2/day as a 4-hour infusion on days 1 to 3, and mitoxantrone at a dose of 8 mg/m2/day over 30 minutes intravenously on day 1. Treatment cycles were repeated every 4 weeks for a total of 4 cycles. In patients with peripheral lymphocyte counts greater than 20 × 109/L (20 000/mm3) and/or a larger tumor mass (ie, bulky disease greater than 10 cm), a cytoreductive prephase could be performed, comprising cyclophosphamide at a dose of 200 mg/m2 as a 1-hour infusion over 3 to 5 days.
Patients achieving a CR or PR who were randomized for R maintenance received 2 courses of R to be given 3 and 9 months after completion of salvage therapy. Each course of R consisted of 4 doses of 375 mg/m2/day given for 4 consecutive weeks. Patients who were randomized for observation only received no further treatment.
Evaluation and response criteria
Response to initial therapy was assessed after the first 2 cycles of therapy and 4 weeks after the completion of the fourth course. Response evaluation comprised a physical examination, ultrasound of the abdomen, and CT scans of previously involved areas. In patients otherwise fulfilling the criteria of a CR, a bone marrow biopsy was performed.
Response duration was monitored every 3 months by physical examination, ultrasound of the abdomen, and peripheral blood counts. CT scans of previously involved areas were repeated every 6 months.
Response was defined according to the International Working Group criteria.20 Hence, CR comprised the elimination of all lymphoma manifestations for at least 4 weeks, including the bone marrow, while PR was defined as a reduction of disease manifestations by at least 50% for more than 4 weeks. The appearance of new nodal or extranodal manifestations or the enlargement of pre-existing lymphoma manifestations by more than 25% were considered as progression. Response duration was defined from the end of successful therapy to documentation of progression or death, time to progression was defined as the interval between the start of treatment and documentation of progressive disease, and survival was defined as the interval between enrollment into the study to death.
The frequency and severity of side effects was recorded according to National Cancer Information Center (NCIC) Common Toxicity Criteria (CTC).
Statistics
The comparison of R maintenance with no further treatment was designed to test whether R maintenance could reduce the relative risk of relapse by 50%. On this basis, a 1-sided triangular sequential test with a working significance level of .05 was applied. This procedure allowed for the detection of the assumed superiority of R maintenance over that of no further treatment with a probability of 95%, and also allowed for the halting of recruitment as soon as the level of significance was reached. The sequential procedure was designed to be equivalent in power and working significance level to a fixed sample test with 91 events. A further explorative analysis was done on an intention-to-treat basis for histologic subgroups and for the evaluation of overall survival. The Fisher test was used for comparison of binary responses and the log-rank test and univariate Cox regression for the analysis of time-censored observations.
Study conduct
The study was carried out in accordance with the modified Helsinki declaration.
All patients gave their written informed consent after having been informed about the purpose and investigational nature of the trial. Prior to initiation, the study received approval by the responsible ethics committees of the participating institutions.
Results
A total of 319 patients from 109 participating institutions were enrolled into the trial between November 1998 and April 2005. In June 2001, the applied 1-sided sequential test showed a significant advantage for R-FCM over FCM alone, and further randomization for this question was stopped after 147 patients.6 All subsequent patients received R-FCM. A number of patients (195) were randomized for R-maintenance versus no further treatment, and randomization was stopped in May 2005 after the respective statistical test showed a significant advantage for the R-maintenance arm. In detail, the following results were obtained.
Patient characteristics
Of the 195 patients, 125 patients (64%) had FL, 56 patients (29%) were diagnosed with MCL, and 14 patients (7%) presented with other histologies. After correction for the results of reference histology, 113 patients (58%) had FL, 66 patients (34%) had MCL, and 16 patients (8%) had other lymphoma subtypes. A number of patients (176) were evaluable for response to therapy, response duration, and toxicity at the time of this report. In 19 patients the documentation was incomplete. In 14 patients documentation was incomplete at the time of this analysis, one patient moved to another institution, in another case the patient record was inaccessible, and in 3 patients the documented staging after initial therapy was not sufficient.
The median age was 62 years, with a range from 35 to 80 years. More than half (61%) of patients were 60 years of age or older. All patients had advanced stage III or IV disease and were in need of treatment before entering the study. All patients had previously received at least one type of chemotherapy. The median time from initial diagnosis to study entry was 35 months.
A small number of patients (38) received FCM alone for initial therapy, while 138 patients were treated with R-FCM (Table 1).
Treatment results
Response to initial therapy. The first 147 patients entering the trial were randomized for FCM or R-FCM, and in case of response, subsequently for R-maintenance versus observation only. As depicted in Table 2, R-FCM resulted in significantly higher response rates in FL and MCL, and randomization was terminated in June 2001. All subsequent patients received R-FCM, and the favorable response rates were confirmed (Table 2).
Response duration and survival. Of 267 evaluable patients, a total of 207 patients achieved a CR or PR after FCM (n = 39) or R-FCM (n = 168), and 176 patients were subsequently randomized for R maintenance versus observation only (Table 1). The second randomization was not carried out in 35 patients who had responses to initial therapy: 14 patients terminated initial therapy early, 12 patients or their physicians refused further therapy, 5 patients had initial allergic reactions to R, and 4 patients developed new concomitant diseases. There were also 4 patients who did not achieve a PR after initial therapy but were randomized by violating the protocol. However, in all randomized patients, the progression-free survival after initial therapy was evaluated according to an intention-to-treat analysis.
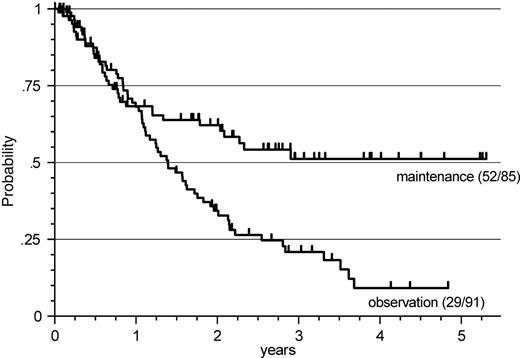
After a median observation time of 26 months (range, 1-64 months), a significantly longer response duration was observed for the total group of patients on the R-maintenance arm, with the median not being reached in this evaluation, compared with an estimated median of 17 months for patients receiving no further treatment (P < .001) (Figure 1). This analysis, however, includes 38 patients who had received FCM alone for initial therapy, and also includes patients with FL and MCL.
Response duration for R maintenance and observation. A significantly longer response duration was observed for the total group of patients on the R-maintenance arm (P < .001) with the median not being reached in this evaluation, compared with an estimated median of 17 months for patients receiving no further treatment.
Response duration for R maintenance and observation. A significantly longer response duration was observed for the total group of patients on the R-maintenance arm (P < .001) with the median not being reached in this evaluation, compared with an estimated median of 17 months for patients receiving no further treatment.
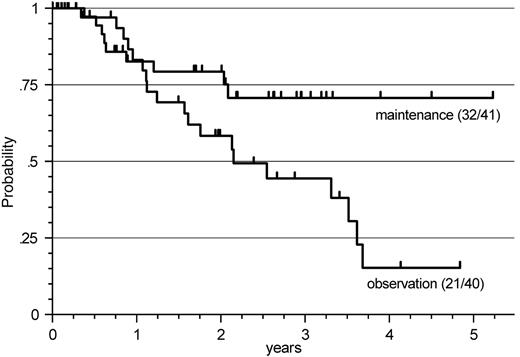
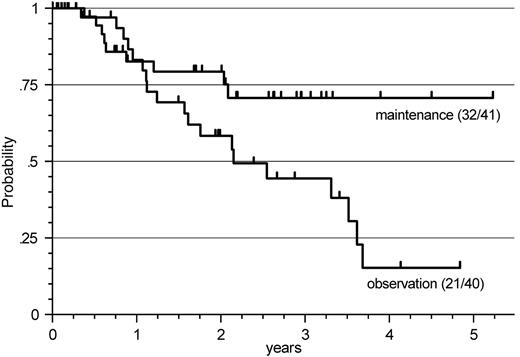
Response duration after R-FCM in patients with FL. When restricting the analysis to patients with FL and to initial therapy with R-FCM (n = 81), a significant prolongation of response duration by R maintenance compared with that by observation only was observed, with the median not being reached in this evaluation, compared with an estimated median of 26 months, respectively (P = .035) (Figure 2).
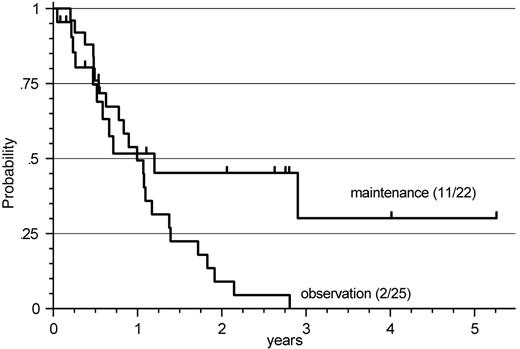
Response duration after R-FCM in patients with MCL. When applying this analysis to patients with MCL and initial R-FCM therapy (n = 47), a beneficial effect of R-maintenance was also observed (P = .049). Although the median response duration did not differ between R maintenance and observation only (14 months versus 12 months), a higher proportion of patients in this evaluation experienced ongoing remissions beyond 2 years (45% vs 9%) (Figure 3).
Survival. For the total group of patients, and also for the groups of patients with FL and MCL, the median survival has not been reached in this evaluation (October 2005) in either study arm. The estimated proportion of patients alive at 3 years is 77% after R maintenance and 57% after observation only (P = .100).
Side effects
Treatment-associated side effects related to the infusion of R were observed in 8% of the maintenance cycles and were mostly mild to moderate. R therapy was discontinued early in one patient only because of a severe allergic reaction. Side effects according to the CTC were monitored during response duration every 3 months for both arms, and no significant differences were observed (Table 3).
Response duration after R-FCM in patients with FL. A significant prolongation of response duration by R-maintenance compared with observation only was observed (P = .035), with the median not being reached in this evaluation, compared with an estimated median of 26 months.
Response duration after R-FCM in patients with FL. A significant prolongation of response duration by R-maintenance compared with observation only was observed (P = .035), with the median not being reached in this evaluation, compared with an estimated median of 26 months.
Response duration after R-FCM in patients with MCL. A significant prolongation of response duration by R-maintenance compared with observation only was observed (P = .049), with median response durations of 14 months versus 12 months but a higher proportion of ongoing remissions beyond 2 years of 45% versus 9%.
Response duration after R-FCM in patients with MCL. A significant prolongation of response duration by R-maintenance compared with observation only was observed (P = .049), with median response durations of 14 months versus 12 months but a higher proportion of ongoing remissions beyond 2 years of 45% versus 9%.
Discussion
The current study clearly indicates that maintenance therapy with R results in a significant improvement of response duration in patients with recurring or refractory FL and, to a lesser degree, also in patients with MCL who responded to salvage therapy with an R-chemotherapy combination. These data show a sustained efficacy of R through all phases of treatment and demonstrate that the antilymphoma activity of R in remission is not compromised by adding R to induction chemotherapy. The duration of response on R-maintenance after R-chemotherapy in fact exceeds previously reported results on the duration of second remissions substantially21 and may even allow speculation that a potent prior R-chemotherapy combination may provide the best condition for a subsequent R-maintenance therapy. While Ghielmini et al16 and Hainsworth et al17,18 already reported a beneficial effect of R maintenance after response to initial single-agent R treatment, the current data allow for the expansion of this favorable experience to more intensive treatment regimens and particularly to the setting of R-chemotherapy combinations.
These results were most recently confirmed by a European Organization for Research on the Treatment of Cancer (EORTC) intergroup study that used a similar trial design.12 In this study, patients with recurring FL were initially randomized for salvage therapy with CHOP versus R-CHOP and subsequently underwent a second randomization for no further treatment versus R maintenance, which consisted of R at a dose of 375 mg/m2/day to be given as a single infusion every 3 months for a total of 2 years. An interim analysis revealed a significantly higher CR rate after R-CHOP compared with CHOP (30% vs 18%; P < .001) and a significant prolongation of response duration (30 months vs 20 months; P < .001).22 A subsequently reported subgroup analysis of 189 patients who had uniformly received R-CHOP for remission induction showed, in addition, a significantly longer median response duration for patients on the R-maintenance arm of 51 months compared with 15 months for patients receiving no further treatment (P < .001).12 Hence, both studies consistently reveal a long-lasting beneficial effect of R maintenance on response duration after salvage therapy with different R-containing regimens.
In the present study by the GLSG and in the EORTC intergroup trial, different schedules of R were applied in remission. While in the present trial R was administered once a week for 4 consecutive weeks, given at 3 and 9 months after entering remission, the EORTC intergroup study applied single infusions of R every 3 months over a period of 2 years. While Hainsworth et al17,18 and Hochster et al15 also used the “4 doses every 6 months” schedule in their studies, Ghielmini et al16 repeated single infusions of R every 2 months a total of 4 times. To date it remains unanswered which of these regimens might be most effective and if they differ in their antilymphoma activity. In addition, it is currently unclear how long R should be given in remission, and if the standard dose of 375 mg/m2/day is the most appropriate dose. Based on pharmacokinetic analyses and assuming a serum target level of R of 25 μg/mL for maintained antilymphoma activity, single applications of R at a dose of 375 mg/m2/day to be repeated every 2 to 3 months appear most appropriate.23,24
Independent of these considerations, which need to be the subject of future studies, all available data consistently show a highly beneficial long-term effect of R maintenance. They also show that prolonged application of R in remission is safe and associated with little or almost no clinical side effects. In the current study no significant side effects were encountered during R maintenance therapy.
Based on the efficacy and safety of R-maintenance therapy in patients with relapsed or refractory FL as convincingly demonstrated by the present study and the EORTC intergroup trial, it seems deeply warranted to investigate the impact of R-maintenance during first-line therapy as well. The only currently available study by Hochster et al15 reveals a significant prolongation of response duration and even survival by R-maintenance versus observation only in patients with advanced-stage FL who had received CVP for first-line chemotherapy without R. Whether R maintenance is also effective in first-line therapy after R-chemotherapy combinations, and whether the application of R during all phases of therapy might be superior to restricting the addition of R either to initial chemotherapy or to maintenance in remission after first-line chemotherapy without R remains unanswered.
In the setting of recurring and refractory FL, however, the evidence from the present study and the EORTC intergroup trial is convincing in demonstrating a beneficial effect of R when applied both during salvage chemotherapy and for remission maintenance. These results have substantial implications on the management of patients with recurring FL since they indicate that long-lasting remissions can be achieved in most patients, providing extended periods of freedom from disease and treatment. Therefore, less therapy than that provided to date may be needed over time to maintain remissions and a high quality of life in patients with recurring FL. These data could encourage researchers to reconsider the overall treatment approaches to patients with advanced-stage FL lymphoma, both concerning the forms and intensities of initial therapy and of treatment modalities in remission. Different options can be discussed. For initial therapy, choices range from singleagent R therapy to several R-chemotherapy combinations of different intensities such as CVP, CHOP, or others. Even chemotherapy alone followed by R maintenance may be considered. For treatment in remission, options include no further treatment, different schedules of R maintenance, or even myeloablative therapy with subsequent autologous stem cell transplantation. Based on the current knowledge, it appears that these possibilities may not be handled in a competitive but rather in a complementary setting. Hence, their use may be tailored according to the general condition of the individual patient, the risk profile of the underlying disease, and not least according to the patient's own preference. The challenge for the future is to design the most appropriate approach to well-defined subgroups of patients with advancedstage FL, taking best use of the different therapeutic options and the increasing knowledge about the biologic and prognostic heterogeneity of the disease. One easily available parameter to aid in selecting among different treatment approaches may include the distinction of prognostic subgroups as defined by the Follicular Lymphoma International Prognostic Index (FLIPI).25 While this score was developed in the pre-R era of antilymphoma therapy, its relevance was recently confirmed for R-chemotherapy regimens as well.26
The challenges in meeting individual patient's needs and selecting among different treatment modalities in the management of patients with advanced-stage FL is a reflection of the great achievements that have most recently been made in the treatment of these disorders. A substantial part of these achievements is associated with the use of R in different phases of therapy. Maintenance is the most recently added modality, and subsequent studies should optimize R maintenance by addressing open questions such as its duration, the most effective schedule, and also the most appropriate dose.
Authorship
Contribution: W.H., R.F., and M.U. wrote the manuscript; W.H., M.U., and M.D. planned the study and wrote the study protocol; R.F., M.D., H.-P.B., R.R., H.W., C.P., J.F.S., B.M., A.H., T.L., F.H., H.E., and W.H. treated and documented the patients; R.F. and M.U. organized and monitored the study; and M.U. performed the statistical evaluation.
Conflict-of-interest disclosure: W.H. received honoraria and research funding from Roche. The remaining authors declare no competing financial interests.
R.F. and M.U. contributed equally to the work. A complete list of the participating institutions of the GLSG appears as a data supplement to the online version of this article.
Prepublished online as Blood First Edition Paper August 31, 2006; DOI 10.1182/blood-2006-04-016725.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
This work was supported in part by a grant by Deutsche Krebshilfe (T14/96/Hi 1, project no. 70-2208-Hi 2) and by the Competence Network Malignant Lymphoma supported by the German Ministry for Research and Technology.