Abstract
CD40-activated B cells (CD40-B cells) have previously been introduced as an alternative source of antigen-presenting cells for immunotherapy. CD40-B cells can prime naive and expand memory T cells, and they can be generated in large numbers from very small amounts of peripheral blood derived from healthy individuals or cancer patients alike. Administration of CD40-B cells as a cellular adjuvant would require these cells to migrate toward secondary lymphoid organs and attract T cells in situ, processes guided by specific chemokines and chemokine receptors. Here, we demonstrate that primary, human CD40-B cells express a pattern of adhesion molecules and chemokine receptors necessary for homing to secondary lymphoid organs and have the capacity to migrate to cognate ligands. Furthermore, we show that CD40-B cells express important T-cell attractants and induce strong T-cell chemotaxis. These findings further support the use of CD40-B cells as cellular adjuvant for cancer immunotherapy.
Introduction
Numerous potential tumor-rejection antigens have been identified in the past.1 The majority of vaccination strategies use cellular adjuvants to direct T-cell responses against these target structures. Dendritic cells (DCs) as “nature's adjuvant” are a major focus due to their natural capacity to take up and present antigen and home to secondary lymphoid organs, where they effectively induce T-cell responses.2 However, difficulties to generate large amounts of DCs necessary for vaccination and to identify the optimal DC subtype have sparked an interest in alternative antigen-presenting cells (APCs).
B cells have been demonstrated to present antigen to T cells in vivo.3 CD40 activation dramatically improves antigen presentation by normal and malignant B cells and has therefore been studied as an approach to generate autologous nonartificial APCs for immunotherapy.4,5 CD40-activated B cells (CD40-B cells) not only expand antigen-specific CD4+ and CD8+ T cells, but also prime naive T cells in vitro.6-9 Most importantly, CD40-B cells can be generated in large amounts from small samples of peripheral blood from healthy donors and cancer patients under good manufacturing practice (GMP) conditions.6 CD40-B cells are therefore used by a growing number of investigators as APCs in vitro and potentially represent a complementary cellular adjuvant in DC-based immunotherapy in particular in pediatric patients10 and patients with low marrow reserve.8,9,11
Unlike dendritic cells, it is not the B cells' physiologic role to survey peripheral tissues and home to secondary lymphoid tissue upon antigen uptake. It therefore remains unclear whether they express the respective factors and receptors that are necessary for homing to draining lymph nodes and/or to attract T cells (eg, in the T-cell areas of secondary lymphatic organs).12 The requirements necessary for successful migration of antigen-presenting cells to secondary lymphoid organs have most extensively been studied for DCs. These cells mature upon encounter of antigen and certain inflammatory stimuli, down-regulate CCR1, CCR2, CCR5, CCR6, and CXCR-1, and up-regulate surface CXCR-4 and CCR-7 expression within 6 to 8 hours.13,14 CCR1, CCR2, CCR5, CCR6, and CXCR-1 are receptors necessary for homing to inflammatory sites, while CXCR4 and CCR7 have an antagonistic function and mediate chemotaxis to secondary lymphoid organs.13,15,16 Of interest, monocyte-derived DCs, as currently applied in cancer vaccine trials, lack l-selectin (CD62L), a key molecule implicated in the initial phase of migration through high endothelial venules. As shown in animal and human studies, homing of these cells can be very limited.12,17,18 Accordingly, transfection of CD62L into mature DCs has been shown to improve homing properties.19
Here, we determined the expression of these important homing receptors on CD40-B cells and investigated whether CD40-B cells are equipped with the necessary receptors and ligands for migration to secondary lymphoid organs. To successfully interact with T cells, APCs not only need to locate to T-cell areas of lymph nodes but also must actively attract T cells. To determine the potential of CD40-B cells to actually physically interact with T cells and not only relocate to their vicinity, we assessed the expression of important T-cell attractants and determined the capacity of CD40-B cells to induce T-cell migration.20
Materials and methods
Generation of CD40-activated B cells and dendritic cells
Flow cytometry
Chemokine receptor expression was determined by flow cytometry using standard procedures. Antibodies were purchased from R&D Systems (Minneapolis, MN), with the exception of CCR5 (Pharmingen, San Jose, CA) and CD20 (Dako, Carpinteria, CA).
RNA preparation, microarray hybridization, and microarray data processing
CD40-activated B cells were harvested on day 14 of culture and stored at –80°C. Target preparation and array hybridization were performed on the HG-U133A platform (Affymetrix, Santa Clara, CA) as described previously.22
Reverse-transcriptase–polymerase chain reaction (RT-PCR)
Detection of chemokines was carried out using the TaqMan Master technology (Roche Applied Science, Mannheim, Germany) according to the manufacturer's recommendations.
Chemotaxis assay
For B-cell migration, 5 × 105 CD40-B cells were transferred into the upper chamber of 5-μm pore size transwell plates (Costar, Cambridge, MA). Varying amounts of chemokines (R&D Systems) were added to the lower chamber. Migration of cells was assessed after 2 and 3 hours using a Coulter Z2 counter (Beckman Coulter, Fullerton, CA) and a FACS Canto (Becton Dickinson, Heidelberg, Germany). For analysis of T-cell migration, magnetic-activated cell sorter (MACS)–purified CD4+ or CD8+ T cells were used.
Results and discussion
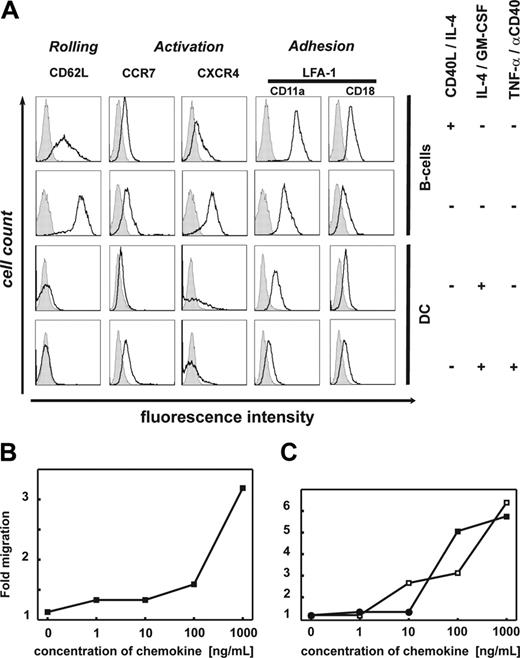
We assessed whether CD40-B cells express chemokine receptors up-regulated on mature DCs and associated with homing of APCs to secondary lymphoid organs (CXCR4 and CCR7) or homing to inflammatory sites.13,23,24 To better understand the overall homing properties, we performed comprehensive gene-expression profiling of B cells before (n = 4) and after (n = 4) 14 days of CD40L/IL-4 culture using the Affymetrix technology as well as flow cytometric analysis. In contrast to immature or mature DCs, CD40-B cells express the full lymph node homing triad CD62L, CCR7/CXCR4, and leukocyte function antigen-1 (LFA1, CD11a/CD18) on transcriptional and protein level (Figure 1A; also Table S1, available on the Blood website; see the Supplemental Tables link at the top of the online article). These receptors are crucial for homing to secondary lymphoid organs.12 They are implicated in rolling, activation, and adhesion to high endothelial venules via addressin (PNAD), SLC/CCL21, and ICAM-1, respectively, in order to enable extravasation.25 While CD62L, CXCR4, and CCR7 were moderately down-regulated on CD40-B cells after the 14-day culture period, surface expression of CD11a/CD18 had increased.26 This expression pattern was stable over at least 6 weeks of B-cell culture (data not shown). Resting and CD40-B cells lacked significant expression of CCR1, CCR2, CCR3, CCR4, CXCR1, CXCR2, CXCR3, CXCR5, and CXCR6. Upon activation, CCR5, CCR6, and CXCR3 were down-regulated (data not shown). The expression pattern of the homing molecules CD62L, CCR7/CXCR4, and LFA-1 on CD40-B cells is comparable with naive T cells and B cells.13,26,27 Expression of these molecules in the absence of CXCR5 suggests that CD40-B cells might be able to enter the T-cell areas, but not germinal centers,28 and therefore could colocalize with T cells in the T-cell–rich areas of secondary lymphoid organs.
Lymph-node homing phenotype of CD40-activated B cells. (A) Expression of lymph node homing receptors on CD40-B cells. First row: Expression by CD40-activated B cells after 14 days of culture; similar results were obtained at later time points. Second row: Expression by unstimulated B cells. Third row: Expression by immature monocyte-derived DCs. Fourth row: Expression by monocyte-derived DCs upon maturation with anti-CD40L/TNF-α. One representative experiment of at least 8 is shown. (B-C) Migration of CD40-B cells. Total number of CD40-B cells in bottom chamber of Costar transwell plate after 2-hour migration through a 5-μm filter. Migration was determined for different concentrations of chemoattractants: (B) Ligand of CXCR4: CXCL12. (C) Ligands of CCR7: CCL21 (□) and CCL19 (▪). Mean values are shown; at least 6 independent experiments were performed.
Lymph-node homing phenotype of CD40-activated B cells. (A) Expression of lymph node homing receptors on CD40-B cells. First row: Expression by CD40-activated B cells after 14 days of culture; similar results were obtained at later time points. Second row: Expression by unstimulated B cells. Third row: Expression by immature monocyte-derived DCs. Fourth row: Expression by monocyte-derived DCs upon maturation with anti-CD40L/TNF-α. One representative experiment of at least 8 is shown. (B-C) Migration of CD40-B cells. Total number of CD40-B cells in bottom chamber of Costar transwell plate after 2-hour migration through a 5-μm filter. Migration was determined for different concentrations of chemoattractants: (B) Ligand of CXCR4: CXCL12. (C) Ligands of CCR7: CCL21 (□) and CCL19 (▪). Mean values are shown; at least 6 independent experiments were performed.
Using a standard migration assay, we next determined whether CCR7 and CXCR4 are functional and mediate chemotaxis. In the absence of chemokines, CD40-B cells showed very little spontaneous migration. However, in the presence of increasing concentrations of CXCL12, the ligand for CXCR4, migration of CD40-B cells was significantly increased by 3.2-fold over background at a concentration of 1000 ng/mL (Figure 1B). CXCL12 is expressed in secondary lymphoid organs and has been demonstrated to mediate migration of mature DCs.29 Similarly, increasing concentrations of CCL19 and CCL21, which are expressed in T-cell areas of lymph nodes and by lymphatic endothelial cells, increased CCR7-mediated migration of CD40-B cells 5.9-fold (CCL19) and 6.2-fold (CCL21) (Figure 1C).13,30 Migration was shown to be directed only toward increasing chemokine concentrations excluding random, activation-induced locomotion (data not shown). Taken together, these data suggest that ex vivo–generated CD40-B cells have the capacity to migrate in response to chemokines important for homing to secondary lymphoid organs.
To induce immunity, antigen-presenting cells need to physically encounter T cells in a coordinated migration, which is particularly regulated by chemokine gradients in the T-cell areas of secondary lymphoid organs.12 The induction of T-cell chemotaxis by APCs has been studied extensively for DCs. Maturation stimuli such as LPS, dsDNA, and IL-1β induce significant alterations of chemokine profiles and ensure efficient antigen presentation to T cells. In order to determine the profile of chemoattractants secreted by CD40-B cells, we determined the expression of known T-cell attractants and chemokines produced by mature DCs. Expression of chemokines CCL17 (TARC), CCL5 (RANTES), CCL22 (MDC), and CXCL10 (IP-10) was detected using comprehensive gene-expression analysis (Table S2) and RT-PCR (data not shown).
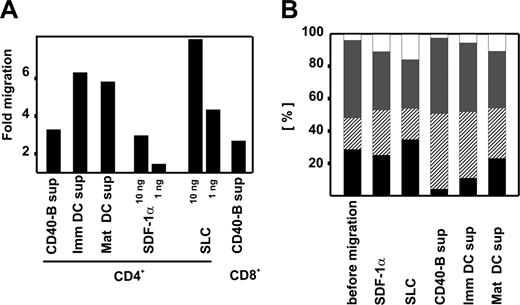
To assess whether this array of chemokines expressed by CD40-B cells actually induces T-cell migration, we performed standard transwell migration assays. Highly purified CD4+ or CD8+ T cells were isolated from peripheral blood, and chemotaxis was determined in response to supernatant from CD40-B cells. T cells were transferred into the upper chamber containing fresh B-cell medium and allowed to migrate for 2 hours. When supernatant from CD40-B cells was added to the lower chamber, T-cell migration increased 3.1-fold for CD4+ and 2.5-fold for CD8+ T cells, clearly demonstrating the chemotactic potential of CD40-B cells. As controls, CXCL12 and CCL21 as well as supernatant from monocyte-derived immature and mature DCs were used (Figure 2A). Migration was shown to be directed toward increasing concentrations of supernatant, thus excluding random, activation-induced locomotion (data not shown).
Blocking experiments using single monoclonal antibodies against the chemokine receptors of interest did not lead to significant blockade of migration (data not shown), supporting the notion that numerous chemokine-chemokine receptor interactions are responsible for strong T/B-cell interactions and/or that chemokine-independent mechanisms play a role (data not shown).
CD40-activated β cells induce T-cell migration. (A) Migration of T cells induced by supernatant from CD40-B cell cultures. CD4+ and CD8+ T cells were migrated for 2 hours through a 5-μm filter into the lower chamber of transwell plates containing supernatant from immature and mature monocyte-derived DCs and CD40-B cells as well as chemokines SDF-1α and SLC. Mean values of migration above background (cells spontaneously migrating to normal culture medium) in at least 7 independent experiments are shown. (B) Migration of T-cell subpopulations to supernatants from APC cultures. CD4+ were migrated for 2 hours through a 5-μm filter into the lower chamber of transwell plates containing SLC, SDF-1α, and supernatants from CD40-B, immature, and mature monocyte-derived DCs. T-cell subpopulations before and after migration are shown. From bottom to top: T naive (▪), T effector memory (▨), T central memory ( ), and T effector memory RA+ (□). Mean values of 3 independent experiments are shown.
), and T effector memory RA+ (□). Mean values of 3 independent experiments are shown.
CD40-activated β cells induce T-cell migration. (A) Migration of T cells induced by supernatant from CD40-B cell cultures. CD4+ and CD8+ T cells were migrated for 2 hours through a 5-μm filter into the lower chamber of transwell plates containing supernatant from immature and mature monocyte-derived DCs and CD40-B cells as well as chemokines SDF-1α and SLC. Mean values of migration above background (cells spontaneously migrating to normal culture medium) in at least 7 independent experiments are shown. (B) Migration of T-cell subpopulations to supernatants from APC cultures. CD4+ were migrated for 2 hours through a 5-μm filter into the lower chamber of transwell plates containing SLC, SDF-1α, and supernatants from CD40-B, immature, and mature monocyte-derived DCs. T-cell subpopulations before and after migration are shown. From bottom to top: T naive (▪), T effector memory (▨), T central memory ( ), and T effector memory RA+ (□). Mean values of 3 independent experiments are shown.
), and T effector memory RA+ (□). Mean values of 3 independent experiments are shown.
Phenotypic analysis of migrated T cells demonstrated that SDF-1α and SLC did not induce migration of a particular subgroup, while supernatants from CD40-B as well as immature and mature monocyte-derived DCs promoted migration of effector memory T cells. Correspondingly, chemotaxis of naive T cells was reduced under these conditions, in particular when supernatant from CD40-activated B cells was used (Figure 2B). Based on these findings, a combined approach using DCs for priming and CD40-B cells for frequent, high-dose expansion of antigen-experienced cells might be envisioned.
These data strongly suggest that in vitro–generated CD40-activated B cells are equipped with the respective chemokines and receptors, allowing them to home to secondary lymphoid organs to attract T cells.
Prepublished online as Blood First Edition Paper, December 15, 2005; DOI 10.1182/blood-2004-01-0113.
M.v.B.-B. was supported by the José-Carreras Leukämiestiftung and the Deutsche Krebshilfe (Max-Eder Nachwuchsgruppenprogramm). B.M. was supported by the Deutsche Forschungsgemeinschaft. This work was further supported by NIH grants P01-CA-66996 and P01-CA-78378 (L.M.N.), a Special Fellowship of the Leukemia & Lymphoma Society (J.L.S.), a Translational Research Award by the Leukemia & Lymphoma Society (J.L.S.), and a grant from the Deutsche Forschungsgemeinschaft (M.v.B.-B., J.L.S.).
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.