The spleen plays a central role in the pathophysiology of several potentially severe diseases such as inherited red cell membrane disorders, hemolytic anemias, and malaria. Research on these diseases is hampered by ethical constraints that limit human spleen tissue explorations. We identified a surgical situation—left splenopancreatectomy for benign pancreas tumors—allowing spleen retrieval at no risk for patients. Ex vivo perfusion of retrieved intact spleens for 4 to 6 hours maintained a preserved parenchymal structure, vascular flow, and metabolic activity. Function preservation was assessed by testing the ability of isolated-perfused spleens to retain Plasmodium falciparum-infected erythrocytes preexposed to the antimalarial drug artesunate (Art-iRBCs). More than 95% of Art-iRBCs were cleared from the perfusate in 2 hours. At each transit through isolated-perfused spleens, parasite remnants were removed from 0.2% to 0.23% of Art-iRBCs, a proportion consistent with the 0.02% to 1% pitting rate previously established in artesunate-treated patients. Histologic analysis showed that more than 90% of Art-iRBCs were retained and processed in the red pulp, providing the first direct evidence of a zone-dependent parasite clearance by the human spleen. Human-specific physiologic or pathophysiologic mechanisms involving clearing or processing functions of the spleen can now be experimentally explored in a human tissue context.
Introduction
The spleen plays a key role in innate and adaptative immune responses, in selective clearance of aged or abnormal red blood cells (RBCs), and in removal of pathogens present in the blood.1 It is also central to the pathophysiology of several frequent and potentially severe diseases such as inherited red cell membrane disorders, hemolytic anemias, and malaria.2-5 Detailed studies on the mechanisms involved are hampered by 2 linked factors: the human spleen displays anatomic and physiologic features not observed in rodent models, and the human spleen is difficult to explore.1,6 The risk of life-threatening intraperitoneal bleeding makes spleen biopsy or needle-aspirate an unethical method for human research purposes.7-8 Histology studies from postmortem or splenectomy samples provide crucial information, but their scope is limited to late or complicated disease stages.9-10 So far, explorations of the human spleen have been restricted to “black box” approaches, in which intrasplenic tissue processing was inferred from the kinetics of elements circulating in the peripheral blood.11-17 Humanized mouse models provide an interesting research tool, but are limited by the rapid clearance of normal human RBCs and by the different spleen anatomy, vascularization/circulatory bed, and cellular subsets compared with humans.1,6,18-21
The spleen is one of the few organs that may be removed while healthy. Left pancreas resection for tumor removal usually includes a splenectomy for vascular-related constraints.22 Retrieval of intact spleen is then possible without modifying the patient's medical and surgical care, making experimental study of the human spleen clearing and processing functions during ex vivo perfusion theoretically feasible. However, unlike other isolated-perfused organs such as the heart, liver, or kidney, no simple macroscopic marker such as contractility, or bile/urine flow, is available to evaluate the preservation of spleen functions. Studies in splenectomized Plasmodium falciparum malaria patients have shown that most drug-exposed parasite clearance is spleen dependent.15 Furthermore, work in the last decades defined methods for accurate antimalarial drug efficacy assessment that provide tools for quantifying spleen-clearing function. Drug-exposed parasite clearance kinetics was established from clinical observations, in particular from artesunate-treated patients.16,23 Pitting, a phenomenon whereby erythrocytes containing inclusion bodies squeeze through the narrow interendothelial slits of the venous sinus wall, leaving a fraction of the cell containing the nondeformable inclusion bodies trailing behind on the reticular meshwork side, was shown to account for a proportion of drug-exposed P falciparum-infected RBC clearance.1,14,16,24,25 In order to use clearance of artesunate-treated P falciparum-infected red blood cells (Art-iRBCs) as a physiologic readout of spleen function, we measured clearance kinetics and pitting rate of Art-iRBCs in isolated-perfused spleens. We show here that ex vivo perfusion maintains these clearing functions at physiologic levels for several hours. Retrieved spleens are then available for detailed histology examination at the end of experiments, providing an opportunity to study the anatomic distribution and fate of perfused Art-iRBCs.
Patients, materials, and methods
Human spleen retrieval
Spleens were retrieved downstream of surgical interventions involving patients with left pancreas tumors, during which the spleen had to be removed for vascular-related constraints. Medical and surgical care was not modified and patient consent was obtained by the surgical team. Regulatory steps were according to the French law that guarantees patient safety, confidentiality, and protection from advertisement or economic incentive (art. L.1211-L.1211-4, L.1211-3, L.1211-6 of the French Code of Public Health Regulations). The project was reviewed by the Necker Hospital investigational review board. The only captured clinical information was age, sex, ABO Rhesus blood group, and surgical indication. All documents were identified with a code number. Only the surgical team kept the list containing the spleen codes and patient names. At the end of each experiment, a detailed written macroscopic description of the spleen was provided to the referent pathologist along with photographs and more than 4 formaldehyde-fixed fragments from different parts of the spleen. Retrieved spleens were devoid of any macroscopic or microscopic alteration. Upon a 30- to 90-minute period of warm ischemia linked to the surgical procedure, the main splenic artery and vein were cannulated. The spleens were flushed with cold Krebs-albumin solution (25 mmol NaHCO3, 118 mmol NaCl, 4.7 mmol KCl, MgSO4, 1.2 mmol 7 H2O, 1.2 mmol NaH2PO4, CaCl2, 1.2 mmol 2 H2O, 7 mmol glucose, and 5 g human serum albumin [albumax], for 1 L sterile water) for transport to the laboratory. Once in the laboratory (cold ischemia time, 60-90 minutes), the spleen was connected to the perfusion device.
Ex vivo perfusion device
Preliminary establishment of the optimal perfusion conditions was performed with Dr Francis Paulmier and his team (INRA Nouzilly France) with 12 piglet spleens perfused with uninfected pig RBCs. Subsequently, 6 human spleens were retrieved, 3 of them perfused with human uninfected RBCs only, and 3 with human uninfected RBCs and Art-iRBCs, as explained in “Setting-up of model, assessment by metabolic markers, and vascular imaging,” using a final perfusion device adapted from that described by Pabst et al26 (Figure 1). Major modifications of this device were a reduction of dead volume allowing the closed-circuit perfusion of a total volume of 150 mL, and the use of a manufactured glass warming chamber (Christian Roujoux, Institut Pasteur, Paris, France) allowing easy access to the spleen hilum while maintaining most of the capsule surface in a 37°C environment. A peristaltic pump (ISM 920 pump from Ismatec, Labortechnik, Glattbrugg-Zürich, Switzerland; and Masterflex 06419-17 tube from Tygon, Saint-Gobain, France) provided flow into sterile tubing (Transfusion kit; Transfumed, Sendal, Almaraz, Caceres, Spain). The perfusate was aspirated from a cylindric reservoir (Rubbermaid 300 mL; Curver, Luxembourg) through a bubble-trap (Transfusion kit; Transfumed) then pushed through 2 m of silicone gas-permeable tubing (Tygon 3350 Silicon 1/8 × 3/16; Cole-Parmer Instrument Company, Vermont Hills, IL) around which a 3% CO2/100% O2 gas phase was maintained through a constant 1 L/min gas flow from a pressurized tank (Air Liquide, Paris La Défense, France). Prior to entry into the spleen through a manufactured ligated glass catheter (Christian Roujoux), the perfusate was warmed using a manufactured glass heating coil (Christian Roujoux) equilibrated to 37.5°C as a result of a permanent water flow (Masterflex Easydrive pump and Masterflex tubes 06419-25 from Tygon) from a water bath. Cells, reagents, and samples for analysis were added into/removed from the reservoir in which a rotating (120 rotations per minute) magnet prevented cell sedimentation. Progressive warming of isolated-perfused spleens from 6°C to 10°C to 37°C was performed by increasing the Krebs-albumin medium flow from 1 mL/min to 100 to 150 mL/min over 40 to 60 minutes. Krebs-albumin medium was changed at least twice during this adaptation period (600-1000 mL total volume) until most of the patient's RBCs were flushed from the spleen. At steady state, perfusate flow was 1 mL/g spleen/min, and temperature of the spleen capsule was maintained between 36.7°C and 37.2°C. In order to improve oxygen delivery to isolated-perfused spleens, O+ uninfected RBCs at .05 (5%) hematocrit level were added and allowed to circulate for 30 to 60 minutes before introducing Art-iRBCs (2.5%-6% parasitemia). Spleen aspect, gas flow through the artificial lung, perfusate aspect/color, as well as “arterial” pressure (ie, upstream of the spleen) were constantly monitored, and were recorded every 5 to 10 minutes. The arterial pressure was maintained between 60 to 120 mmHg. Five minutes before introducing Art-iRBCs, the spleen was rinsed again with 400 mL Krebs-albumin medium until the perfusate hematocrit level was less than .001 (0.1%)—in order to limit the influence of Art-iRBC dilution into uninfected RBCs on Art-iRBC concentration kinetics. After perfusion of Art-iRBCs, the initial hematocrit level was .016 (1.6%) or .04 (4%). Hematocrit, K+, and Na+ concentrations were recorded every 30 to 60 minutes. Glycemia was evaluated every 10 minutes using a dipstick glucometer (Accu-Chek and Glucotrend Premium; Roche Diagnostics, Mannheim, Germany). Glucose in water (150 g/L) was added as required to maintain glycemia between 1 and 2 g/L.
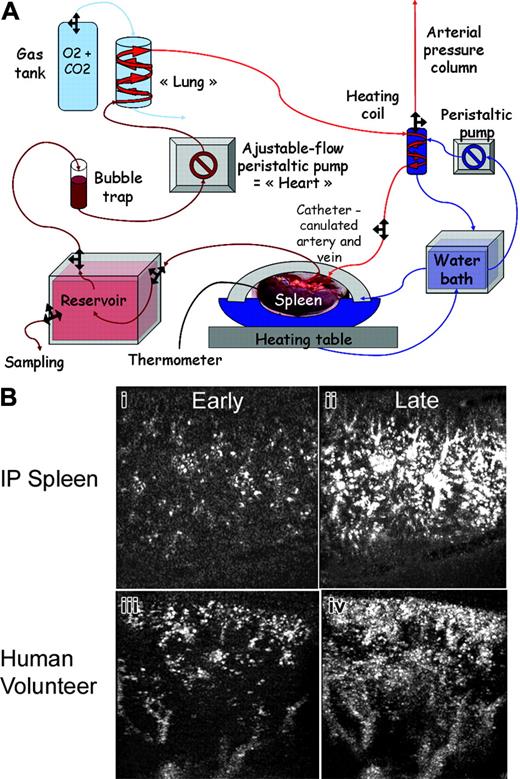
Description and vascular evaluation of the model. (A) Schematic diagram of the experimental set-up of the human IP-spleen. A peristaltic pump provides flow into polypropylene tubing. The perfusate is aspirated from the reservoir through a bubble-trap and then pushed into 2 m of silicone gas-permeable tubing around which a 3% CO2/100% O2 atmosphere is maintained through a constant 1 L/min gas flow. Prior to entry into the spleen through a glass catheter, the perfusate is warmed using a 37.5°C-equilibrated heating coil. Cells, reagents, and samples for analysis are introduced into or removed from the reservoir. (B) Qualitative comparison of patient and IP-spleen parenchymal enhancement using contrast ultrasonography. Early and late parenchymal aspect after bolus injection of contrast agent, from an IP-spleen (Bi-ii) and a representative human volunteer (Biii-iv) showing the same progressive homogenous enhancement. For technical reasons, the ultrasonographic section is from anterior capsule to posterior capsule in IP-spleens, whereas it is from capsule to hilum in patients.
Description and vascular evaluation of the model. (A) Schematic diagram of the experimental set-up of the human IP-spleen. A peristaltic pump provides flow into polypropylene tubing. The perfusate is aspirated from the reservoir through a bubble-trap and then pushed into 2 m of silicone gas-permeable tubing around which a 3% CO2/100% O2 atmosphere is maintained through a constant 1 L/min gas flow. Prior to entry into the spleen through a glass catheter, the perfusate is warmed using a 37.5°C-equilibrated heating coil. Cells, reagents, and samples for analysis are introduced into or removed from the reservoir. (B) Qualitative comparison of patient and IP-spleen parenchymal enhancement using contrast ultrasonography. Early and late parenchymal aspect after bolus injection of contrast agent, from an IP-spleen (Bi-ii) and a representative human volunteer (Biii-iv) showing the same progressive homogenous enhancement. For technical reasons, the ultrasonographic section is from anterior capsule to posterior capsule in IP-spleens, whereas it is from capsule to hilum in patients.
Ultrasonographic evaluation
The spleen was covered with transparent Saran wrap to prevent evaporation and ultrasonography gel leakage into the perfusate. At different time points, ultrasonography was performed (Philips HDI 5000; Philips, Bothell, WA). A 1-mL bolus of microbubbles (Sonovue; Bracco, Milan, Italy) was added into the reservoir and the parenchymal enhancement recorded using microvascular imaging analysis (MVI; Philips). In a separate prospective clinical study, human volunteers were administered a 6-mL bolus of microbubbles and the same ultrasonographic methodology was applied. The results of this study will be reported elsewhere (J.-M.C., manuscript in preparation). The qualitative characteristics of parenchymal enhancement of isolated-perfused spleens was compared with that observed in patient spleens.
Functional validation
P falciparum parasites (strain FUP/CB) were cultured in O+ human red blood cells in RPMI medium supplemented with 5% albumax/5% AB+ human serum. Ring-stage parasites were incubated for 8 to 12 hours with artesunate (0.1 μg/mL) prior to centrifugation and introduction into the perfusate. At that time, the parasites (Art-iRBCs) were no longer viable as assessed by the absence of any reinvasion in culture. At different time points, 100 to 200 μL was collected from the perfusate and centrifuged, and the pellet was used for thin smears and erythrocyte membrane immunofluorescence (EMIF).
EMIF
EMIF was performed as described by Perlmann et al.27 Briefly, infected red blood cell monolayers were prepared from aliquots of the perfusate and fixed in 1% glutaraldehyde for 20 seconds (Sigma, St Louis, MO). Labeling of ring-injected erythrocyte surface antigen (RESA) was performed with sera from hyperimmune African adults (1:200 serum dilution in PBS/1% BSA) followed by Alexafluor 488-conjugated goat anti-human IgG (diluted 1:500; Molecular Probes, Eugene, OR). Parasite nuclei were stained with 10 μg/mL Hoechst 33342 (Molecular Probes). Slides were mounted with Vectashield medium (Vector laboratories, Burlingame, CA). Images were acquired on a Zeiss Axiovert 200 M microscope, using an Axiocam HRc camera controlled by Zeiss Axiovision software (all from Carl Zeiss, Heidelberg, Germany).
Histologic evaluation
At the end of the experiments, the spleen was gently perfused with 70 to 100 mL buffered 4% formaldehyde for 4 to 5 minutes (flow maintained below 20 mL/min, pressure below 15 cm H20) to allow the homogenous fixation of the tissue. Blocks (10 × 10 × 5 mm) were fixed in 4% buffered formaldehyde. Slides (3-μm thick) were stained with Giemsa for microscopic examination. Pictures were acquired on a Nikon E 800 microscope, using a Nikon digital still DXM 1200 camera controlled by an ACT-1 version 2 Nikon software (all from Nikon, Tokyo, Japan). Images were visualized with a Plan Apo 40 ×/0.95 DiCM or a Plan Apo 100 ×/1.40 oil-immersion DiCM objective lens (Nikon). Giemsa-stained slides were photographed at × 400 magnification and read after the superimposition of a counting grid. Brown dots larger than 0.5 μm were counted and localized on 20 photographs for each spleen. Immunochemical analysis was performed essentially as described.6 Briefly, after appropriate preparation, sections were incubated with either anti-CD68 mouse monoclonal antibody (M0876, dilution 1:50 in PBS/BSA; Dako, Glostrup, Denmark) or a convalescent patient's serum (dilution 1:75 in PBS/BSA). For detection of anti-CD68 and convalescent patient antibodies, rabbit anti-mouse IgG linked to peroxidase and a goat anti-human IgG linked to alkaline phosphatase were used, both at a 1:1000 dilution in PBS/BSA. The enzymatic complexes were revealed using aminobenzidine for peroxidase and fast blue for alkaline phosphatase.
Results
Setting-up of model, assessment by metabolic markers, and vascular imaging
The perfusion system was first established with spleens obtained from 12 anaesthetized piglets, as parameters such as spleen size and major vessel sections are similar to those of humans. The piglet spleens allowed for adjustment of the parameters to achieve appropriate physiologic metabolic and circulatory features (“Patients, materials, and methods”). The isolated human spleens were then set up in the experimental system shown Figure 1A. A typical experiment consisted of perfusing first Krebs-albumin solution, then red blood cells for 0.5 to 1 hour, and finally infected RBCs for 2 hours. The perfusion time for Krebs and RBCs was adjusted for each spleen until a steady state was reached, based on glucose consumption, stable flow, and arterial pressure. At the end of the experiment, the isolated-perfused human spleen was fixed and processed for histology. The physiologic and functional characteristics of the isolated-perfused spleen were assessed using several approaches. Ultrasonography at steady state showed a homogenous echostructure of the parenchyma, devoid of hyperechogenic or hypoechogenic structures such as air bubbles or large ischemic zones. Injection of the contrast agent (microbubbles) induced a progressive parenchymal enhancement in the isolated-perfused spleen (Figure 1Bi-ii), qualitatively similar to that observed in human volunteers (a representative example is shown Figure 1Biii-iv). Doppler analysis showed large permeable vessels, often localized in pairs, through which flows ran in opposite directions (data not shown). At steady state, the arterial pressure was maintained at 60 to 120 mmHg. During each experiment, the Na+ and K+ concentrations varied by less than 20%. There was significant glucose consumption, reflecting metabolic activity, so that glucose levels were monitored every 10 to 20 minutes, and extra glucose was added to maintain 1- to 2-g/L levels. Repeatedly measured arterial and venous O2 and CO2 partial pressures were within physiologic limits. From artery to vein, O2 partial pressure decreased by more than 80 mmHg, whereas CO2 partial pressure increased by more than 8 mmHg, showing O2 consumption and CO2 production, as expected to occur in an appropriately perfused and metabolically active organ. This points to a good spleen resistance to short periods of warm ischemia.
Clearance rate of infected red blood cells exposed to a lethal dose of artesunate (Art-iRBCs)
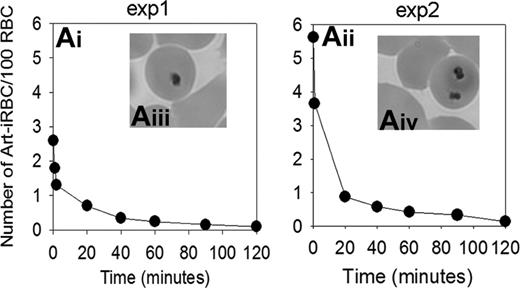
Perfusion of ring stage-infected RBCs previously exposed to a lethal dose of artesunate for 8 to 12 hours in vitro was started 10 to 30 minutes after a stable 1 mL/g per minute flow was reached. The concentration of artesunate used was in the range of the peak plasma level observed in patients treated intramuscularly with the drug.28 Typical morphology of Art-iRBCs containing dead parasite remnants with a shrunken, dense cytoplasm and pyknotic nucleus is shown in the Figure 2 (Aiii,iv).15 Sequential Giemsa-stained thin films of the circulating cells showed that Art-iRBC counts decreased with a half-life of 17 to 18 minutes, with an overall clearance time of approximately 120 minutes (2 experiments with differing initial parasitemia are shown in Figure 2). At that time, more than 95% of the parasite input could no longer be observed in the perfusate, indicating an efficient and rapid clearance. As a control, no change in parasitemia was observed when infected RBCs circulated through a device to which no spleen had been connected (data not shown). Parasite clearance time was in the order of magnitude of that observed in humans (Table 1), provided that a 20-fold correcting factor was applied to take into account that a given Art-iRBC crosses the spleen every 20 minutes in patients, but does so every minute in isolated-perfused spleens.16
Experimental pitting rate in isolated-perfused spleens compared with published data in artesunate-treated patients16
. | Experiment 1 . | Experiment 2 . | DHA-treated patients data from Newton et al16 . |
|---|---|---|---|
| Total volume of red blood cells (RBCs) in IP-spleen perfusate or in patient blood, mL | 13 | 10 | 1500-2000 |
| iRBCs in IP-spleen perfusate or in patient blood | |||
| Initial concentration, % of RBCs | 2.6 | 5.63 | 7.63 |
| Total volume, mL* | 0.338 | 0.563 | > 100 |
| Total no. | 3.38 × 109 | 5.63 × 109 | > 1012 |
| Parasite clearance time (corrected value), h† | ≥ 2 (≥40) | ≥2 (≥40) | 60 |
| Peak concentration of once-infected RBCs (O-iRBCs), % of RBCs‡ | 0.31 | 1.19 | 1.8 (0.8-8.8) |
| Estimated global proportion of Art-iRBCs pitted during the experiment, %§ | 11.9 | 21.1 | NA |
| Estimated cumulative no. Art-iRBCs pitted|| | 4.03 × 108 | 11.33 × 108 | NA |
| Time to peak of O-iRBCs (corrected value), h† | 1 (20) | 1.5 (30) | 45 |
| Calculated no. of Art-iRBCs pitted per h, × 108 | 4.03 | 7.55 | 21 (5-126) |
| Estimated amount of Art-iRBCs pitted per spleen transit¶, × 106 | 6.71 | 12.59 | 26 (6-177) |
| Pitting rate per body transit, % of iRBCs | NA | NA | 0.003 (0.001-0.05) |
| Calculated pitting rate per spleen transit, % of iRBCs** | 0.2 | 0.23 | 0.06 (0.02-1)†† |
. | Experiment 1 . | Experiment 2 . | DHA-treated patients data from Newton et al16 . |
|---|---|---|---|
| Total volume of red blood cells (RBCs) in IP-spleen perfusate or in patient blood, mL | 13 | 10 | 1500-2000 |
| iRBCs in IP-spleen perfusate or in patient blood | |||
| Initial concentration, % of RBCs | 2.6 | 5.63 | 7.63 |
| Total volume, mL* | 0.338 | 0.563 | > 100 |
| Total no. | 3.38 × 109 | 5.63 × 109 | > 1012 |
| Parasite clearance time (corrected value), h† | ≥ 2 (≥40) | ≥2 (≥40) | 60 |
| Peak concentration of once-infected RBCs (O-iRBCs), % of RBCs‡ | 0.31 | 1.19 | 1.8 (0.8-8.8) |
| Estimated global proportion of Art-iRBCs pitted during the experiment, %§ | 11.9 | 21.1 | NA |
| Estimated cumulative no. Art-iRBCs pitted|| | 4.03 × 108 | 11.33 × 108 | NA |
| Time to peak of O-iRBCs (corrected value), h† | 1 (20) | 1.5 (30) | 45 |
| Calculated no. of Art-iRBCs pitted per h, × 108 | 4.03 | 7.55 | 21 (5-126) |
| Estimated amount of Art-iRBCs pitted per spleen transit¶, × 106 | 6.71 | 12.59 | 26 (6-177) |
| Pitting rate per body transit, % of iRBCs | NA | NA | 0.003 (0.001-0.05) |
| Calculated pitting rate per spleen transit, % of iRBCs** | 0.2 | 0.23 | 0.06 (0.02-1)†† |
NA indicates not assessed.
Number calculated assuming an RBC concentration of 1010/mL (10–3/L)
Observed parasite clearance time and time to peak of O-iRBCs were multiplied by 20 [corrected time] to take into account the fact that a given iRBC crosses patient spleen every 20 minutes, but does so every minute through isolated perfused spleens
The concentration of O-iRBCs at any time point was calculated using the following formula: concentration of O-iRBCs in the perfusate = % of iRBCs on Giemsa-stained smears: [number O-iRBCs/number of iRBCs] on EMIF- and Hoechst-stained slides. The peak concentration was the highest observed value in each experiment. Because the peak concentration of O-iRBCs depends on parasitemia on Giemsa smears, it may not be perfectly synchronous with the peak proportion of O-iRBCs among EMIF-positive cells. This occurred during experiment 1
The estimated pitting rate was calculated by dividing the peak concentration of O-iRBCs by the initial iRBC concentration, an acceptable assumption since the hematocrit level remained stable during experiments
Indicates number of O-irBCs produced. This minimal value was estimated under the hypothesis that no O-iRBC clearance occurred before the peak O-iRBC concentration was reached
Assuming that the whole RBC pool crossed IP-spleens once per minute
Pitting rate per spleen transit = number of Art-iRBCs pitted during the experiment: number of spleen transits to reach the peak (ie, 60 for experiment 1 and 90 for experiment 2)
Pitting rate was calculated per body transit in patients.16 It takes approximately 20 body transits for a given iRBC to cross the spleen once
Kinetics of Art-iRBC in the perfusate. (Ai-iii) Art-iRBC concentration kinetics in the perfusate in 2 separate IP-spleens. The fast decrease during the first 2 minutes reflects the dilution of initial culture with uninfected RBCs just prior to perfusion. (Aiii-iv) Typical morphology of Art-iRBC containing dead parasite remnants (DPRs) on Giemsa-stained smears from the perfusate.
Kinetics of Art-iRBC in the perfusate. (Ai-iii) Art-iRBC concentration kinetics in the perfusate in 2 separate IP-spleens. The fast decrease during the first 2 minutes reflects the dilution of initial culture with uninfected RBCs just prior to perfusion. (Aiii-iv) Typical morphology of Art-iRBC containing dead parasite remnants (DPRs) on Giemsa-stained smears from the perfusate.
Pitting of Art-iRBCs
Red blood cells leaving the reticular meshwork of the red pulp must cross the sinus wall to enter the venous system. During this process, erythrocytes containing inclusion bodies undergo pitting.1,10,24
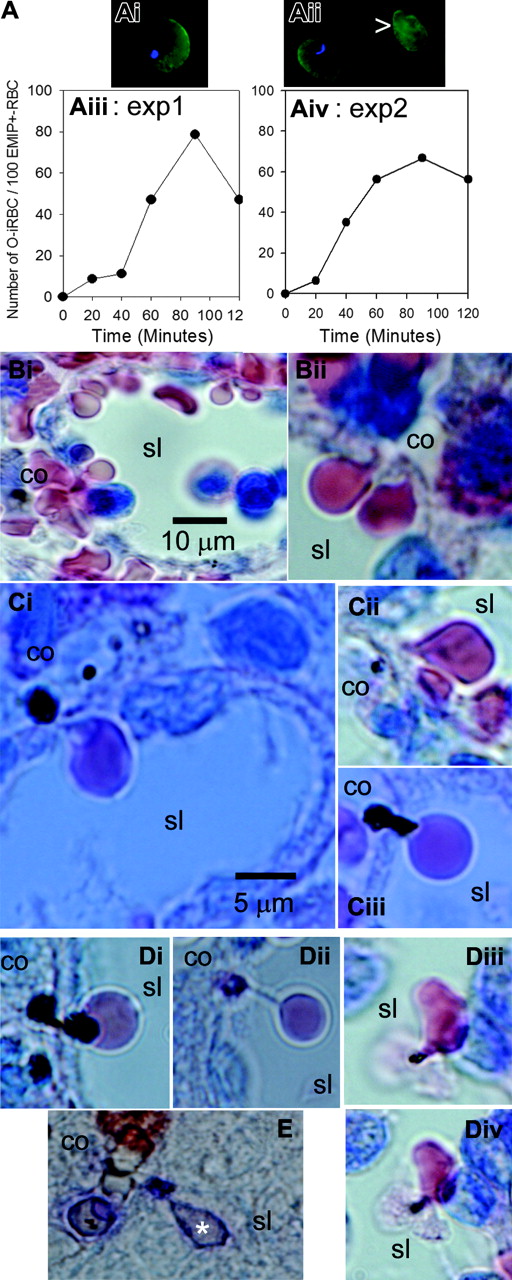
Pitting of infected RBCs by the isolated-perfused spleen was studied using EMIF, an immunofluorescence assay targeting parasite molecules deposited in the RBC membrane of ring stages, mainly the plasmodial antigen called RESA.27 After they have been pitted, once-infected artesunate-treated RBCs (O-iRBCs) exhibit a positive EMIF reaction, but lack an intraerythrocytic dead parasite and hence are not stained with Hoechst 33342, a nuclear DNA marker. Figure 3Aiii-iv shows that the proportion of such O-iRBCs progressively increased in the perfusate, reflecting an active pitting process. O-iRBCs accounted for 66% to 80% of the RESA-positive RBCs after 90 minutes. The pitting rate per transit through isolated-perfused spleens (0.2%-0.23%) was similar to that deduced in human patients (0.02%-1%).16 Total numbers of infected RBCs pitted per hour were also in the same range (Table 1). On Giemsa-stained histologic sections, uninfected RBCs were observed as they squeezed to cross the sinus walls from the cords to the sinus lumen (Figure 3Bi-ii). Typical pictures of the pitting process were observed with Art-iRBCs (Figure 3Ci-ii), along with less conventional patterns such as stringlike formations linking O-iRBCs to their “just-pitted” dead parasite remnant (Figure 3Di) or “being-pitted” large dead parasite remnants with a bipolar cordal-luminal aspect (Figure 3Ciii,Dii). In some instances, incompletely pitted infected RBCs were observed in the sinus lumen, with their “almost-pitted” dead parasite remnant still attached to the RBC membrane, sometimes in close contact with luminal cells (Figure 3Diii-iv). Pitting of Art-iRBCs was also observed by immunochemistry using a human convalescent serum, with the immunoglobulins detected by alkaline-phosphatase-labeled anti-human Ig antibodies (Figure 3E). Overall, pitted infected RBCs represented 12% to 21% of the initial Art-iRBC loads (Table 1), indicating that other clearance mechanism(s) were involved.
Direct phagocytosis of Art-iRBCs in the cords
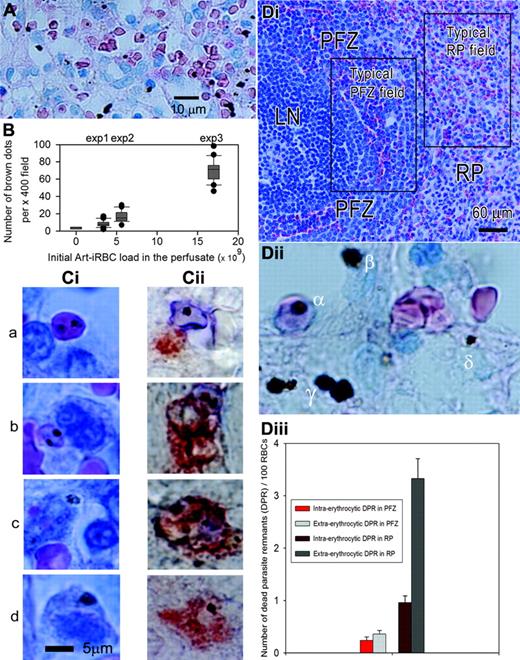
On Giemsa-stained sections of the spleen, dead parasite remnants appeared as brown dots either within intact Art-iRBCs (intraerythrocytic dead parasite remnants) or outside RBCs (extraerythrocytic dead parasite remnants) (Figure 4A,Dii). Counting brown dots per × 400 field of Giemsa-stained sections (as seen in Figure 4A-B) showed that the number of brown dots in the spleen tissue at the end of experiments was related to the number of Art-iRBCs initially introduced in the system (one-way analysis of variance and multiple comparison tests, Bonferroni correction with 3 independent spleens and 20 sections/spleen, P < .05 for each paired comparison). In contrast, a low (1-5 dots per × 400 field) background was observed in the red pulp of an isolated-perfused spleen through which no Art-iRBCs had been perfused. This confirmed that the brown dots observed on sections of Art-iRBC-perfused spleens were attributable to dead parasite remnants. Extraerythrocytic dead parasite remnants accounted for 65.2% of dead parasite remnants (mean count for 3 experiments, 20 × 400 fields counted/experiment; range, 53%-79.5%) showing that, over 2 hours, isolated-perfused spleens had not only retained but also efficiently and rapidly processed the majority of Art-iRBCs. Intact Art-iRBCs were observed either free or colocalizing with cord cells (Figure 4A,Cia). Spleen sections double-stained with a serum from a convalescent P falciparum patient and anti-CD68 antibodies (Figure 4Cii) showed the same pattern, namely dead parasite remnants either inside intact Art-iRBCs or inside CD68+ macrophages, sometimes in close proximity with RBC fragments (Figure Ci-iic). A variety of morphologic patterns was observed, from fully intact Art-iRBCs in close contact with (Figure 4Ciia)—or apparently engulfed by (Figure 4Ciib)—macrophages to fragmented Art-iRBCs and dead parasite remnants within macrophages (Figure 4Ciic). Fragmentation of extracellular Art-iRBCs was very rarely observed. Taken together, these data strongly suggest that direct phagocytosis of intact Art-iRBCs by macrophages took place in isolated-perfused spleens, a process leading to intramacrophagic dead parasite remnants (Figure 2Cid,Ciid). Analysis of the localization of extraerythrocytic dead parasite remnants (see Figure 4Dii for definitions) showed that 83.7% (range, 79%-87%) colocalized with cord cells. Only 4.4% (range, 1.9%-6.8%) of extraerythrocytic dead parasite remnants were free, whereas 11% (range, 9%-13%) colocalized with sinus wall cells.
Kinetics and morphologic aspects of pitting. EMIF-positive iRBCs (EMIF+-iRBC) (Ai) were distinguished from once-infected RBCs (O-iRBC, Aii arrow) by the presence of intraerythrocytic Hoechst-labeled dead parasite remnants (DPRs). The proportion of O-iRBCs in the perfusate increased during experiments (Aiii-iv). On Giemsa-stained histologic sections, uninfected RBCs were observed as they squeezed to cross the sinus walls (Bi-ii) from cords (co) to sinus lumens (sl). During pitting of Art-iRBCs, small DPRs were left on the cordal side of the sinus wall—a typical aspect of the pitting process (Ci-iii)—whereas large DPRs had a bispherical cordal-luminal aspect (Di). Some DPRs retained in the sinus wall were still linked to their once-host cell by a string-like formation (Dii) and incompletely pitted Art-iRBCs were observed in sinus lumens, with a membrane-bound DPR in contact with a luminal cell (Diii-iv). (E) Aspect of pitting as observed by immunochemistry using a human convalescent serum, the Ig being detected by alkaline-phosphatase labeled reagent.
Kinetics and morphologic aspects of pitting. EMIF-positive iRBCs (EMIF+-iRBC) (Ai) were distinguished from once-infected RBCs (O-iRBC, Aii arrow) by the presence of intraerythrocytic Hoechst-labeled dead parasite remnants (DPRs). The proportion of O-iRBCs in the perfusate increased during experiments (Aiii-iv). On Giemsa-stained histologic sections, uninfected RBCs were observed as they squeezed to cross the sinus walls (Bi-ii) from cords (co) to sinus lumens (sl). During pitting of Art-iRBCs, small DPRs were left on the cordal side of the sinus wall—a typical aspect of the pitting process (Ci-iii)—whereas large DPRs had a bispherical cordal-luminal aspect (Di). Some DPRs retained in the sinus wall were still linked to their once-host cell by a string-like formation (Dii) and incompletely pitted Art-iRBCs were observed in sinus lumens, with a membrane-bound DPR in contact with a luminal cell (Diii-iv). (E) Aspect of pitting as observed by immunochemistry using a human convalescent serum, the Ig being detected by alkaline-phosphatase labeled reagent.
Art-iRBC retention and processing are zone dependent
Specific microcirculatory structures of the perifollicular zone, namely small blood-filled spaces densely packed with erythrocytes in the proximity of a proportion of lymphoid nodules as well as cords and sinus lumens of the red pulp were readily identified on Giemsa-stained histologic sections (Figure 4Di).1,6 Dead parasite remnants at the end of the experiment were more numerous in the red pulp than in the perifollicular zone, both as intraerythrocytic (0.96% vs 0.24%, 2 sample t test by zone between raw values, P < .001) or extraerythrocytic dead parasite remnants (3.33% vs 0.36%, 2 sample t test by zone between raw values, P <.001) (Figure 3C). This shows that the red pulp—which occupies 75% of the total spleen volume—displayed more than 90% of Art-iRBC retention. Processing of Art-iRBCs was also more efficient in the red pulp than in the perifollicular zone.6 Indeed, extraerythrocytic dead parasite remnants were more numerous than intraerythrocytic dead parasite remnants in the red pulp (3.33% vs 0.96%, 2 samples t test by zone, P < .001) but not in the perifollicular zone (0.36% vs 0.24%, 2 sample t test by zone, P = .187). Hence, most Art-iRBCs were retained and processed in the red pulp, the perifollicular zone appearing more as a transit zone than a retention/processing area for Art-iRBCs. Of interest, predominant Art-iRBC retention and processing in the red pulp strongly confirm the suitable quality of the perfusion of this peculiar zone in isolated-perfused spleens.
Art-iRBC retention and processing. (A-B) Aspect and quantification of brown dots on Giemsa-stained sections showing that they correspond to dead parasite remnants. Brown dots colocalized with red blood cells (intraerythrocytic dead parasite remnants; Dii, α) or not (extraerythrocytic dead parasite remnants). Extraerythrocytic dead parasite remnants could be further classified as extracellular (Dii, β), intracellular in cord cells (Dii, γ)or intracellular in sinus wall cells (Dii, δ). Most extraerythrocytic dead parasite remnants colocalized with cord cells (A,Dii). (B) Mean number of brown dots per × 400 field on Giemsa-stained section as box plots for 4 different spleens in which different parasite loads had been introduced. (Ci-ii) Aspect and localization of Art-iRBCs and extraerythrocytic dead parasite remnants at a cellular scale, analyzed either by Giemsa-staining (Ci) or by immunohistochemistry (Cii). Fragmented Art-iRBCs colocalized with cord cells/macrophages (Ci-ii, row c). (D) Microcirculatory structures of the spleen were identified on Giemsa-stained sections (Di) allowing differential counting of intraerythrocytic and extraerythrocytic dead parasite remnants (Dii) on × 400 fields. To adjust for circulatory space on each field, dead parasite remnants numbers are expressed as mean (and standard error of the mean) for 100 RBCs in each zone (Diii). For statistical analysis, see “Art-iRBC retention and processing are zone dependent.”
Art-iRBC retention and processing. (A-B) Aspect and quantification of brown dots on Giemsa-stained sections showing that they correspond to dead parasite remnants. Brown dots colocalized with red blood cells (intraerythrocytic dead parasite remnants; Dii, α) or not (extraerythrocytic dead parasite remnants). Extraerythrocytic dead parasite remnants could be further classified as extracellular (Dii, β), intracellular in cord cells (Dii, γ)or intracellular in sinus wall cells (Dii, δ). Most extraerythrocytic dead parasite remnants colocalized with cord cells (A,Dii). (B) Mean number of brown dots per × 400 field on Giemsa-stained section as box plots for 4 different spleens in which different parasite loads had been introduced. (Ci-ii) Aspect and localization of Art-iRBCs and extraerythrocytic dead parasite remnants at a cellular scale, analyzed either by Giemsa-staining (Ci) or by immunohistochemistry (Cii). Fragmented Art-iRBCs colocalized with cord cells/macrophages (Ci-ii, row c). (D) Microcirculatory structures of the spleen were identified on Giemsa-stained sections (Di) allowing differential counting of intraerythrocytic and extraerythrocytic dead parasite remnants (Dii) on × 400 fields. To adjust for circulatory space on each field, dead parasite remnants numbers are expressed as mean (and standard error of the mean) for 100 RBCs in each zone (Diii). For statistical analysis, see “Art-iRBC retention and processing are zone dependent.”
Discussion
Exploring the abdominal, prone-to-bleed, difficult-to-sample human spleen has often resembled studying a black box.11 The retrieval of healthy spleen after selected surgical interventions offered the opportunity to set up and validate a functional isolated-perfused human spleen system. The quantitative readout for the clearing function of the isolated-perfused human spleens provided results in close similarity with in vivo data obtained from malaria patients in terms of clearance kinetics, qualitative tissular processing, and pitting rate (Figure 2; Table 1).16,29 The pitting rate we are referring to in the present analysis is different from the rate of circulating pitted RBCs used as the gold standard for assessing spleen filtering function in patients with potential asplenia. “Classic” pitted RBCs result from macrophage-mediated removal of (nonmicrobial) intracellular remnants as evidenced by small surface depressions that can be visualized using differential interference microscopy (Nomarski optics).30 Because this pitting process removes part of the red cell membrane, pitted RBCs become less deformable and are cleared from the circulation by the spleen. This clearing function is impaired when the spleen is absent or not functional. It is commonly accepted by hematologists that a rate of circulating pitted RBCs more than 2% is an indication of a higher risk for overwhelming infection due to functional asplenia.31,32 By contrast, in the context of treated malaria patients, RBCs from which parasites have been removed (or pitted) are called once-infected red blood cells (O-iRBCs) and visualized as RESA-positive cells lacking a parasite remnant.16 Since O-iRBCs are not detected in the peripheral blood of asplenic malaria patients, their presence is a marker of spleen-clearing functions.15 Therefore the, “pitting rate” as we defined it in this paper (ie, spleen production of O-iRBCs) was an appropriate assessment of preserved function in isolated-perfused spleens. Yet, O-iRBCs are damaged RBCs, most likely with a loss of surface and/or a damaged membrane. Their 2- to 5-day life span is shorter than that of normal noninfected red blood cells.14,16 It is likely that if the isolated-perfused experiment were to be prolonged for several days, O-iRBCs would eventually disappear and the only red cells remaining in the circulating perfusate would be the RESA-negative “never-infected” RBCs.
Functional clearance of Art-iRBCs by the isolated-perfused spleen indicates that the organ retains clearing and processing functions and thus that the black box can now be informatively opened for hypothesis-driven tissue investigations. The zone dependence of Art-iRBC retention (Figure 4) is in keeping with the classic paradigm that RBC quality control is more a function of the slow microcirculation of the red pulp than of the fast microcirculation of the perifollicular zone.6,11 We provide here the first experimental confirmation of this paradigm in the context of interactions between a human pathogen and the human spleen tissue. The accumulation of Art-iRBCs in the red pulp might be interpreted as a purely rheologic filtration similar to that observed with carbonized plastic beads.33 This explanation is unlikely, however, since in vitro exposure to artesunate results in infected red blood cells with a normal or almost normal elongation index as assessed by laser diffraction.14 Furthermore, pitting would have hardly occurred with rigid infected RBCs. Taken together, our clearing and processing data confirm that pitting of Art-iRBCs is an intrasplenic process in humans, the remnant being removed as the RBC crosses the wall of red pulp sinuses.14-16 Pitting accounted for a fraction of parasite clearance, estimated as 12% to 21% of the initial parasite load. The close correspondence between this proportion of Art-iRBC cleared by pitting—as estimated from kinetic analysis of the perfusate cells—on the one hand and the proportion of extraerythrocytic dead parasite remnants deposited into the sinus wall cells on the other hand (11%) is striking. It is tempting to speculate that most extraerythrocytic dead parasite remnants observed into or in close proximity to the sinus wall were generated by pitting, while cordal dead parasite remnants were created by phagocytosis of intact Art-iRBCs. This latter aspect is reminiscent of the physiologic clearance of senescent RBCs, which is handled through elimination of intact RBCs rather than lysed debris.34 Hence, our results show that pitting is probably not the only clearance mechanism of Art-iRBCs and that direct phagocytosis of Art-iRBCs may account for a substantial part of parasite clearance, a mechanism already observed in the spleen of a patient who died from P falciparum malaria.29 One complementary hypothesis is that incomplete/abortive pitting triggers intrasinusal phagocytosis, resulting in a 2-step mechanism of innate clearance. Of importance, Art-iRBC clearance observed here—either through phagocytosis or pitting—did not require any significant spleen priming and was independent from most serum factors, since perfusion was performed in Krebs-human albumin medium.
We used P falciparum-infected RBCs as markers for spleen filtering functions. In return, human isolated-perfused spleens may now help explore the pathogenesis of P falciparum malaria, the most frequent and severe health problem in which the spleen plays a central role.35 In malaria-endemic countries, splenectomy increases the incidence of both fever episodes and parasitemia, and also possibly that of severe malaria episodes.36-38 The spleen modulates the cytoadherent properties of P falciparum-infected RBCs.5 In naive P falciparum-infected patients receiving hyperimmune immunoglobulins, the most dramatic decrease of parasitemia was accompanied by an acute splenomegaly.39 Yet, how the spleen innately selects—or induces changes in—some subpopulations of infected RBCs, and how it clears infected RBCs in the presence of hyperimmune immunoglobulins, remains controversial. The experimental challenge of human isolated-perfused spleens with circulating blood stage parasites along with defined effectors should lead to a better understanding of the mechanisms of naturally acquired protection and to identification of their target antigens. This novel tool fills a gap in malaria vaccine development in providing a relevant functional assay for erythrocytic-stage parasite clearance that may help prioritize vaccine development efforts. Developing the erythrocytic-stage component of the envisioned multistage antimalaria vaccine would greatly benefit from a functional screening model equivalent to the human sporozoite challenge for the pre-erythrocytic vaccine development.40 While sterile immunity may be difficult to achieve with a vaccine targeting the erythrocytic stage, a marked reduction of parasitemia may represent an interesting end point for a blood stage vaccine. This is, however, difficult to monitor in phase 2 vaccine trials as, for ethical reasons, even a minimal P falciparum parasitemia in patients or volunteers must be promptly treated. In this respect, challenging human isolated-perfused spleens with infected RBCs in the presence of antibodies/serum from phase 1 volunteers might provide critical information on the vaccine capacity to induce antibodies efficiently controlling parasite density, a key element in protection against clinical malaria.
Several diseases in which blood cell and/or microorganism clearance and processing by the spleen are an issue might benefit from the model. The clearance of damaged, aging, or genetically variant RBCs as well as sequestration crisis in sickle cell disease or red cell removal in hemolytic (eg, autoimmune) anemias are examples of physiologic or pathologic mechanisms that could be explored using this new tool.
Prepublished online as Blood First Edition Paper, December 29, 2005; DOI 10.1182/blood-2005-10-4094.
Supported by the Institut Pasteur Transverse Research Programs (PTR no. 85), by the “Fonds dédié pour les maladies parasitaires,” and by a grant from Fondation pour la Recherche Médicale to V.B.
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We are indebted to Dr Catherine Pastor and Dr Filomena Conti for early guidance in the world of isolated-perfused organs, Dr Francis Paulmier (INRA, Nouzilly, France) and his team for access to piglet spleens, Christian Roujoux for glassware manufacturing, Dr Francis Remerand for sharing expertise on vascular physiology, and Bénédicte Bénédic for excellent technical assistance. We reserve a special mention to Dr Nathalie Chabbert for creative input at crucial steps of this project.
The authors have no conflicting financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal