Most deaths in beta-thalassemia major result from cardiac complications due to iron overload. Differential effects on myocardial siderosis may exist between different chelators. A randomized controlled trial was performed in 61 patients previously maintained on subcutaneous deferoxamine. The primary end point was the change in myocardial siderosis (myocardial T2*) over 1 year in patients maintained on subcutaneous deferoxamine or those switched to oral deferiprone monotherapy. The dose of deferiprone was 92 mg/kg/d and deferoxamine was 43 mg/kg for 5.7 d/wk. Compliance was 94% ± 5.3% and 93% ± 9.7% (P = .81), respectively. The improvement in myocardial T2* was significantly greater for deferiprone than deferoxamine (27% vs 13%; P = .023). Left ventricular ejection fraction increased significantly more in the deferiprone-treated group (3.1% vs 0.3% absolute units; P = .003). The changes in liver iron level (-0.93 mg/g dry weight vs -1.54 mg/g dry weight; P = .40) and serum ferritin level (-181 μg/L vs -466 μg/L; P = .16), respectively, were not significantly different between groups. The most frequent adverse events were transient gastrointestinal symptoms for deferiprone-treated patients and local reactions at the infusion site for deferoxamine. There were no episodes of agranulocytosis. Deferiprone monotherapy was significantly more effective than deferoxamine over 1 year in improving asymptomatic myocardial siderosis in beta-thalassemia major.
Introduction
Iron-induced heart failure and arrhythmia are the most common causes of death in beta-thalassemia major, accounting for up to 71% of deaths in older series1-3 and 67% of deaths in a more recent report, despite improvements in overall survival.4 There is a need therefore to identify myocardial siderosis early and improve treatments for heart complications. Myocardial siderosis can be identified by cardiovascular magnetic resonance (CMR) measurement of myocardial T2*, which is highly sensitive to tissue iron concentration, and this has been validated in the United Kingdom5,6 and independently in the United States.7 Patients having increased myocardial siderosis have been shown to be at increased risk of left ventricular (LV) systolic and diastolic dysfunction,5,7-9 arrhythmias,7 and heart failure.5,10 In one series, patients presenting with heart failure had a mean T2* of 5.1 ms,11 which is substantially below the normal lower limit of 20 ms,5 and another series showed 89% of patients presenting in heart failure having a T2* less than 10 ms.10 Myocardial T2* in non-iron loaded individuals is not affected by impaired LV function, age, or infarction.12 The T2* technique is fast13 and is highly reproducible between different MR scanners.5,14-17 T2* calibration has been achieved for the liver in humans and5,18 in the heart in animals19 and will soon be available for the human heart.20 Therefore, myocardial T2* can currently be used to determine changes in human myocardial iron but not the absolute myocardial iron concentration. The T2* technique shows improvement in myocardial siderosis in response to intravenous deferoxamine treatment in acute heart failure,11 and a case-controlled study found significantly less myocardial siderosis and improved LV ejection fraction (EF) in thalassemia patients treated with deferiprone compared with deferoxamine.21 These data suggest that myocardial T2* has value for comparing the cardiac efficacy of chelators. We therefore compared these 2 treatments as monotherapy in a prospective multicenter, randomized, controlled trial, using the change over 1 year in myocardial T2* as the primary outcome measure to determine whether deferiprone had superior cardiac efficacy compared with deferoxamine.
Patients, materials, and methods
Patient recruitment
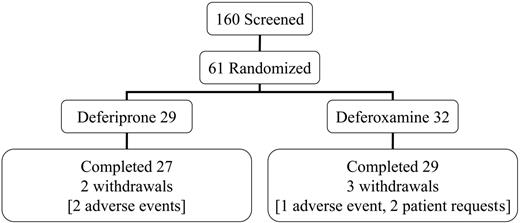
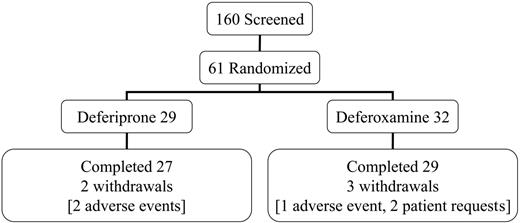
This open-label trial was conducted in 4 centers in Italy and Greece. All patients had homozygous beta-thalassemia major, were regularly transfused and chelated with subcutaneous deferoxamine monotherapy, and had no symptoms of heart failure prior to screening. The mean dose of deferoxamine in the randomized patients prior to entry into the trial was 39 ± 8 mg/kg/d for 5.7 d/wk (equivalent to 32 mg/kg/d). Patients aged 18 years or older were screened (n = 160) and selected if the myocardial T2* was abnormal (< 20 ms) but not severe (< 8 ms) and if the LV EF was greater than 56%. No patient with symptomatic heart failure was eligible for the trial. This rationale excluded patients with no significant cardiac siderosis and allowed patients with severe myocardial siderosis or LV dysfunction to receive the best current clinical management. Sixty-one patients were randomized (Figure 1). The reasons for screening failure (n = 99) were usually a myocardial T2* outside the required range (< 8 ms in 11%; > 20 ms in 76%). Additional less common exclusions were LV EF less than 56% (2%); liver enzymes greater than 3 times upper limit (3%); unsuitable psychological condition (1%); age younger than 18 or older than 36 years (8%); claustrophobia (3%); pretransfusion hemoglobin (Hb) level less than 90 g/L (9 g/dL; 3%); refused or unable to participate (7%). Eighteen patients failed more than one criterion. Each center's ethics committee approved the study and patients gave signed informed consent. Secondary trial end points included cardiac volumes and function, liver iron concentration (LIC), and serum ferritin concentrations.
Cardiovascular magnetic resonance
The T2* sequence was installed in Athens (GE CVi; GE Healthcare, Slough, United Kingdom) and Cagliari (GE Signa Echo-speed; GE Healthcare). Validation at each site included scanning of phantoms of known T2* value, the scanning of 5 patients at each site twice for local reproducibility, and rescanning within 3 days after flying to London for comparison with a reference scanner (Siemens Sonata; Siemens, Erlangen, Germany). We predefined a limit of acceptability of 15% variation in T2* for each site compared with London. The variability compared with London was 9.7% in Athens and 1.6% in Cagliari. Site interstudy variability was 3.5% and 2.4%, respectively. These results15 and full details of the T2* MR sequence have been published.13 Myocardial T2* was analyzed using dedicated software (Thalassemia-Tools; Cardiovascular Imaging Solutions, London, United Kingdom) with consistent regions of interest in the ventricular septum which avoid susceptibility artefacts,5,22 as previously described.13,14 Cardiac volumes were acquired using contiguous steady-state free-precession short-axis cines from base to apex,23 a technique with excellent reproducibility.24 Measurements were scheduled for 3 to 10 days after transfusions. A core laboratory in London analyzed all CMR scans using dedicated software (CMRtools; Cardiovascular Imaging Solutions). All measurements were made in London by 2 reviewers in consensus who were blinded to treatment allocation. Trial analyses were not fed back to the centers during the trial.
Other iron measurements
LIC was assessed at baseline and 12 months using a superconducting quantum interference device (SQUID).25 All the measurements were centralized at the Turin University facility (Model 5700 3-Channel SQUID; Tristan Technologies, San Diego, CA).26 Prior to the start of the trial, SQUID was considered the best noninvasive technique for measuring liver iron level. Serum ferritin concentration was measured at baseline and every 3 months. Serum was separated, labeled, and stored frozen at -20°C until being shipped to a central laboratory, where it was measured by microparticle enzyme immunoassay (AXSYM System; Abbott Diagnostics, Abbott Park, IL).
Screening, randomization, completion, and withdrawal patient numbers.
Safety assessments
Patients were monitored weekly for absolute neutrophil count (ANC) and any adverse events. Serum alanine transaminase (ALT) levels were measured quarterly, serum zinc levels were measured at baseline and every 6 months, and serum creatinine levels were measured at baseline and 12 months.
Statistical analysis
The statistical analysis plan was defined prior to breaking the randomization code and locking of the database of the study. All continuous parameters were analyzed using the 2-sample t test, using a P value of .05 as the threshold for statistical significance. Since tissue iron is linearly related to the inverse of T2*, this measure was log transformed prior to analysis to linearize the relationship and provide an unbiased estimate of relative change from baseline for both treatments. Data are presented as mean plus or minus standard deviation (SD), except for the T2* data, which used the geometric mean (antilog of the mean of the log data) plus or minus the coefficient of variation (CV), defined as √[eMSE - 1], where MSE is the mean square error (equivalent to the variance of the mean in log scale). Proportions of patients between the treatment groups were compared by the Fisher exact test. Trend analysis over time for ferritin and ALT data were performed using repeated-measure analysis of variance (ANOVA; MIXED in SAS, SAS Institute, Cary, NC). Sample size calculations indicated that in the single-center setting, 32 patients would show a 5% difference in myocardial T2* between drugs for a type 1 error of 0.05 and a power of 80%. This sample size was increased to 60 to allow for reproducibility deterioration in the multicenter setting and to allow for 20% dropouts. All analyses presented are based on intention to treat. Last observation carried forward was used to fill in the missing data for withdrawn patients in the efficacy analyses. Deferiprone compliance was measured using the Medication Event Monitoring System device (Aardex, Zug, Switzerland) and calculated as the percent of openings with an interval longer than 4 hours recorded, divided by number of doses prescribed. Deferoxamine compliance was calculated as the percentage of completed infusions, as determined by the Crono pumps, divided by the number of infusions prescribed. Statistical analysis was performed using SAS Institute (for PC, release 8.2).
Results
Patient characterization
The baseline characteristics are shown in Table 1. The groups were well matched for the primary end point of myocardial siderosis. Matching was good for most other measures including liver iron level and transfusional iron input, but significant differences were present for the serum ferritin level, hemoglobin level, and white cell count.
The target dose for subcutaneous deferoxamine was 50 mg/kg/d for at least 5 days per week. The actual dose prescribed for deferoxamine was 43 mg/kg for 5.7 d/wk (equivalent to 35 mg/kg/d for 7 days per week). This is equal to the recommended dose of 35 mg/kg/d for stable patients with similar ferritin levels from the product data sheet and slightly higher than the 40 mg/kg/d for 5 d/wk from clinical recommendations,27 though lower than the maximal dose sometimes used in clinical practice. Oral deferiprone was initiated at 75 mg/kg/d and increased to the target of 100 mg/kg/d. The actual prescribed dose of deferiprone was 92 mg/kg/d. Compliance was similar (deferiprone 94% ± 5.3%; deferoxamine 93% ± 9.7%; P = .81). Five patients withdrew, 3 taking deferoxamine (1, deterioration of cardiac function; 2, personal reasons) and 2 taking deferiprone (elevated liver enzymes, which in 1 case was probably caused by cytomegalovirus hepatitis with increased IgM levels to CMV that fell but with negative polymerase chain reaction [PCR]).
Myocardial T2*
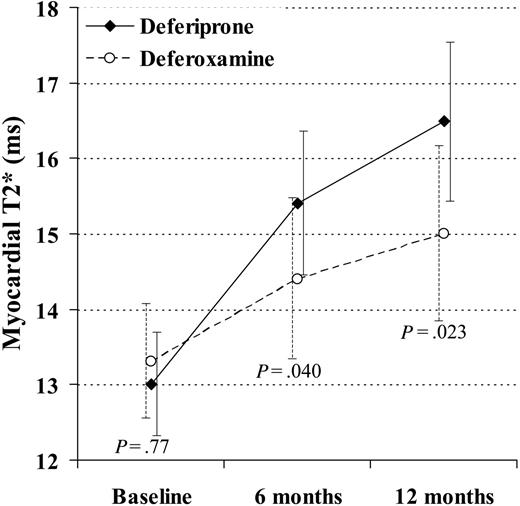
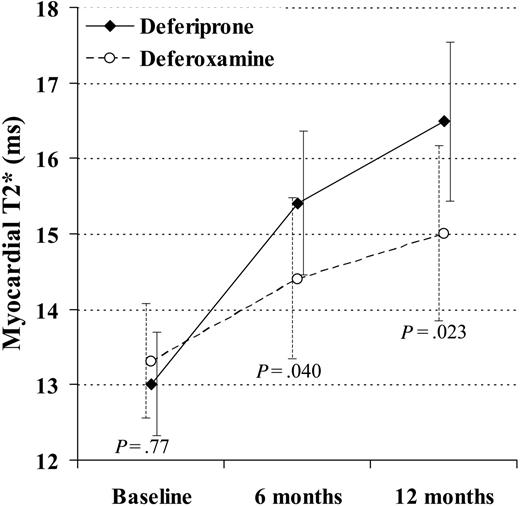
Myocardial T2* rose with deferiprone at 6 and 12 months to 15.4 ms (+18%; CV 38%; P < .001) and 16.5 ms (+27%; CV 38%; P < .001). The myocardial T2* rose with deferoxamine at 6 and 12 months to 14.4 ms (+9%; CV 37%; P = .003) and 15.0 ms (+13%; CV 39%; P < .001). The difference in the change between drugs was significant at 6 months (ratio of geometric mean, 1.09; P = .040) and at 12 months (ratio, 1.12; P = .023; Figure 2). A rise in T2* and EF was seen in 19 (66%) deferiprone-treated patients and 14 (45%) deferoxamine-treated patients.
Because of the baseline differences between groups in serum ferritin level, white cell count, and hemoglobin level, further analysis of these factors and their interaction with the outcome of the primary end point was performed. The predefined statistical plan called for parametric analysis of the serum ferritin level, but on inspection of the data, we found 2 extreme outliers in the deferoxamine-treated group with ferritin values of 9259 and 9300 μg/L, and the Shapiro-Wilk test indicated that the group was not normally distributed (P < .001). Accordingly, a log transformation was applied and this was successful in normalizing the ferritin data (P = .76). In addition, the differences in white cell count and hemoglobin level were examined, as both were significantly higher in the deferoxamine-treated group. These differences appear to have resulted from the difference in the number of splenectomized patients in the 2 therapy arms (34% in the deferoxamine-treated group vs 14% in the deferiprone-treated group). The splenectomized patients had significantly higher corrected white cell counts than nonsplenectomized patients (P < .001). Data review showed 4 splenectomized patients in the deferoxamine-treated group had baseline corrected white cell counts greater than 20 × 109/L. The larger number of splenectomized patients in the deferoxamine-treated group could also explain the higher hemoglobin level in the deferoxamine-treated group because of decreased red blood cell (RBC) consumption to maintain the same hemoglobin level despite similar mean transfusional input between the 2 groups (151.6 ± 43.4 mL/kg/y in the deferiprone-treated group vs 144.3 ± 44.4 mL/kg/y in the deferoxamine-treated group; P = .52). Therefore, we conducted a multivariate analysis of the effect on the myocardial T2* of baseline differences in log serum ferritin level and splenectomy status. This showed that the baseline ferritin level does not have a significant impact on the improvement in T2* (P = .66), but splenectomy was significantly predictive (P = .002). Thus splenectomized patients exhibited greater T2* improvement than nonsplenectomized patients. The antilog of the estimated effect size (e0.1850 = 1.20), which is equal to the ratio of geometric mean between splenectomized and nonsplenectomized patients, indicates that the splenectomized patients were 20% better in improving T2* than nonsplenectomized patients. After controlling for the baseline ferritin values and the splenectomy status, the difference in T2* favoring deferiprone remained significant and its significance was substantially greater (P = .002). Since the deferoxamine-treated group had more splenectomized patients than the deferiprone-treated group, an even greater difference in mean change from baseline in T2* in favor of deferiprone would have been expected had there been an equal number of splenectomized patients in the 2 treatment groups.
Cardiac function and volumes
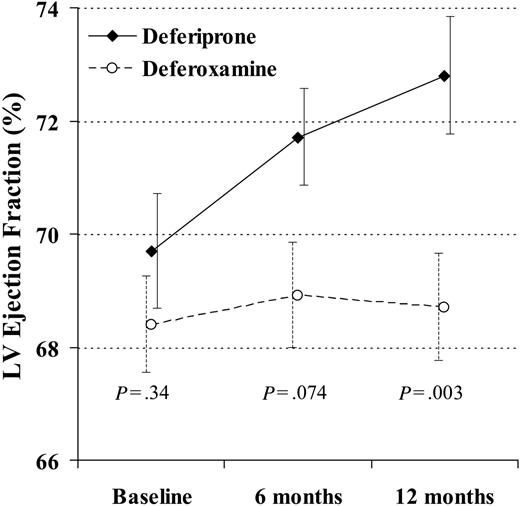
At 12 months, there was a significant difference favoring deferiprone for reduction in end-systolic volume (-6.4 ± 6.8 mL vs -0.6 ± 7.9 mL; P = .004) and a borderline difference in reduction of end-diastolic volume (-7.8 ± 13 mL vs -1.2 ± 13 mL; P = .060). The EF rose with deferiprone by absolute units of 2.0% ± 2.7% at 6 months (P < .001) and 3.1% ± 3.6% at 12 months (P < .001). The EF rose with deferoxamine by 0.52% ± 3.5% at 6 months (P = .42) and 0.32% ± 3.4% at 12 months (P = .66). The difference in the change between groups was borderline at 6 months (P = .074) and significant at 12 months (P = .003; Figure 3). The percent change in T2* and EF with deferiprone was significantly correlated (r2 = 0.21; P = .012), although this was relatively weak and not useful to predict the EF change from the change in T2*. There was no correlation with deferoxamine (r2 = 0.036; P = .49).
The change in myocardial T2* at 6 and 12 months was significantly greater in the patients taking deferiprone rather than deferoxamine. In this analysis, 29 deferiprone-treated patients and 31 deferoxamine-treated patients are included (1 deferoxamine-treated patient was excluded because T2* and ejection fraction were not measured at 6 and 12 months, and the 4 dropouts were included using last observation carried over). The vertical axis shows the geometric mean of T2*. Standard error bars are shown.
The change in myocardial T2* at 6 and 12 months was significantly greater in the patients taking deferiprone rather than deferoxamine. In this analysis, 29 deferiprone-treated patients and 31 deferoxamine-treated patients are included (1 deferoxamine-treated patient was excluded because T2* and ejection fraction were not measured at 6 and 12 months, and the 4 dropouts were included using last observation carried over). The vertical axis shows the geometric mean of T2*. Standard error bars are shown.
The change in left ventricular (LV) ejection fraction at 12 months was significantly greater in the patients taking deferiprone rather than deferoxamine. In this analysis, 29 deferiprone-treated patients and 31 deferoxamine-treated patients are included (1 deferoxamine-treated patient was excluded because T2* and ejection fraction were not measured at 6 and 12 months, and the 4 dropouts were included using last observation carried over). Standard error bars are shown.
The change in left ventricular (LV) ejection fraction at 12 months was significantly greater in the patients taking deferiprone rather than deferoxamine. In this analysis, 29 deferiprone-treated patients and 31 deferoxamine-treated patients are included (1 deferoxamine-treated patient was excluded because T2* and ejection fraction were not measured at 6 and 12 months, and the 4 dropouts were included using last observation carried over). Standard error bars are shown.
Other iron measures
One patient in the deferiprone-treated group did not have an LIC at baseline and there were 3 patients (1 deferiprone and 2 deferoxamine) who did not have an LIC value at 12 months. The last observation carried over technique could not be used for those 3 patients and they were excluded from the analysis at 12 months. Therefore, LIC was assessed in 27 and 30 patients for the deferiprone- and deferoxamine-treated groups, respectively. LIC fell with deferiprone by 0.93 ± 2.9 mg/g dry weight at 12 months (-10.1%; P = .11) compared with a fall with deferoxamine by 1.54 ± 2.5 mg/g dw at 12 months (-24.4%; P = .002). The difference between groups at 12 months was not significant (P = .40). The change in ferritin level at 6 months with deferiprone and deferoxamine was 151 ± 713 μg/L and -314 ± 921 μg/L (P = .033 between groups) and at 12 months -181 ± 826 μg/L and -466 ± 739 μg/L (-10.1% and -16.7% change from baseline values, respectively; P = .16 between groups). There was no difference in trend of serum ferritin level over time between groups (P = .41).
Vital signs
Weight gain was greater with deferiprone at 6 months (1.6 ± 1.8 kg vs -0.22 ± 2.7 kg; P = .004) and at 12 months (2.2 ± 3.0 kg vs 0.1 ± 2.7 kg; P = .004). There were no significant differences between groups in other vital signs.
Other laboratory measures
The difference between the deferiprone- and deferoxamine-treated groups in change of ALT level at 12 months was not significant (22.9 ± 48.6 U/L vs 4.7 ± 38.2 U/L; P = .11). This includes the 2 patients who dropped out for increased serum enzyme levels. There was no significant difference in trend of ALT level over time between groups (P = .32). The difference in percentage of patients with ALT greater than twice the upper limit was not significant at baseline or at 12 months.
There was no significant difference between groups in the change of zinc level at 12 months (-0.80 ± 2.8 μM vs 0.23 ± 2.3 μM; P = .12). There was no significant difference between groups in the change in creatinine level at 12 months (3.24 ± 10.5 μM vs 0.06 ± 12.7 μM; P = .29). There were no significant differences between groups in other laboratory data, including ANC.
Adverse events
The most frequent adverse events with deferiprone were gastrointestinal symptoms (nausea, vomiting, or abdominal pain), which occurred in 19 (66%) patients. These usually occurred in the first weeks of treatment, were mild to moderate, and usually resolved within a median of 3 days (range, 1-17 days) without discontinuation or decreasing the dose of deferiprone. Deferoxamine adverse events included reactions at the infusion site, which occurred in 12 (38%) patients. Joint problems, including pain and/or swelling, occurred in 8 (28%) deferiprone-treated patients and in 6 (19%) deferoxamine-treated patients (P = .30). Nine (31%) deferiprone-treated patients reported increased appetite (P < .001). One episode of neutropenia (1.01 × 109 neutrophils/L) occurred in one deferiprone-treated patient. The event resolved within 3 days (2.69 × 109 neutrophils/L) without discontinuation or decreasing the dose of deferiprone. There were no episodes of agranulocytosis.
Discussion
Heart disease, which results from myocardial iron deposition associated with lifelong blood transfusions and increased gut iron uptake, is the most common cause of death in beta-thalassemia major. Recently, it has become possible to target therapy for myocardial siderosis using myocardial T2*, allowing comparison of the myocardial efficacy of iron chelators. Using myocardial T2*, continuous intravenous deferoxamine has been shown to reduce myocardial siderosis in acute heart failure,11 although iron clearance was considerably slower from heart than liver. In the current trial, all screened patients had no cardiac symptoms and were receiving chronic deferoxamine monotherapy, and those who were subsequently randomized either continued deferoxamine at an increased dose (+4 mg/kg/d; P = .001) following entry into the trial with standardization of treatment or were switched to deferiprone monotherapy. We found a significant reduction in our primary trial end point of myocardial siderosis in the deferoxamine-treated group (T2* increased by 13% at 12 months; P < .001), which probably resulted from the increased dose of deferoxamine and/or improved compliance. Despite this improvement in the control group, the deferiprone-treated group showed superior efficacy in reducing myocardial siderosis (T2* increased by 27% at 12 months, P < .001; P = .023 vs deferoxamine-treated group at 12 months). These data accord with a previous retrospective study showing lower myocardial iron with deferiprone.21 Although the clinical efficacy of raising myocardial T2* for asymptomatic patients remains to be determined, it would appear that reduced levels of myocardial iron are a favorable response.
The deferiprone-treated group also exhibited significant improvement in secondary trial end points compared with deferoxamine, with a reduced end-systolic volume and increased EF. In this trial, we excluded patients with an EF less than 56% by CMR, which is the lower limit for healthy nonanemic subjects.28 We believed this was sufficient evidence that significant LV systolic dysfunction was present and that the best current medical treatment should be offered outside the trial setting. However, “normal” cardiac volumes and function in thalassemia are not well defined. Compared with healthy nonanemic subjects, thalassemia patients' hearts have larger end-diastolic volumes and increased cardiac output,7,29,30 which allows for increased perfusion for tissue oxygenation requirements. In addition, the ejection fraction in thalassemia major patients with no iron loading (T2* > 20 ms) is higher than in controls with a lower limit of up to 63%.5,7,30 Thus, because the EF in thalassemia without myocardial siderosis is higher than in healthy nonanemic individuals, we believe that our trial included patients with subclinical LV dysfunction. The increase in EF seen in this trial accords with this view and suggests that deferiprone was effective in relieving this dysfunction. Reduced EF is linked with adverse survival not only in thalassemia31 but also in coronary artery disease32 and heart failure,33 and improved prognosis occurs with treatments that improve EF.34,35 Deferiprone also reduced end-systolic volume, another prognostic parameter in large36 and normal-sized hearts.37 Heart failure treatments known to improve prognosis reduce the end-systolic volume,38 although this has not been shown directly in thalassemia patients. These prospective randomized data are also consistent with a previous study showing significantly superior EF with deferiprone.21
It therefore appears that deferiprone could reduce the risk of progression to iron-related cardiomyopathy by removing more cardiac iron than subcutaneous deferoxamine, and the LV function and volume improvements in patients with subclinical LV dysfunction could yield prognostic benefit. Lower levels of myocardial siderosis would allow an increased reserve for any future periods of iron loading and the potential for greater resistance to catastrophic heart failure that can be seen in thalassemia patients with intercurrent infection. These interpretations accord with longitudinal studies of EF in thalassemia31 and previous retrospective survival data using deferiprone or deferoxamine.39 With more than 4 years of follow-up, cardiac dysfunction was newly diagnosed in 4% of the deferiprone-treated patients and 20% of the deferoxamine-treated patients (P = .007). This was accompanied by no deaths with deferiprone but 3 cardiac-related deaths with deferoxamine.
These findings suggest differences in the in vivo myocardial efficacy of these drugs. Deferoxamine is an important chelator40-42 but is a large positively charged molecule, relatively lipophobic, and one that undergoes conformation change on binding of iron, which may lead to intracellular trapping.43 These properties potentially limit its ability to chelate intracellular iron, unless there is an active excretion pathway, as postulated for deferoxamine in hepatocytes.43,44 By contrast, deferiprone is a small, neutral molecule with greater lipophilicity and therefore has significantly greater potential to chelate intracellular iron.45,46 This suggests that deferiprone may have preferential access to intracellular iron in tissues such as the myocardium, and in vitro and animal data support this hypothesis.47 Because of these variations in action, combination therapy with these chelators, for which there is supportive in vitro47 and clinical evidence,48 may be attractive. Combination therapy is being subjected to a further randomized controlled trial for cardiac efficacy.49
Three previous randomized trials have compared the myocardial effects of chelators. Maggio et al50 compared deferiprone (75 mg/kg/d) with deferoxamine (50 mg/kg/d for 5 d/wk). No difference was found between groups in serum ferritin level or T2-weighted myocardial signal intensity ratio (SIR). Peng et al51 compared deferiprone (75 mg/kg/d) with deferoxamine (50 mg/kg/d for ≥ 5 d/wk). Deferiprone resulted in a significantly larger improvement in SIR and EF. Galia et al52 compared deferiprone (75 mg/kg/d) with deferoxamine (50 mg/kg/d for 6 d/wk). No significant differences between groups were demonstrated in myocardial SIR or EF. Our current trial differs from these reports in several ways that might explain the efficacy of deferiprone that we found. First, the current trial used deferiprone at 100 mg/kg/d. Second, compliance was high in the current study but was not quantified in the previous reports and may have been lower. Third, the prior studies used heart SIR to measure myocardial siderosis, which is not an absolute measure and is sensitive to noise, limiting both its reproducibility and interpretation. Fourth, the current trial is multicenter and not single center. Our results are consistent with another randomized controlled trial from Olivieri et al53 that has only been published in abstract form; deferiprone (75 mg/kg/d) resulted in a significant increase in T2 relaxation time, which was not matched by deferoxamine (50 mg/kg/d).
The patients in the current study were well matched at baseline for the primary end point of myocardial T2*, and they were also well matched for the secondary end point of liver iron. However, the accuracy of SQUID may vary between machines, and direct comparison of absolute liver iron levels with studies relating our single-center SQUID results to those from other centers may not be straightforward. Despite these issues, it is worth noting that at baseline our patients had a median LIC of 4.30 mg/g dw, with only 8% having an LIC greater than 15 mg/g dw. Therefore, the patients in the current study would appear to be reasonably well chelated by liver criteria. In the current study, the 1.5 mg/g dw reduction in LIC over 12 months with deferoxamine was significant, whereas the 0.94 mg/g dw reduction with deferiprone did not achieve significance, although the between groups comparison was not significant. This is the first randomized controlled study to compare changes in LIC at the highest dose of deferiprone currently approved in Europe (100 mg/kg/d). Deferiprone appeared to prevent liver iron accumulation, even if the mean decrease appeared marginally less effective than deferoxamine. The patients in our study showed a difference at baseline for the secondary end point of serum ferritin level. The explanation for this difference is the presence of 2 extreme outliers in the deferoxamine-treated group with very high ferritin levels. After log transformation to achieve a normal distribution, no difference between groups at baseline was present. This lack of difference accords with the lack of baseline statistical difference in the direct measures of liver or myocardial iron. After controlling for the baseline serum ferritin status (> 2500 μg/L or ≤ 2500 μg/L) in a covariate analysis, the difference in myocardial T2* at month 12 between the treatment groups remained significant (P = .032), favoring the deferiprone treatment group, and there was no difference in trend of serum ferritin level over time between treatment groups.
Compliance with deferiprone at 100 mg/kg/d was excellent with no new drug-related complications compared with previous lower-dose trials. Contrary to other trials where deferiprone was interrupted at the first sign of neutropenia (< 1.5 × 109/L), we allowed continuation of deferiprone if the neutrophil count remained above 1.0 × 109/L. One patient experienced one episode of neutropenia, which resolved without discontinuing deferiprone. No agranulocytosis was seen. Two deferiprone-treated patients discontinued the study due to increased liver enzymes, which returned to baseline levels upon interruption of therapy. One deferoxamine-treated patient developed arrhythmias and a reduction in EF, though still in the normal range, and an increase in the LV and atrial diameters and was withdrawn from the study for initiation of intensive chelation therapy. Two other deferoxamine-treated patients discontinued the study for personal reasons.
Limitations
The results of this trial are relevant to patients with no symptoms of heart failure and who are older than 18 years. Evidence that these findings apply to symptomatic patients or children awaits trials in these specific populations.
Conclusions
At the doses used in this prospective trial, deferiprone was superior to deferoxamine over 1 year in improving myocardial siderosis in asymptomatic patients with beta-thalassemia major and this confirms previous retrospective data. This suggests that deferiprone has superior access to myocardial iron stores compared with deferoxamine, which accords with in vitro data. The combination of detection of myocardial siderosis by T2* CMR and the superior effect of deferiprone in removal of cardiac iron allows a rational paradigm of early management of cardiac siderosis in patients maintained on long-term deferoxamine, and this should translate into improved survival.
Prepublished online as Blood First Edition Paper, December 13, 2005; DOI 10.1182/blood-2005-07-2948.
Supported by Apotex Research Inc.
Several of the authors (D.J.P., V.B., A.P., E.D.G., R.G.) have declared a financial interest in Apotex, whose product, deferiprone, was studied in the present work. Several of the authors (D.J.P., A.P., R.G.) have declared a financial interest in Novartis, whose product, deferoxamine, was studied in the present work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
This trial is registered in www.clinicaltrials.gov as NCT00105495.
We thank Drs David Lee and Yu-Chung Tsang for expert statistical advice on this study and the following doctors for their important contributions. Cagliari: Gildo Matta, Carlo Dessi, Annalisa Agus, Simona Piras, G. B. Melis, P. Bina; Torino: Filomena Longo, Giulio Lupo; Aghia Sophia Children's Hospital: Christina Fragodimitri, Fotis Karabatsos, Antonia Hatziliami, Jacqueline Youssef, Ekaterini Dokou, Helen Berdoussi, Helen Architektonidou, Dimitris Tsoussis; Royal Brompton Hospital: David Firmin, Lisa Anderson. We also thank Prof John Porter for his constructive criticism of the manuscript.