Abstract
Dendritic cells (DCs) exhibit distinct functional properties at immature and mature states. To identify genes preferentially regulated in monocyte-derived immature DCs (imDCs), 13 000-element microarrays were hybridized with RNA isolated from imDCs, mature DCs (mDCs), monocytes, and macrophages and a TGF-β–induced protein (βig-h3) was identified as being most prominently up-regulated in imDCs. By polymerase chain reaction (PCR), little βig-h3 mRNA was detected in monocytes and macrophages, but it was abundant in imDCs. On DC activation with LPS, βig-h3 mRNA became diminished, and in tissues, βig-h3 mRNA was abundantly expressed in lymphoid-rich tissues such as the spleen, bone marrow, small intestines, and colon. βig-h3 was expressed in 293T cells and purified as a 70-kDa protein and, by Western blotting, βig-h3 was predominantly detected in the medium of imDCs. We demonstrate that βig-h3 binds to macrophages and imDCs but not to mDCs and activates the Rac GTPase in macrophages, stimulating macrophage membrane ruffling and enhancing macrophage endocytosis. imDC endocytosis was also inhibited by purified anti–βig-h3 antibodies. Therefore, βig-h3 appears to be selectively up-regulated in imDCs to regulate antigen uptake through endocytosis.
Introduction
Dendritic cells (DCs) are potent antigen-presenting cells (APCs) that play critical roles in the activation of naïve T cells and cross-presentation of antigens to CD8 T cells.1-3 The potency of DCs as APCs is, to a certain extent, mediated by the ability of these cells to perform antigen uptake and T-cell stimulation at distinct maturation stages.4,5 Immature DCs (imDCs), present mainly in peripheral tissues, are potent in antigen uptake, using mechanisms such as receptor-dependent endocytosis and receptor-independent macropinocytosis.1-3,6 Many microbial structures and inflammatory stimuli can activate imDCs, causing DCs to undergo a complex maturation program when the cells increase their expression of surface major histocompatibility complex (MHC) and costimulatory molecules, such as CD80/CD86, CD54, and CD40, and produce proinflammatory cytokines.1-3 These maturing DCs also migrate to the T-cell area of draining lymph nodes, guided by their surface chemokine receptor CCR7.7-11 In contrast to imDCs, mature DCs (mDCs) exhibit reduced endocytic activities and are poor in antigen uptake.1-5 imDCs can expand regulatory T cells.12 Although mDCs can directly activate CD8+ T cells, imDCs can expand antigen-specific CD8 regulatory T cells.13,14 imDCs also expand CD4+CD25+ regulatory T cells.15 Therefore, besides antigen uptake, imDCs can also actively regulate adaptive immunity.
The molecular mechanisms underlying the functional properties of imDCs are poorly understood. For example, although imDCs are potent in macropinocytosis,4,5 how these cells acquire this macropinocytotic activity is unclear. Macropinocytosis requires membrane ruffling to form sealed macropinosomes,16 and 2 independent studies have shown that activation of the small GTPase Rac is required for imDC macropinocytosis.17,18 One of these studies also implicated constitutively active cdc42 in DC macropinocytosis.18 Rac and cdc42 are members of the Rho subfamily of small GTPases that are activated on GTP binding.19 It remains to be determined how imDCs acquire the ability to sustain Rac or cdc42 activation. In addition, although imDCs are known to expand regulatory T cells, the underlying mechanisms regulating this activity have not yet been determined. In the present study, we focus on the identification of genes that are associated with immature DCs.
Materials and methods
Reagents
LPS (Escherichia coli O55:B5) was purchased from Sigma-Aldrich (St Louis, MO). Human interleukin 4 (IL-4), granulocyte-macrophage colonystimulating factor (GM-CSF), and macrophage colony-stimulating factor (M-CSF) were obtained from R&D Systems (Minneapolis, MN). The following mouse monoclonal antibodies were obtained from Ancell (Bayport, MN): CD1a (FITC), CD14 (PE), CD40 (FITC), and CD54 (FITC). Antibodies for CD80 (PE), CD86 (PE), and CD83 (FITC) were purchased from BD PharMingen (San Diego, CA).
Culturing and activation of DCs and macrophages
Monocytes were isolated from peripheral-blood buffy coat as previously described,20 and isolated monocytes were confirmed to be approximately 95% pure based on the expression of surface CD14. The cells were cultured for 6 days at 37°C in the presence of IL-4 and GM-CSF (each at 20 ng/mL) to generate imDCs4 and cultured with M-CSF (20 ng/mL) to generate macrophages.
To activate imDCs and macrophages, the cells were harvested and cultured for 48 hours at 1 × 106/mL with LPS (0.5 μg/mL). The activation of DCs and macrophages was judged by surface expression of CD83 and CD54, respectively.20 Monocytes and macrophages also express surface CD83, but this is transient and lost within 24 hours.20 Briefly, cells were washed in PBS and then incubated for 30 minutes with specific antibodies or, as controls, isotype mouse IgG. The cells were washed 3 times in FACS wash (PBS containing 2.5% [vol/vol] bovine calf serum [BCS] and 0.05% [wt/vol] NaN3) and fixed in PBS containing 1% (wt/vol) paraformaldehyde (pH 7.6) before analysis on a FACSCalibur using CellQuest software (Becton Dickinson Immunocytometry Systems, San Jose, CA).
Preparation of RNA samples and microarray hybridization
RNA was isolated from fresh monocytes, macrophages, imDCs, and mDCs using TRIzol (Life Technologies, Bethesda, MD) followed by a repeated extraction with TRIzol to yield RNA with an A260/A280 ratio more than 1.8. For each cell type, RNA samples were pooled equally from 2 to 3 donors to prepare microarray probes. For each microarray hybridization, 2 to 3 μg total RNA was used following a single round of linear mRNA amplification.21 All samples were compared against a standard commercially available mRNA reference pool (Stratagene, La Jolla, CA) that had been similarly amplified. cDNA microarrays were fabricated using an SDDC-2 microarrayer (Virtek, Waterloo, CA) following standard procedures,22 using cDNA clones obtained from various commercial vendors (Incyte, Research Genetics). Samples were fluorescently labeled using Cy3 dye; the reference was labeled with Cy5. After hybridization, microarray images were captured using a CCD-based microarray scanner (Applied Precision, Issaquah, WA). Genes listed in Table 1 were further verified by sequencing.
Genes that are preferentially up- or down-regulated in DCs
Gene names (HUGO symbols) . | Accession no. . | Monocytes . | Macrophages . | imDCs . | mDCs . |
|---|---|---|---|---|---|
| TGF-β induced, 68 kDa (TGFBI) | BC004972 | -0.46 | 0.09 | 1.72 | -1.34 |
| Coagulation factor XIII A1 polypeptide (F13A1) | AF418272 | -0.82 | -0.55 | 2.20 | -0.83 |
| M-CSFR (CSFIR) | X03663 | 0.10 | 0.42 | 1.08 | -1.59 |
| Laminin β3 (LAMB3) | D37766 | -1.52 | 0.19 | 1.87 | -0.54 |
| CD1A antigen (CD1A) | M28825 | -1.10 | -1.17 | 1.56 | 0.71 |
| CCR1 (CCR1) | D10925 | -0.06 | 0.67 | 0.52 | -1.12 |
| Rho GDI β (ARHGDIB) | L20688 | -0.45 | -0.20 | 1.06 | -0.41 |
| p56Dok2(DOK2) | AF034970 | -0.40 | -0.54 | 1.20 | -0.26 |
| ATP-binding cassette/MRP3 (ABCC3) | Y17151 | -1.00 | -0.45 | 1.26 | 0.18 |
| Formin-like protein (FMNL1) | NM_005892 | -0.42 | -0.25 | 0.79 | -0.12 |
| Mannose receptor (MRC1) | X55635 | -1.08 | -0.28 | 1.11 | 0.25 |
| AKAP12 A kinase anchor protein 12 (AKAP12) | AB003476 | -0.84 | -0.08 | 0.85 | 0.08 |
| Chromosome 5 clone (CTC-224D3) | AC008385 | -0.89 | -0.33 | 0.98 | 0.25 |
| Ribosomal protein S16 (RPS16) | NM001020 | -1.24 | -0.03 | 1.13 | 0.14 |
| Mitochondrial gene (ND41) | AF382006 | -0.43 | -0.70 | 1.00 | 0.13 |
| Monoglyceride lipase (MGLL) | AK025983 | -1.35 | 0.14 | -0.90 | 2.11 |
| Activating transcription factor 3 (ATF3) | N39944 | -0.29 | 0.28 | -1.29 | 1.30 |
| Diubiquitin (UBD) | AW270961 | -1.35 | -0.77 | -0.17 | 2.30 |
| Inhibitor of apoptosis protein-1 (MIHC) | AF070674 | -0.33 | -0.97 | -0.55 | 1.85 |
| Peripheral myelin protein 2 (PMP22) | AA128311 | -0.54 | 0.27 | -0.92 | 1.18 |
| RGS1 (RGS1) | S59049 | -0.10 | -1.17* | -0.70* | 1.34 |
| Calmodulin 2 (CALM2) | BF671011 | -1.63 | -0.58 | 0.21 | 2.00 |
| NA | NA | -0.53 | -0.53 | -0.34 | 1.40 |
| Transgelin 2 (TAGLN2) | BE407460 | -0.40 | -0.26 | -0.46 | 1.26 |
| PA28 α (PSME2) | AA310524 | -0.96 | 0.22 | -0.46 | 1.20 |
| CCR7 (CCR7) | L08176 | 0.42 | -1.54 | -0.18 | 1.30 |
| Monoamine oxidase (MAOA) | M69226 | -0.99 | -0.79 | 0.20 | 1.58 |
| UV radiation resistance-associated gene (UVRAG) | AB012958 | -0.95 | -0.70 | 0.28 | 1.37 |
| NA | NA | -0.93 | -0.33 | 0.09 | 1.17 |
| Neuroepithelial cell transforming gene (NET1) | BF197249 | -0.99 | -0.64* | 0.26 | 1.16 |
| Tubulin β5 (TUBB) | BC019924 | -1.31 | -0.08 | 0.35 | 1.04 |
| di-N-acetyl-chitobiase (CTBS) | AW853098 | -0.98 | -0.43 | 0.34 | 1.06 |
| TARC (CCL17) | NM_002987 | -1.18 | -1.52* | 1.03 | 1.17 |
| Kunitz serine protease inhibitor (SP1NT2) | AB006534 | -1.51 | -0.81 | 0.92 | 1.40 |
| Cyclin-dependent kinase inhibitor 1A (p21) (CDKN1A) | U09579 | -1.20 | -0.51 | 0.45 | 1.26 |
| Fatty acid-binding protein 4 (FABP4) | AW631118 | -0.54 | -1.00 | 1.18 | 0.54 |
| Mitochondrial isoleucine tRNA synthetase (FLJ10326) | AA262600 | -0.90 | -0.41 | 1.18 | 0.62 |
| Cdc23 (CDC23) | BC017713 | -0.88 | -0.61 | 0.69 | 0.79 |
| YEATS domain containing 2 (YEATS2) | AK022903 | 0.10 | -1.32 | 1.01 | 0.46 |
| CD4 antigen (CD4) | U47924 | -1.09 | -0.29 | 0.66 | 0.72 |
| Interferon gamma receptor 2 (IGNGR2) | U05875 | -0.98 | -0.40 | 0.43 | 0.95 |
| Synaptogyrin 2 (SYNGR2) | AA465253 | -0.87 | -0.51 | 0.56 | 0.82 |
| KIAA0586 gene product (KIAA0586) | AB0I1158 | -0.97 | -0.40 | 0.47 | 0.91 |
| WASP-interacting protein (WASPIP) | NM_003387 | -0.65 | -0.45 | 0.63 | 0.68 |
| ARP2/3 subunit 4 (ARPC4) | R56649 | -0.73 | -0.29 | 0.67 | 0.35 |
| M-ficolin (FCN1) | NM_002003 | 1.11 | 1.29 | -1.48 | -1.38 |
| Solute carrier family 21 (SLCO1A2) | NM_005075 | 0.87 | 1.51 | -1.38 | -1.50 |
| Kreisler maf-related leucine zipper homolog (MAFB) | NM_005461 | 1.65 | 1.06 | 0.08 | -2.09 |
| CD16a (FCGR3A) | X52645 | 0.64 | 2.02 | -1.27 | -1.39 |
| ILT5 (LILRB3) | AF009644 | 0.37 | 1.49 | -0.62 | -1.23 |
| Complement C3 (C3) | NM_000064 | 0.68 | 1.43 | -1.32 | -0.79 |
| 1-8U (IFITM3) | BE906728 | 1.34 | 0.75 | -0.61 | -1.48 |
| ILT2 (LAIR1) | NM_002287 | 0.20 | 1.41 | -0.76 | -1.12 |
| G-CSFR1 (CSF3R) | M59818 | 0.44 | 0.99 | -1.03 | -0.74 |
| S100 calcium-binding protein A9 (S100A9) | X06233 | 0.53 | 0.82 | -0.72 | -0.95 |
Gene names (HUGO symbols) . | Accession no. . | Monocytes . | Macrophages . | imDCs . | mDCs . |
|---|---|---|---|---|---|
| TGF-β induced, 68 kDa (TGFBI) | BC004972 | -0.46 | 0.09 | 1.72 | -1.34 |
| Coagulation factor XIII A1 polypeptide (F13A1) | AF418272 | -0.82 | -0.55 | 2.20 | -0.83 |
| M-CSFR (CSFIR) | X03663 | 0.10 | 0.42 | 1.08 | -1.59 |
| Laminin β3 (LAMB3) | D37766 | -1.52 | 0.19 | 1.87 | -0.54 |
| CD1A antigen (CD1A) | M28825 | -1.10 | -1.17 | 1.56 | 0.71 |
| CCR1 (CCR1) | D10925 | -0.06 | 0.67 | 0.52 | -1.12 |
| Rho GDI β (ARHGDIB) | L20688 | -0.45 | -0.20 | 1.06 | -0.41 |
| p56Dok2(DOK2) | AF034970 | -0.40 | -0.54 | 1.20 | -0.26 |
| ATP-binding cassette/MRP3 (ABCC3) | Y17151 | -1.00 | -0.45 | 1.26 | 0.18 |
| Formin-like protein (FMNL1) | NM_005892 | -0.42 | -0.25 | 0.79 | -0.12 |
| Mannose receptor (MRC1) | X55635 | -1.08 | -0.28 | 1.11 | 0.25 |
| AKAP12 A kinase anchor protein 12 (AKAP12) | AB003476 | -0.84 | -0.08 | 0.85 | 0.08 |
| Chromosome 5 clone (CTC-224D3) | AC008385 | -0.89 | -0.33 | 0.98 | 0.25 |
| Ribosomal protein S16 (RPS16) | NM001020 | -1.24 | -0.03 | 1.13 | 0.14 |
| Mitochondrial gene (ND41) | AF382006 | -0.43 | -0.70 | 1.00 | 0.13 |
| Monoglyceride lipase (MGLL) | AK025983 | -1.35 | 0.14 | -0.90 | 2.11 |
| Activating transcription factor 3 (ATF3) | N39944 | -0.29 | 0.28 | -1.29 | 1.30 |
| Diubiquitin (UBD) | AW270961 | -1.35 | -0.77 | -0.17 | 2.30 |
| Inhibitor of apoptosis protein-1 (MIHC) | AF070674 | -0.33 | -0.97 | -0.55 | 1.85 |
| Peripheral myelin protein 2 (PMP22) | AA128311 | -0.54 | 0.27 | -0.92 | 1.18 |
| RGS1 (RGS1) | S59049 | -0.10 | -1.17* | -0.70* | 1.34 |
| Calmodulin 2 (CALM2) | BF671011 | -1.63 | -0.58 | 0.21 | 2.00 |
| NA | NA | -0.53 | -0.53 | -0.34 | 1.40 |
| Transgelin 2 (TAGLN2) | BE407460 | -0.40 | -0.26 | -0.46 | 1.26 |
| PA28 α (PSME2) | AA310524 | -0.96 | 0.22 | -0.46 | 1.20 |
| CCR7 (CCR7) | L08176 | 0.42 | -1.54 | -0.18 | 1.30 |
| Monoamine oxidase (MAOA) | M69226 | -0.99 | -0.79 | 0.20 | 1.58 |
| UV radiation resistance-associated gene (UVRAG) | AB012958 | -0.95 | -0.70 | 0.28 | 1.37 |
| NA | NA | -0.93 | -0.33 | 0.09 | 1.17 |
| Neuroepithelial cell transforming gene (NET1) | BF197249 | -0.99 | -0.64* | 0.26 | 1.16 |
| Tubulin β5 (TUBB) | BC019924 | -1.31 | -0.08 | 0.35 | 1.04 |
| di-N-acetyl-chitobiase (CTBS) | AW853098 | -0.98 | -0.43 | 0.34 | 1.06 |
| TARC (CCL17) | NM_002987 | -1.18 | -1.52* | 1.03 | 1.17 |
| Kunitz serine protease inhibitor (SP1NT2) | AB006534 | -1.51 | -0.81 | 0.92 | 1.40 |
| Cyclin-dependent kinase inhibitor 1A (p21) (CDKN1A) | U09579 | -1.20 | -0.51 | 0.45 | 1.26 |
| Fatty acid-binding protein 4 (FABP4) | AW631118 | -0.54 | -1.00 | 1.18 | 0.54 |
| Mitochondrial isoleucine tRNA synthetase (FLJ10326) | AA262600 | -0.90 | -0.41 | 1.18 | 0.62 |
| Cdc23 (CDC23) | BC017713 | -0.88 | -0.61 | 0.69 | 0.79 |
| YEATS domain containing 2 (YEATS2) | AK022903 | 0.10 | -1.32 | 1.01 | 0.46 |
| CD4 antigen (CD4) | U47924 | -1.09 | -0.29 | 0.66 | 0.72 |
| Interferon gamma receptor 2 (IGNGR2) | U05875 | -0.98 | -0.40 | 0.43 | 0.95 |
| Synaptogyrin 2 (SYNGR2) | AA465253 | -0.87 | -0.51 | 0.56 | 0.82 |
| KIAA0586 gene product (KIAA0586) | AB0I1158 | -0.97 | -0.40 | 0.47 | 0.91 |
| WASP-interacting protein (WASPIP) | NM_003387 | -0.65 | -0.45 | 0.63 | 0.68 |
| ARP2/3 subunit 4 (ARPC4) | R56649 | -0.73 | -0.29 | 0.67 | 0.35 |
| M-ficolin (FCN1) | NM_002003 | 1.11 | 1.29 | -1.48 | -1.38 |
| Solute carrier family 21 (SLCO1A2) | NM_005075 | 0.87 | 1.51 | -1.38 | -1.50 |
| Kreisler maf-related leucine zipper homolog (MAFB) | NM_005461 | 1.65 | 1.06 | 0.08 | -2.09 |
| CD16a (FCGR3A) | X52645 | 0.64 | 2.02 | -1.27 | -1.39 |
| ILT5 (LILRB3) | AF009644 | 0.37 | 1.49 | -0.62 | -1.23 |
| Complement C3 (C3) | NM_000064 | 0.68 | 1.43 | -1.32 | -0.79 |
| 1-8U (IFITM3) | BE906728 | 1.34 | 0.75 | -0.61 | -1.48 |
| ILT2 (LAIR1) | NM_002287 | 0.20 | 1.41 | -0.76 | -1.12 |
| G-CSFR1 (CSF3R) | M59818 | 0.44 | 0.99 | -1.03 | -0.74 |
| S100 calcium-binding protein A9 (S100A9) | X06233 | 0.53 | 0.82 | -0.72 | -0.95 |
Fifty-five genes are listed according to their up- or down-regulation in imDCs or mDCs, or both. The identities of these genes are identified by HUGO symbols (in parentheses and italics) and accession numbers. The expression values represent the average of 3 experiments except for those few values labeled with an asterisk, which denotes an average value of 2 experiments. These 55 genes were listed as 4 groups according to expression values in imDCs or mDCs (or both) identified with underlining.
CCR indicates CC chemokine receptor; p56Dok2, docking protein; RGS, regulator of G-protein signaling; NA, not annotated; PA28α, proteasome activator subunit 1; TARC, thymus and activation-regulated chemokine; ILT, immunoglobulin-like transcript; and I-8U, interferon-induced transmembrane protein 3.
Data processing and analysis
Fluorescence intensities corresponding to individual microarrays were uploaded into a centralized Oracle 8i database, accessible at www.omniarray.com. Establishment of various data sets and gene retrievals were performed using standard SQL queries, and are available from www.omniarray.com/dendritic_cells. All genes and arrays were mean-normalized to facilitate interarray comparisons. Hierarchic clustering was performed using the program Xcluster (Stanford, Palo Alto, CA) and visualized using the program Treeview.23 To identify genes that were differentially regulated across all 4 cellular populations, array elements were chosen that consistently exhibited more than 3-fold regulation across at least 3 arrays.
PCR
RNA was isolated from monocytes, macrophages, imDCs, and mDCs. RNA was also isolated from cultured monocytes at different time points of differentiation into imDCs, that is, at 0, 1, 2, 4, and 6 days. RNA from 19 different human tissues was purchased from BD Clontech (Palo Alto, CA). cDNA was synthesized from these RNA samples using the reverse transcription-for-polymerase chain reaction (RT-for-PCR) kit (BD Clontech). βig-h3 mRNA was detected by 35 cycles of RT-PCR using βig-h3–specific primers (5′-gctctagacgcgtcgtcccgctccat/cccttcgaagttggtgatggtggagatga3′). As a control, the β-actin mRNA was similarly amplified using specific primers (ggaaggaaggctggaaga/ggcgtgatggtgggcatg). The PCR products were separated on 1% (wt/vol) agarose gels and visualized using ethidium bromide. For real-time PCR, the following primers were used (5′-3′): βig-h3 (gactttgaaccgtatcctg/gagtagctcatcaatgtagt) and β-actin (aaatcgtgcgtgacattaagg/agcactgtgttggcgtacag). PCR was performed on a Light Cycler (Roche Diagnostics, Basel, Switzerland) using the DNA Master SYBR Green I kit. The reactions were carried out for 45 cycles in 20 μL volume containing 4 mM MgCl2, 0.5 μM of each primer, 2 μL DNA Master SYBR Green I, and 2 μL cDNA template. After a starting 10 minutes at 95°C, each following cycle consisted of 10 seconds at 95°C, 10 seconds at 56°C, and 10 seconds at 72°C, and finished by gradual product melting up to 95°C. The specificity of the PCR product was determined by melting curve analysis.
Cloning and expression of βig-h3
The βig-h3 cDNA was amplified, by RT-PCR, from imDC RNA with βig-h3–specific primers (5′-gctctagacgcgtcgtcccgctccat/cccttcgaaatgcttcatcctctctaataac-3′) and cloned into the XbaI and SfuI sites of the pcDNA3.1 myc-His plasmid (Invitrogen, Carlsbad, CA) in frame with 3′myc/His-coding sequences (pβig-h3-MH). The human embryonic kidney 293T cells (American Type Culture Collection, Manassas, VA) were cultured in DMEM supplemented with 10% (vol/vol) BCS (HyClone, Logan, UT), 100 U/mL penicillin, and 100 μg/mL streptomycin and transfected with the pβig-h3-MH vector (50 μg/T75 flask) using calcium phosphate.24 After 6 hours, the media were changed to serum-free DMEM containing bovine serum albumin (BSA; 100 μg/mL). The media were harvested in 2 days and incubated overnight at 4°C with 0.5 mL Ni-NTA-agarose (Qiagen, Valencia, CA). In some experiments, iodoacetamide was added to 2.5 mM before incubation with the beads. The beads were packed in a column and washed extensively with a wash buffer (20 mM Tris, pH 8.0, 500 mM NaCl, and 10 mM imidazole). Bound βig-h3 was eluted in 0.15-mL fractions using an elution buffer (20 mM Tris, pH 8.0, 500 mM NaCl, and 250 mM imidazole). βig-h3, eluted in fractions 2 to 5, was examined on 10% (wt/vol) sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) gels by Coomassie blue staining or Western blotting using a mouse monoclonal anti-myc or polyclonal anti–βig-h3 antibody. The latter was prepared by immunization of 6-week-old female Balb/c mice, through the peritoneal route, with purified βig-h3 (30 μg/mouse) in Freund complete adjuvant. After 2 boosters at 2-week intervals, sera were collected. Purified βig-h3 was quantified using the Bio-Rad Protein Assay kit (Bio-Rad, Hercules, CA) and dialyzed against PBS using the Pierce Microdialyzer System 100 (Pierce, Rockford, IL). Soluble CD14 was similarly expressed and purified.
Western blotting
Monocytes, macrophages, imDCs, and mDCs were cultured for 24 hours in the serum-free CD hybridoma medium (Gibco, Grand Island, NY) and media were collected. 293T cells were transfected in 6-well plates with pβig-h3-MH or pcDNA3.1 (4 μg/well) as described in “Cloning and expression of βig-h3.”After 6 hours, the cells were washed and cultured for another 24 hours in serum-free DMEM containing BSA (100 μg/mL) to collect media. The media were subjected to SDS-PAGE on 10% (wt/vol) gels under reducing conditions (1% [vol/vol] 2-mercaptoethanol). On electroblotting, βig-h3 was detected with a mouse polyclonal anti–βig-h3 antibody (1:1000 dilution). The blots were developed with alkaline phosphatase-conjugated goat anti–mouse IgG (1:5000 dilution; Sigma-Aldrich). Signals were visualized using the Immune-Star substrate pack (Bio-Rad).
Endocytosis
Endocytosis was examined as previously described.4 Cells were washed and resuspended in fresh medium at 1 × 106/mL. Purified βig-h3 or CD14 was added to the cell culture to 10 μg/mL and incubated for 30 minutes at 37°C before addition of FITC-dextran (FITC-DX) or lucifer yellow (LY; 1 mg/mL; Molecular Probes, Eugene, OR). In some experiments, purified βig-h3 was preincubated for 30 minutes with polymyxin B (20 μg/mL) before incubation with macrophages that were then incubated with the fluorescent dye molecules. imDCs were also preincubated with affinity-purified anti–βig-h3 IgG (2 μg/mL) for 1 hour before incubation for another 10, 20, or 30 minutes with the dye molecules (20 μg/mL). The antibody was purified by affinity chromatography using immobilized βig-h3 on cyanogen bromide-activated agarose (Sigma-Aldrich). After washing 4 times, the cells were analyzed by flow cytometry. In all experiments, cells incubated with the probes at 4°C were used as negative controls.
Detection of membrane ruffles by fluorescence microscopy
Membrane ruffling was determined using rhodamine-labeled phalloidin as previously reported.25 Macrophages were cultured for 24 hours on glass coverslips. After stimulation for 30 minutes with purified βig-h3 or CD14 (10 μg/mL) or left unstimulated, the cells were fixed for 15 minutes in 3.7% (wt/vol) formaldehyde in PBS and permeabilized for 5 minutes in 0.2% (vol/vol) Triton X-100. The cells were then incubated for 30 minutes with rhodamine-phalloidin (5 U/mL) and were, after washing, viewed using a LSM510 laser-scanning microscope equipped with a 63×/1.4 oil-immersion objective lens and using Zeiss LSM Image Browser software (Carl Zeiss, Jena, Germany).
Rac and cdc42 activation assay
Macrophages cultured in 6-well plates were stimulated for 30 minutes with βig-h3 or CD14 at 10 μg/mL or left unstimulated. The cells were lysed in a buffer consisting of 20 mM Tris (pH 7.5), 300 mM NaCl, 2 mM EDTA, 2 mM EGTA, 1% (vol/vol) Triton X-100, 10 μg/mL leupeptin, 10 μg/mL aprotinin, and 2 mM PMSF and, after centrifugation, the lysates were incubated for 1 hour at 4°C with the p21-binding domain (PBD) of p21-activated kinase 1 (PAK-1) immobilized on agarose (Upstate Biotechnology, Lake Placid, NY).26 As a positive control, lysate of unstimulated macrophages was incubated for 15 minutes with GTPγS (0.1 mM) before incubation with PAK-1 PBD-agarose. The agarose beads were washed in the lysis buffer and boiled in SDS-PAGE sample buffer. The supernatants were subjected to Western blotting using Rac and cdc42 antibodies. As controls, equal volumes of the different cell lysates (20% of total lysate) were similarly analyzed by Western blotting.
Binding of βig-h3 to the cell surface
Cells were washed and resuspended at 2 × 107/mL in cold RPMI containing 20 mM Tris (pH 7.4), 1 mM CaCl2, and 1 mM MgCl2 (RPMI-Tris). The cells (50 μL) were incubated with purified βig-h3 (50 μg/mL) for 2 hours at 4°C with gentle rocking. On washing twice in the binding buffer, cells were blocked for 30 minutes with 20% (vol/vol) goat serum in RPMI-Tris and then incubated for 30 minutes on ice with a mouse anti-myc antibody (10 μg/mL) or an isotype IgG. The cells were washed twice and then incubated for 30 minutes with PE-labeled goat anti–mouse IgG. After 2 washes, the cells were fixed in 1% (wt/vol) paraformaldehyde in PBS (pH 7.6) and analyzed by flow cytometry. The cells were also spotted on glass coverslips and examined using a BX51 Olympus fluorescence microscope equipped with a UPFL600I 63× oil-immersion objective lens (NA, 0.65-1.25). Images were captured using Olympus Micro Image software (Olympus, Tokyo, Japan).
Results
Characterization of monocytes and derived macrophages and DCs
The differentiation of CD14+ monocytes into macrophages and DCs in vitro has been well documented.4 We isolated monocytes using an adhesion method, which yields monocytes of about 95% purity (Figure 1), and cultured these cells into macrophages and DCs. Although both monocytes and macrophages are CD14hiCD1a– (Figure 1A), the cultured imDCs exhibited little surface CD14 and acquired CD1a (Figure 1A). On activation, DCs but not macrophages sustained surface CD83 expression as previously reported (Figure 1A).20 Compared with macrophages, DCs also exhibited more significant increase in CD40, CD80, and CD86 expression on activation. These 4 types of cell were used for microarray studies.
Monocyte, DC, and macrophage phenotypic and microarray analysis. (A) Monocytes and in vitro cultured DCs and macrophages were stained for surface expression of the indicated molecules and analyzed by flow cytometry (dotted lines). DCs and macrophages, activated for 48 hours with LPS, were similarly stained (solid lines). Filled histograms represent isotype mouse IgG controls. (B) Schematic illustration of the microarray hybridization plan. RNA was isolated from monocytes, macrophages, imDCs, and mDCs for serial microarray hybridization as indicated with arrow lines, after a single round of linear mRNA amplification.21 (C) Hierarchic clustering of monocytes, macrophages, imDCs, and mDCs. Samples were clustered on the basis of their overall similarity using a truncated 578-gene set (see “Data processing and analysis” for selection criteria) and the results visualized using the Treeview program. Red bars indicate up-regulation, whereas green bars indicate down-regulation. Samples self-associated by cell type of origin (monocytes, blue; macrophages, green; imDCs, pink; mDCs, red). imDCs and mDCs lie in a separate branch from monocytes and macrophages.
Monocyte, DC, and macrophage phenotypic and microarray analysis. (A) Monocytes and in vitro cultured DCs and macrophages were stained for surface expression of the indicated molecules and analyzed by flow cytometry (dotted lines). DCs and macrophages, activated for 48 hours with LPS, were similarly stained (solid lines). Filled histograms represent isotype mouse IgG controls. (B) Schematic illustration of the microarray hybridization plan. RNA was isolated from monocytes, macrophages, imDCs, and mDCs for serial microarray hybridization as indicated with arrow lines, after a single round of linear mRNA amplification.21 (C) Hierarchic clustering of monocytes, macrophages, imDCs, and mDCs. Samples were clustered on the basis of their overall similarity using a truncated 578-gene set (see “Data processing and analysis” for selection criteria) and the results visualized using the Treeview program. Red bars indicate up-regulation, whereas green bars indicate down-regulation. Samples self-associated by cell type of origin (monocytes, blue; macrophages, green; imDCs, pink; mDCs, red). imDCs and mDCs lie in a separate branch from monocytes and macrophages.
Gene-expression profiles of monocytes, macrophages, imDCs, and mDCs are distinct from each other
To identify genes that are preferentially expressed in DCs, especially imDCs, the gene-expression profiles of monocytes, macrophages, imDCs, and mDCs were serially compared using cDNA microarrays containing approximately 13 000 array targets. The hybridization scheme is summarized in Figure 1B. To control for possible experimental variability, the hybridizations for each cell type were performed using 3 independent batches of pooled RNA, leading to a total of 12 samples (3 for each cell type), where each batch was equally pooled from 2 to 3 healthy donors to minimize individual donor variability.
After hybridization and scanning, approximately 8000 array elements were found to exhibit fluorescence signals significantly above background levels. To identify genes in this data set that were specifically regulated across all 4 cell types, we selected array elements that exhibited a minimum of 3-fold regulation across at least 3 of the arrays. This secondary filter resulted in a truncated data set of 578 genes. To identify global patterns of regulation in this reduced data set, a 2-way hierarchic clustering algorithm23 was applied to cluster both the genes and samples in order of their similarity to one another (Figure 1C). The 12 samples were found segregated into 4 broad branches, each branch representing a different cell type. Consistent with their lineages, imDCs and mDCs occupy a separate branch from monocytes and macrophages, suggesting that imDCs and mDCs are more related to one another than to the latter 2 cell types.
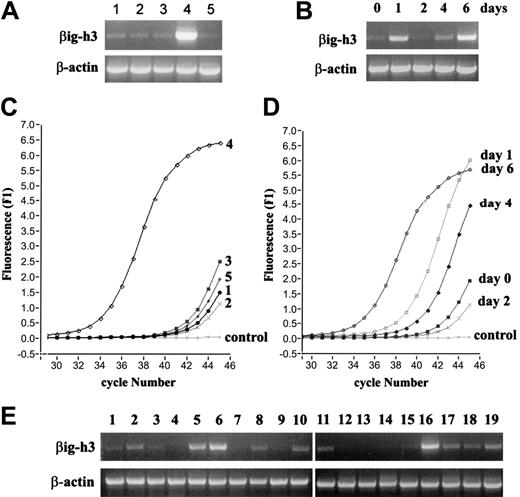
Examination of βig-h3 gene expression by RT-PCR. (A) Preferential βig-h3 gene expression in imDCs. RNA was isolated from: (1) monocytes, (2) macrophages, (3) LPS-activated macrophages, (4) imDCs, and (5) mDCs. RT-PCR was performed using βig-h3–specific and β-actin primers. (B) Monocytes were cultured in the presence of GM-CSF and IL-4 and harvested at the indicated time points up to 6 days. RNA was isolated from these cells and used in RT-PCR. (C) Real-time PCR for βig-h3 expression in the different cell types. RNA samples obtained as in panel A were subjected to 45 cycles of PCR using the Light Cycler and FastStart DNA Master SYBR Green I kit. “Control” indicates that cDNA template was omitted. β-Actin expression in the 5 RNA samples was similarly examined which gave Ct values of 36.48 to 37.15 showing little variation in cDNA inputs between different samples. (D) Real-time PCR analysis of βig-h3 expression during monocyte differentiation into imDCs. The RNA samples are as in panel B. The Ct values for β-actin are 31.92 (day 0), 31.47 (day 1), 32.52 (day 2), 32.03 (day 4), and 31.91 (day 6). These variations do not significantly affect the conclusion drawn based on the βig-h3 expression data. Melting curves for all reactions exhibited single specific PCR product (data not shown). (E) RT-PCR was performed with 19 human tissue RNA samples using βig-h3 primers. The RNA samples are: (1) adrenal gland, (2) bone marrow, (3) brain, (4) fetal brain, (5) colon, (6) small intestine, (7) kidney, (8) liver, (9) fetal liver, (10) lung, (11) placenta, (12) prostate, (13) salivary gland, (14) skeletal muscle, (15) spinal cord, (16) spleen, (17) testis, (18) trachea, and (19) uterus. PCR reactions in panels A-B and E were separated on 1% (wt/vol) agarose gels and visualized using ethidium bromide.
Examination of βig-h3 gene expression by RT-PCR. (A) Preferential βig-h3 gene expression in imDCs. RNA was isolated from: (1) monocytes, (2) macrophages, (3) LPS-activated macrophages, (4) imDCs, and (5) mDCs. RT-PCR was performed using βig-h3–specific and β-actin primers. (B) Monocytes were cultured in the presence of GM-CSF and IL-4 and harvested at the indicated time points up to 6 days. RNA was isolated from these cells and used in RT-PCR. (C) Real-time PCR for βig-h3 expression in the different cell types. RNA samples obtained as in panel A were subjected to 45 cycles of PCR using the Light Cycler and FastStart DNA Master SYBR Green I kit. “Control” indicates that cDNA template was omitted. β-Actin expression in the 5 RNA samples was similarly examined which gave Ct values of 36.48 to 37.15 showing little variation in cDNA inputs between different samples. (D) Real-time PCR analysis of βig-h3 expression during monocyte differentiation into imDCs. The RNA samples are as in panel B. The Ct values for β-actin are 31.92 (day 0), 31.47 (day 1), 32.52 (day 2), 32.03 (day 4), and 31.91 (day 6). These variations do not significantly affect the conclusion drawn based on the βig-h3 expression data. Melting curves for all reactions exhibited single specific PCR product (data not shown). (E) RT-PCR was performed with 19 human tissue RNA samples using βig-h3 primers. The RNA samples are: (1) adrenal gland, (2) bone marrow, (3) brain, (4) fetal brain, (5) colon, (6) small intestine, (7) kidney, (8) liver, (9) fetal liver, (10) lung, (11) placenta, (12) prostate, (13) salivary gland, (14) skeletal muscle, (15) spinal cord, (16) spleen, (17) testis, (18) trachea, and (19) uterus. PCR reactions in panels A-B and E were separated on 1% (wt/vol) agarose gels and visualized using ethidium bromide.
A closer examination of the 578 genes revealed 121 genes that displayed highly consistent signal values across the 3 independent serial hybridizations. Of this 121-gene set, the expression of 55 genes was found to be preferentially increased or decreased in imDCs or mDCs or both (Table 1), whereas another 38 genes were globally increased or decreased in macrophages and DCs compared with the monocyte precursors (Table S1, available on the Blood website; see the Supplemental Tables link at the top of the online article). In addition, 28 genes were preferentially up-regulated in macrophages (Table S2). In the present study, we focus on genes that are preferentially up-regulated in imDCs.
βIg-h3 expression is associated with immature DCs
Among the 15 genes that are preferentially up-regulated in imDCs, the βig-h3 gene (TGFBI) is most prominent (Table 1). Using RT-PCR, we confirmed that βig-h3 mRNA is low in monocytes and macrophages and is abundant in imDCs, suggesting its selective up-regulation in imDCs (Figure 2A). Expression of βig-h3 was diminished when DCs were activated with LPS (Figure 2A), but activated macrophages showed no significant change in their low basal level of βig-h3 mRNA. βig-h3 expression is therefore associated with the immature state of DCs. The expression of βig-h3 mRNA was also examined by RT-PCR following a 6-day course of imDC culturing (0, 1, 2, 4, and 6 days). βig-h3 mRNA was transiently up-regulated within the first 24 hours of culturing but returned to the low basal level at day 2 (Figure 2B). It increased again at day 4 and became highly prominent on day 6 when the cells have acquired imDC properties (Figure 2B). Real-time PCR was performed with these RNA samples and the results showed similar patterns of βig-h3 expression (Figure 2C-D). This result shows that high βig-h3 expression is associated with the immature state of DCs.
βig-h3 mRNA is abundant in lymphoid tissues
To our knowledge, this is the first report of selective βig-h3 up-regulation in imDCs. In previous studies, βig-h3 was detected mainly in cell types other than leukocytes.27 We therefore examined βig-h3 expression in 19 different human tissues by RT-PCR and observed that it was highly expressed in the spleen and bone marrow and also abundant in colon and small intestines, which are rich in lymphoid tissues (Figure 2E). Its expression in the lung, liver, placenta, testis, trachea, and uterus was also significant. Although the cellular origin of the detected tissue βig-h3 mRNA is not clear, its association with lymphoid-rich tissues implies a role in immunologic mechanisms.
βig-h3 is a 70-kDa secreted protein
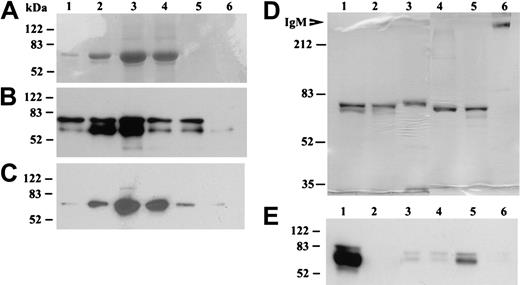
To examine the functional properties of βig-h3, we expressed this protein in 293T cells with C-terminal myc and His tags and purified this protein using Ni-NTA-agarose. βig-h3 was eluted in 6 stepwise fractions, with βig-h3 usually eluting in fractions 2 to 5 as a protein of about 70 kDa (Figure 3A). This 70-kDa protein reacted with a polyclonal anti–βig-h3 antibody (Figure 3B) and a monoclonal anti-myc antibody (Figure 3C). Based on its sequence, a mature βig-h3 polypeptide is expected to contain 668 amino acids (GenBank accession no. NM_000358), which predicts a polypeptide of about 75 kDa, and it is at present not clear why the observed molecular mass of the purified βig-h3 is smaller than this. The lack of potential Asn-linked glycosylation site in βig-h3 and neutral PI (7.6) cannot explain this apparent molecular weight reduction. Under nonreducing conditions, βig-h3 also migrated mainly as a 70-kDa protein irrespective of the presence or absence of iodoacetamide during its purification (Figure 3D). This result shows that under these experimental conditions βig-h3 may not exist as a disulfide-linked oligomer although native βig-h3 purified from tissues was previously observed as a fibrillike structure involving disulfide bonding.28
Purification of recombinant βig-h3 and identification of its secretion by imDCs. (A-C) Elution fractions of βig-h3 from the Ni-NTA-agarose column. 293T medium was mixed with Ni-NTA-agarose and bound βig-h3 was eluted using imidazole collecting 6 fractions (0.15 mL); 25 μL of each fraction was subjected to 10% SDS-PAGE under reducing conditions. The gels were either stained with Coomassie blue (A) or electroblotted. The blots were developed with either an anti–βig-h3 antibody (B) or an anti-myc antibody (C). (D) Purified βig-h3 and, as molecular weight standards, human IgM (Sigma-Aldrich) were subjected to 7.5% SDS-PAGE with (lanes 1-3) or without (lanes 4-6) 2-mercaptoethanol treatment. βIg-h3 was also purified in the presence (lanes 1 and 4) or absence (lanes 2 and 5) of iodoacetamide. As a control, purified human IgM was similarly analyzed (lanes 3 and 6). The gel was stained with Coomassie blue. (E) Media from 293T cells transfected with pβig-h3-MH (lane 1) or pcDNA3.1 (lane 2) or media from monocytes (lane 3), macrophages (lane 4), imDCs (lane 5), and mDCs (lane 6) were subjected to 10% SDS-PAGE, under reducing conditions, followed by Western blotting with a mouse anti–human βig-h3 antiserum. All molecular weight standards are in kilodaltons.
Purification of recombinant βig-h3 and identification of its secretion by imDCs. (A-C) Elution fractions of βig-h3 from the Ni-NTA-agarose column. 293T medium was mixed with Ni-NTA-agarose and bound βig-h3 was eluted using imidazole collecting 6 fractions (0.15 mL); 25 μL of each fraction was subjected to 10% SDS-PAGE under reducing conditions. The gels were either stained with Coomassie blue (A) or electroblotted. The blots were developed with either an anti–βig-h3 antibody (B) or an anti-myc antibody (C). (D) Purified βig-h3 and, as molecular weight standards, human IgM (Sigma-Aldrich) were subjected to 7.5% SDS-PAGE with (lanes 1-3) or without (lanes 4-6) 2-mercaptoethanol treatment. βIg-h3 was also purified in the presence (lanes 1 and 4) or absence (lanes 2 and 5) of iodoacetamide. As a control, purified human IgM was similarly analyzed (lanes 3 and 6). The gel was stained with Coomassie blue. (E) Media from 293T cells transfected with pβig-h3-MH (lane 1) or pcDNA3.1 (lane 2) or media from monocytes (lane 3), macrophages (lane 4), imDCs (lane 5), and mDCs (lane 6) were subjected to 10% SDS-PAGE, under reducing conditions, followed by Western blotting with a mouse anti–human βig-h3 antiserum. All molecular weight standards are in kilodaltons.
To examine whether βig-h3 is secreted by imDCs, serum-free media from imDCs and, as comparison, monocytes, macrophages, and mDCs were examined by Western blotting. As controls, media were also collected from 293T cells transfected with pβig-h3-MH or pcDNA3.1. βig-h3 was prominently detected in the media of imDCs and 293T cells transfected with pβig-h3-MH but not the other cells (Figure 3E). These results show that βig-h3 is secreted by imDCs.
βig-h3 binds to imDCs and macrophages
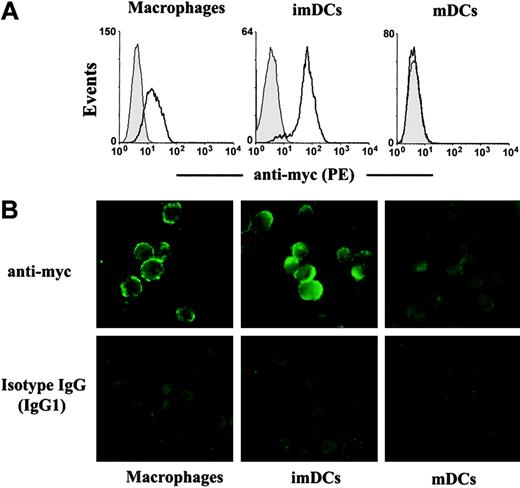
We then examined whether βig-h3 binds to imDCs by flow cytometry. imDCs were washed and then incubated with βig-h3. Bound βig-h3 was detected using an anti-myc antibody followed by fluorescent goat anti–mouse IgG. βig-h3 bound prominently to imDCs and to macrophages (Figure 4A), but showed no detectable binding to mDCs (Figure 4A). When these cells were examined by fluorescence microscopy, βig-h3 was also shown to bind to imDCs and macrophages but not mDCs (Figure 4B). These results raise the possibility that βig-h3 may regulate imDC activities in an autocrine manner and may also regulate macrophage function through paracrine stimulation. Interestingly, we note that the inability of βig-h3 to bind to mDCs may be consistent with its diminished expression in these matured DCs.
Binding of βig-h3 to imDCs and macrophages but not mDCs. Macrophages, imDCs, and mDCs were incubated with purified βig-h3 at 4°C. Bound βig-h3 was detected with a mouse anti-myc antibody followed by PE-labeled goat anti–mouse IgG. (A) The cells were washed and then analyzed by flow cytometry. Filled histograms are signals obtained with isotype IgG. Solid lines indicate signals obtained with the anti-myc antibody. (B) Similarly stained cells were washed and examined under a fluorescence microscope.
Binding of βig-h3 to imDCs and macrophages but not mDCs. Macrophages, imDCs, and mDCs were incubated with purified βig-h3 at 4°C. Bound βig-h3 was detected with a mouse anti-myc antibody followed by PE-labeled goat anti–mouse IgG. (A) The cells were washed and then analyzed by flow cytometry. Filled histograms are signals obtained with isotype IgG. Solid lines indicate signals obtained with the anti-myc antibody. (B) Similarly stained cells were washed and examined under a fluorescence microscope.
βig-h3 stimulates macrophage endocytosis
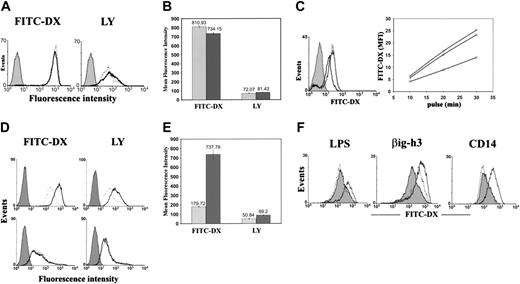
The high βig-h3 expression by imDCs and its ability to bind to imDCs prompted us to examine whether it regulates imDC-related functions. We first examined whether it stimulates imDC endocytosis or macropinocytosis using 2 soluble fluorescent probes, FITC-DX and LY. imDCs are constitutively potent in FITC-DX uptake and also exhibit significant uptake of LY (Figure 5A). The mean fluorescence index (MFI) for FITC-DX and LY was 810.93 and 72.07, respectively (Figure 5B). Prestimulation of imDCs for 30 minutes with purified βig-h3 did not change imDC endocytosis (Figure 5A). However, preincubation of imDCs with affinity-purified anti–βig-h3 antibodies could inhibit subsequent imDC uptake of FITC-DX by approximately 50% (Figure 5C). Nonimmune mouse IgG showed no significant inhibition. Indirectly, this suggests a possible autocrine regulation of imDC endocytosis through secreted βig-h3.
Because βig-h3 binds to macrophages (Figure 4A), we then examined whether it regulates macrophage endocytosis. Macrophages exhibit intermediate levels of FITC-DX and LY endocytosis without stimulation (Figure 5D). Stimulation of macrophages with βig-h3 increased FITC-DX uptake 3-fold (MFI = 737.79; Figure 5E). The uptake of LY was also increased 75% (MFI = 89.2; Figure 5E). In fact, βig-h3–stimulated macrophages exhibited a rate of FITC-DX and LY uptake comparable to imDCs. These results suggest a possible paracrine regulation of macrophage endocytosis by imDCs through βig-h3. In contrast, βig-h3 was unable to stimulate mDC endocytosis. mDCs exhibited low endocytosis that could not be stimulated by βig-h3 (Figure 5D). This is consistent with the lack of βig-h3 binding to mDCs.
LPS stimulates endocytosis.29 This was also observed in this study and it was inhibited by polymyxin B (Figure 5F). However, polymyxin B showed no inhibition of βig-h3–induced endocytosis (Figure 5F). Instead, it slightly enhanced βig-h3–stimulated macrophage endocytosis. Similarly purified CD14 could not stimulate macrophage endocytosis showing insignificant LPS contamination in proteins purified with this protocol. Therefore, βig-h3 stimulates macrophage endocytosis.
βig-h3 induces membrane ruffles
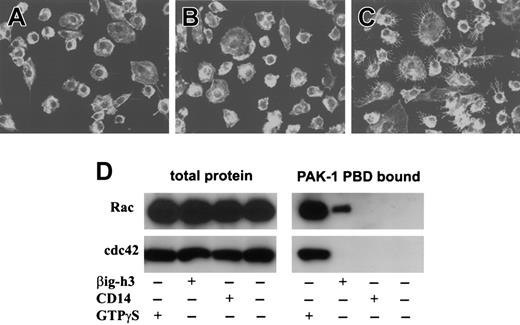
Because βig-h3 stimulates macrophage endocytosis or macropinocytosis, we examined whether it involves the activation of membrane ruffling. Macrophages, stimulated with purified βig-h3, were fixed and stained using phalloidin-rhodamine.25 As shown in Figure 6A, untreated macrophages had few membrane ruffles. Stimulation of macrophages with CD14 did not induce significant membrane ruffles (Figure 6B). However, βig-h3–stimulated macrophages displayed extensive membrane ruffling (Figure 6C). Therefore, βig-h3 probably induces macrophage endocytosis or macropinocytosis through activation of membrane ruffles.
βig-h3 activates the small GTPase Rac but not cdc42
To examine whether βig-h3 activates Rac and cdc42, macrophages were stimulated with βig-h3 and then examined for Rac or cdc42 activation. As positive controls, both Rac-GTPγS and cdc42-GTPγS were precipitated by PAK-1 PBD-agarose (Figure 6D). Although activated Rac was also detected in the lysate of βig-h3–stimulated macrophages, no activated cdc42 was detected (Figure 6D). As negative controls, neither Rac nor cdc42 was precipitated from unstimulated or CD14-stimulated macrophages (Figure 6E). This result shows that βig-h3 stimulation of macrophages activates Rac but not cdc42, implying that Rac activation may be required in βig-h3–elicited macrophage membrane ruffling and endocytosis.
Endocytosis of FITC-DX and LY by imDCs, macrophages, and mDCs. (A) imDCs were incubated for 30 minutes in the presence (solid lines) or absence (dotted lines) of βig-h3 (10 μg/mL). FITC-DX or LY was then added and incubated for 30 minutes. The cells were washed and analyzed by flow cytometry. Filled histograms represent cells incubated with FITC-DX or LY at 4°C (negative controls). (B) Quantification of imDC endocytosis. The MFIs of 3 independent experiments as shown in panel A are presented as mean ± SD. ▦, endocytosis by unstimulated imDCs; ▪, endocytosis by βig-h3–stimulated imDCs. (C) Inhibition of imDC endocytosis by purified anti–βig-h3 mouse IgG. (Left) imDCs were either untreated (solid line) or preincubated for 1 hour with the affinity-purified βig-h3 antibody (bold line) or nonimmune mouse IgG (dotted line). The cells were then incubated with FITC-DX (20 μg/mL) for 20 minutes and were, on washing, analyzed by flow cytometry. Solid histograms represent cells incubated with FITC-DX on ice. (Right) imDCs were either untreated (○) or pretreated with the affinity-purified anti–βig-h3 antibody (□) or nonimmune mouse IgG (▵) and then incubated with FITC-DX for 10, 20, or 30 minutes before flow cytometry. (D) Macrophages (top panels) and mDCs (bottom panels) were incubated with (solid lines) or without (dotted lines) βig-h3 prior to 30 minutes' incubation with FITC-DX or LY. Filled histograms represent cells incubated with FITC-DX or LY for 30 minutes at 4°C. (E) Quantification of macrophage endocytosis. The MFI of 3 independent experiments as shown in panel D are presented as mean ± SD. ▦, endocytosis by macrophages, which were not prestimulated with βig-h3; ▪, endocytosis by βig-h3–stimulated macrophages. (F) Polymyxin B inhibits LPS-stimulated, but not βig-h3–stimulated, macrophage endocytosis of FITC-DX. (Left) LPS was preincubated with (dotted lines) or without (solid lines) polymyxin B before incubation with macrophages. The macrophages were then incubated with FITC-DX and analyzed by flow cytometry. (Middle) βig-h3 was similarly preincubated with (solid line) or without (dotted line) polymyxin B before incubation with FITC-DX. (Right) CD14 (dotted line) was compared with βig-h3 (solid line) in stimulating macrophage endocytosis of FITC-DX. The filled histograms represent FITC-DX endocytosis by unstimulated macrophages.
Endocytosis of FITC-DX and LY by imDCs, macrophages, and mDCs. (A) imDCs were incubated for 30 minutes in the presence (solid lines) or absence (dotted lines) of βig-h3 (10 μg/mL). FITC-DX or LY was then added and incubated for 30 minutes. The cells were washed and analyzed by flow cytometry. Filled histograms represent cells incubated with FITC-DX or LY at 4°C (negative controls). (B) Quantification of imDC endocytosis. The MFIs of 3 independent experiments as shown in panel A are presented as mean ± SD. ▦, endocytosis by unstimulated imDCs; ▪, endocytosis by βig-h3–stimulated imDCs. (C) Inhibition of imDC endocytosis by purified anti–βig-h3 mouse IgG. (Left) imDCs were either untreated (solid line) or preincubated for 1 hour with the affinity-purified βig-h3 antibody (bold line) or nonimmune mouse IgG (dotted line). The cells were then incubated with FITC-DX (20 μg/mL) for 20 minutes and were, on washing, analyzed by flow cytometry. Solid histograms represent cells incubated with FITC-DX on ice. (Right) imDCs were either untreated (○) or pretreated with the affinity-purified anti–βig-h3 antibody (□) or nonimmune mouse IgG (▵) and then incubated with FITC-DX for 10, 20, or 30 minutes before flow cytometry. (D) Macrophages (top panels) and mDCs (bottom panels) were incubated with (solid lines) or without (dotted lines) βig-h3 prior to 30 minutes' incubation with FITC-DX or LY. Filled histograms represent cells incubated with FITC-DX or LY for 30 minutes at 4°C. (E) Quantification of macrophage endocytosis. The MFI of 3 independent experiments as shown in panel D are presented as mean ± SD. ▦, endocytosis by macrophages, which were not prestimulated with βig-h3; ▪, endocytosis by βig-h3–stimulated macrophages. (F) Polymyxin B inhibits LPS-stimulated, but not βig-h3–stimulated, macrophage endocytosis of FITC-DX. (Left) LPS was preincubated with (dotted lines) or without (solid lines) polymyxin B before incubation with macrophages. The macrophages were then incubated with FITC-DX and analyzed by flow cytometry. (Middle) βig-h3 was similarly preincubated with (solid line) or without (dotted line) polymyxin B before incubation with FITC-DX. (Right) CD14 (dotted line) was compared with βig-h3 (solid line) in stimulating macrophage endocytosis of FITC-DX. The filled histograms represent FITC-DX endocytosis by unstimulated macrophages.
Membrane ruffling and Rac activation in βig-h3–stimulated macrophages. Macrophages cultured on glass coverslips were either untreated (A) or stimulated with purified CD14 (B) or βig-h3 (C). The cells were fixed in formaldehyde and examined using a LSM510 laser-scanning microscope and Zeiss LSM Image Browser software. (D) Rac and cdc42 activation in βig-h3–stimulated macrophages. Macrophages were stimulated with βig-h3 or CD14 for 30 minutes or unstimulated before lysis. The cleared cell lysates were incubated with PAK-1 PBD-agarose. As a positive control, the unstimulated cell lysate was incubated with GTPγS before incubation with PAK-1 PBD-agarose. The bound proteins were subjected to Western blotting using anti-Rac and anti-cdc42 antibodies as indicated. As controls, an equal volume of total macrophage lysate for each treatment was also similarly analyzed. The blots were developed using alkaline phosphatase–conjugated goat anti–mouse IgG.
Membrane ruffling and Rac activation in βig-h3–stimulated macrophages. Macrophages cultured on glass coverslips were either untreated (A) or stimulated with purified CD14 (B) or βig-h3 (C). The cells were fixed in formaldehyde and examined using a LSM510 laser-scanning microscope and Zeiss LSM Image Browser software. (D) Rac and cdc42 activation in βig-h3–stimulated macrophages. Macrophages were stimulated with βig-h3 or CD14 for 30 minutes or unstimulated before lysis. The cleared cell lysates were incubated with PAK-1 PBD-agarose. As a positive control, the unstimulated cell lysate was incubated with GTPγS before incubation with PAK-1 PBD-agarose. The bound proteins were subjected to Western blotting using anti-Rac and anti-cdc42 antibodies as indicated. As controls, an equal volume of total macrophage lysate for each treatment was also similarly analyzed. The blots were developed using alkaline phosphatase–conjugated goat anti–mouse IgG.
Discussion
Microarray and other approaches have been used in previous studies to identify genes that are preferentially expressed in DCs and related cell types as summarized by Tang and Saltzman.30 In this report, we compared the gene-expression profiles of monocytes, macrophages, imDCs, and mDCs serially with particular interests in identifying genes that are preferentially regulated in imDCs (Figure 1B). The reliability of the expression data identified for 121 differentially expressed genes is supported by reports of similar expression profiles of many of these genes.30
One subset of genes is related to cell migration or motility. CCR1 and CCR7 were found to be preferentially expressed by imDCs and mDCs, respectively (Table 1). This switch from CCR1 to CCR7 is consistent with previous reports and is in line with DC migration from peripheral to lymphoid tissues on activation.7-11 RGS1 regulates chemokine receptor signaling and it has been shown to be up-regulated during DC maturation (Table 1).31,32 The Wiskott-Aldrich Syndrome protein (WASp) and the subunit 4 of actin-related protein 2/3 (Arp2/3), which are required for the podosome formation at the front edges of imDCs or DC migration,33 are up-regulated in DCs (Table 1). Rho GDP dissociation inhibitor β (RhoGDIβ), which regulates Rho GTPase activation, was selectively up-regulated in imDCs (Table 1).33
The M-CSF receptor was highly up-regulated in imDCs, but down-regulated on activation (Table 1). Indeed, M-CSF was shown to revert imDC, but not mDC, differentiation into macrophages.34 Compared with monocytes, imDCs and mDCs have reduced G-CSF receptor 1 expression (Table 1), which is generally consistent with monocytes typically ceasing to proliferate after 2 days in culture.35 The elevated expression of IFNγ receptor 2 in DCs (Table 1) is consistent with potent IFNγ costimulation of IL-12 production in DCs.36
Surface CD83 is a hallmark of human DC activation,37 but differential CD83 mRNA expression was not observed in the microarray results. In fact, CD83 mRNA was detected in all 4 cell types although, at the protein level, it was only stably expressed on mDCs.20 Monocytes and macrophages only transiently express surface CD83.20
Among genes that are preferentially up-regulated in imDCs, a gene encoding βig-h3 was most prominent (Table 1). Previous studies have described βig-h3 as an extracellular matrix (ECM) protein secreted by nonleukocytes, which regulates cell proliferation, differentiation, and adhesion.27,28 We observed that βig-h3 mRNA was more abundant in lymphoid-rich tissues. At the cellular level, its expression is selectively up-regulated during monocyte differentiation into imDCs. It was also transiently induced after monocytes were cultured for 24 hours with GM-CSF and IL-4 but this did not sustain beyond 2 days (Figure 2B). It is not clear whether this was simply due to transient GM-CSF and IL-4 induction. TGF-β1 could not induce βig-h3 mRNA expression in monocytes (data not shown). Its expression became stabilized from day 4 of the culture when the cells also acquired DC properties. By differential display and microarray, βig-h3 expression in DCs has also been reported by others but not further investigated.38,39 The specific role of βig-h3 expression with regards to DC functions in vivo remains to be investigated.
Our demonstration of imDC-associated βig-h3 expression and its ability to stimulate endocytosis represents a novel observation for both βig-h3 and imDCs/macrophages. The ability of βig-h3 to bind to macrophages, to activate Rac, and to induce membrane ruffles are all in line with its ability to stimulate macrophage endocytosis. In vivo, how βig-h3–stimulated macrophage endocytosis affects macrophage functions remains to be investigated. imDCs are likely to lose βig-h3 production under inflammatory conditions when these cells become mDCs. Therefore, it is possible that imDC stimulates macrophage and its own endocytosis of self-antigens under steady state to promote self-tolerance.
Addition of βig-h3 to the culture did not affect the constitutively potent endocytic activities of imDCs. It is possible that βig-h3 secreted by imDCs binds to imDCs rapidly with high affinity to maintain potent endocytosis. To assess the role of βig-h3 in imDC endocytosis, βig-h3–specific siRNA was used to transfect imDCs. But the transfection experiments were not successful. However, we have demonstrated that imDC endocytosis was inhibited by affinity-purified anti–βig-h3 antibodies. The βig-h3–stimulated macrophage endocytosis was not due to contaminating LPS in the purified βig-h3 but the protein itself. This was demonstrated by the lack of inhibition of βig-h3–stimulated macrophage endocytosis by polymyxin B, which inhibited LPS-stimulated macrophage endocytosis. Similarly purified CD14 exhibited no macrophage endocytosis, enhancement of showing that this protocol of protein expression and purification does not usually result in significant LPS contamination.
The βig-h3 polypeptide is dominated by 4 tandem fas-1 domains, defined based on homology with the Drosophila protein fasciclin-I.40 Although our results suggest a role for βig-h3 in the regulation of macrophage endocytosis, previous studies suggest a role of βig-h3 as an ECM protein that regulates cell adhesion, differentiation, and proliferation.27,41-44 Our demonstration of the lack of disulfide-linked βig-h3 oligomerization is also in contrast with previous detection of native tissue βig-h3 as highly oligomerized fibrils. It is possible that βig-h3 is found in ECM-associated and soluble forms, depending on the tissue compartments or cell types where it is produced, and the 2 forms may exhibit distinct functional properties. An ECM-related βig-h3 function is also supported by the fact that mutations in βig-h3, for example, the residue Arg124His mutation, are associated with lattice cornea dystrophy or granular dystrophy in which βig-h3 was found degraded and abnormally located.45,46 We introduced the Arg124His mutation in the recombinant βig-h3 but it remained potent in stimulating macrophage endocytosis (data not shown). Indeed, immunologic malfunctions have not been reported in these patients.
Although ECM-related functions of βig-h3 have been reported, the abundant βig-h3 mRNA detected in lymphoid tissues and its secretion by imDCs strongly suggests a role for βig-h3 in immune regulation. Our demonstration of βig-h3 stimulation of macrophage endocytosis is consistent with such a role.
Prepublished online as Blood First Edition Paper, December 20, 2005; DOI 10.1182/blood-2005-05-1803.
Supported by National Medical Research Council grants R-182-000-081-213 and R-364-000-019-213 (J.L.) and by a National Cancer Center Core Grant and a Biomedical Research Council Grant 01/1/31/19/209 (P.T.).
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
P.T. thanks Prof Hui Kam Man for support and encouragement.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal