Abstract
Fas (CD95/Apo-1) ligand-mediated apoptosis induction of target cells is one of the major effector mechanisms by which cytotoxic lymphocytes (T cells and natural killer cells) kill their target cells. In T cells, Fas ligand expression is tightly regulated at a transcriptional level through the activation of a distinct set of transcription factors. Increasing evidence, however, supports an important role for posttranscriptional regulation of Fas ligand expression and activity. Lipid rafts are cholesterol- and sphingolipid-rich membrane microdomains, critically involved in the regulation of membrane receptor signaling complexes through the clustering and concentration of signaling molecules. Here, we now provide evidence that Fas ligand is constitutively localized in lipid rafts of FasL transfectants and primary T cells. Importantly, disruption of lipid rafts strongly reduces the apoptosis-inducing activity of Fas ligand. Localization to lipid rafts appears to be predominantly mediated by the characteristic cytoplasmic proline-rich domain of Fas ligand because mutations of this domain result in reduced recruitment to lipid rafts and attenuated Fas ligand killing activity. We conclude that Fas ligand clustering in lipid rafts represents an important control mechanism in the regulation of T cell–mediated cytotoxicity.
Introduction
Induction of cell death by apoptosis not only plays a crucial role in development and tissue remodeling, but also in the killing of target cells by cytotoxic lymphocytes, for example, T cells and natural killer (NK) cells. Two major mechanisms of cell-mediated cytotoxicity have been described: a perforin/granzyme-mediated and a death ligand-mediated pathway (for reviews, see Trambas and Griffiths,1 Russell and Ley,2 and Froelich et al3 ). Death ligands are apoptosis-inducing members of the tumor necrosis factor (TNF) family and include TNFα, Fas (CD95/Apo-1) ligand (FasL), and TNF-related apoptosis-inducing ligand (TRAIL).4 All death ligands induce apoptosis through cognate interaction with their receptors (death receptors), which leads to the formation of a so-called death-inducing signaling complex (DISC) and the activation of caspases, a family of cysteine-aspartate proteases intimately involved in the cellular disintegration during apoptosis. Death ligands can be expressed by a variety of cell types; however, they are strongly expressed in activated T cells and NK cells. The role and regulation of the apoptosis-inducing activity is particularly well documented for FasL. FasL-mediated killing has mainly been implicated in cell-autonomous suicide of T cells,5-7 clearance of activated leukocytes,8 killing of virus-infected target cells,9 and tissue damage associated with immunopathologies such as hepatitis,9,10 graft-versus-host disease,11 and experimental allergic encephalomyelitis.12
FasL expression is tightly regulated at a transcriptional level. In T cells, a variety of transcription factors are involved in the regulation of FasL gene induction, including NFAT, Egr-2 and -3, NF-κB, AP-1, c-Myc, and Sp1 (for reviews, see Brunner et al13 and Kavurma and Khachigian14 ). Although transcriptional regulation of FasL is central for the appropriate expression in T cells on activation via target cells, increasing evidence indicates that FasL is also critically regulated at a posttranscriptional level. For example, cell surface FasL is rapidly cleaved by metalloproteases, providing an efficient mechanism for limiting its apoptosis-inducing activity.15,16 Similarly, we and others recently found that FasL can be stored in secretory lysosomes and is released to the cell surface on restimulation of the cell in a protein synthesis-independent manner.17,18 Biochemical and molecular analysis of this process revealed that intracellular storage of FasL and activation-induced transportation to the cell surface is regulated by its cytoplasmic tail.17,19 FasL contains a unique proline-rich domain, which is required for the sorting of FasL to secretory lysosomes. This proline-rich domain is thought to bind to SH3 domains of various signaling molecules. Thus, it has been shown that this domain associates with Fyn, Lck, Grb2, Nck, and other proteins, which thereby may regulate its subcellular transportation and localization (for a review, see Janssen et al20 ).
Lipid rafts are cholesterol- and sphingolipid-rich microdomains of the plasma membrane (reviewed by Cherukuri et al21 and Simons and Toomre22 ). The high content of saturated fatty acid side chains in lipid rafts allows a tight packaging of these membrane microdomains and associated membrane receptors and signaling molecules. Due to the close proximity of signaling molecules in lipid rafts they have been proposed as signaling platforms. Accordingly, signaling molecule clustering in lipid rafts critically modulates antigen receptor signaling in B and T cells. Recent evidence also supports a role of lipid rafts in apoptosis induction via death receptors (reviewed by Muppidi et al23 and Hueber24 ). For example, activation of TNF receptor 1 induces its recruitment to lipid rafts and this translocalization to these membrane microdomains is crucial for diverging the signal toward NF-κB or ERK1/2 signaling or apoptosis induction.25,26 Similarly, the Fas receptor is localized in lipid rafts, which appears to be important for its apoptosis-inducing activity in thymocytes27 and T cells.28 This role of lipid rafts in Fas signaling may, however, be cell type–specific, because other studies have found no evidence for lipid raft-mediated amplification of the Fas signaling pathway.25
Here, we now provide evidence that the ligand of Fas is also localized in lipid rafts of FasL-transfected cells and primary T cells. We further show that raft localization is critical for FasL activity because disruption of lipid rafts abrogates its killing activity. Raft localization of FasL appears to be mediated by the proline-rich domain in the cytoplasmic tail and mutants of FasL with altered SH3-binding domains show reduced raft localization and apoptosis-inducing activity. We conclude that posttranscriptional modification of FasL through concentration in lipid rafts is essential for its apoptosis-inducing activity.
Materials and methods
Cell lines and reagents
Human embryonic kidney fibroblasts 293T cells were obtained from the American Type Culture Collection (Manassas, VA); the murine T-cell hybridoma line A1.1 has been described previously.29 Stably Fas-transfected murine leukemic L1210 cells were a kind gift from P. Golstein (Marseille, France). All cell lines were cultured in Iscove modified Dulbecco medium (IMDM) containing 5% fetal calf serum (FCS), 2 mM l-glutamine, 100 U/mL penicillin, and 100 μg/mL streptomycin. All media components were purchased from Sigma (St Louis, MO). Phorbol myristate acetate (PMA), ionomycin, and n-octyl-glucopyranoside were from Calbiochem (VWR, Lucerne, Switzerland). Methyl-β-cyclodextrin (MbCD), filipin, and squalestatin were purchased from Sigma.
Generation of human T-cell blasts
Peripheral blood mononuclear cells were isolated from buffy coat preparations of healthy donors by Ficoll-Hypaque density gradient centrifugation. Cells were then activated with phytohemagglutinin-C (1 μg/mL) overnight, followed by a 7-day culture in complete IMDM containing 10% FCS, supplemented with rhIL-2 (100 U/mL; Chiron, Amsterdam, The Netherlands).
Plasmids
The generation of GFP-FasL constructs (full-length, Δ37 and Δ67; kindly provided by G. Griffiths, Oxford, United Kingdom) has been described previously.17,19 All constructs were subcloned without GFP tag into pcDNA3.0 and used for further experiments. pEF-Lck and p18S-Fyn plasmids were a kind gift from G. Bayer (Innsbruck, Austria).
Transfections
293T cells were transfected using the calcium phosphate method. After overnight incubation with the plasmids, the cells were washed with PBS and maintained for at least another 24 hours in complete IMDM before use in experiments.
Western and dot blotting
Proteins were separated on a denaturing 12% acrylamide gel, followed by transfer to nitrocellulose filter and probing with the following primary antibodies: anti-FasL (clone G247-4, 1:500; PharMingen, San Diego, CA), anti–flotillin-2 (1:5000; PharMingen), antitubulin (clone B-5-1-2, 1:5000; Sigma), anti-Lck (1:500; PharMingen), anti-Fyn (1:500; PharMingen), and anti–Zap-70 (clone 99F2, 1:1000; Cell Signaling Technology, Beverly, MA). As secondary antibodies, horseradish peroxidase-labeled goat antimouse and goat antirabbit (both 1:4000; Sigma) were used. Binding was detected by chemiluminescence using a Super Signal West Pico Kit (Pierce, Rockford, IL). In some experiments, the intensity of Western blot bands was analyzed by densitometry.
For GM1 dot blots, samples were dotted in nitrocellulose and GM1 was probed using the horseradish peroxidase-labeled β-subunit of cholera toxin (CTX, 1:5000, Sigma), followed by detection using chemiluminescence as described.
Isolation of lipid rafts
Lipid rafts were isolated using the 2 methods described previously.25 All rafts isolation steps were performed at 4°C. Briefly, cells were lysed in MN buffer (25 mM MES, 150 mM NaCl, pH 6.5) containing 0.5% to 1% Triton X-100, depending on the cell type. For the short method cleared lysates from 3 to 6 × 106 cells were centrifuged at 50 000g for 50 minutes. The supernatant was used as the nonraft fraction. The raft-enriched pellet was solubilized in 50 mM n-octyl-glucopyranoside and cleared from insoluble material by centrifugation. For the sucrose density gradient method, 2 mL of cleared lysate from 2 × 107 cells was mixed with an equal volume of 90% sucrose in MN buffer and overlaid with 4 mL 35% and 4 mL 5% sucrose in MN buffer. The gradient was centrifuged at 100 000g for 16 to 20 hours. Then 1-mL fractions were harvested with a syringe starting from the top. Typical raft markers (eg, GM1 and flotillin) were present in fractions 3 to 5, whereas nonraft protein mainly appeared in fractions 8 to 12.
Disruption of lipid rafts
Dilutions of MbCD and filipin were made in complete medium containing no FCS. Cells were washed twice in PBS to remove any serum followed by 30 minutes of incubation with MbCD (0-10 mM)25 or filipin (0-2 μg/mL).30 Cells were then washed with PBS and cultured in complete medium containing 5% FCS.
Squalestatin was diluted in complete medium containing 5% cholesterol-free FCS. Cholesterol-free FCS was prepared as previously described.31 Cells were cultured in medium with or without squalestatin (0-10 μM) for 48 hours before harvesting.32
Cell viability was confirmed using annexin V staining.33
Patching of lipid rafts
For visualization of lipid rafts, FasL-transfected 293T cells were stained with Alexa 555-labeled β subunit of CTX (1:3000, Molecular Probes, Eugene, OR), and cell surface FasL with anti-FasL (NOK-1.1, 1:300, eBioscience, San Diego, CA) or isotype control for 20 minutes at 4°C, followed by detection with goat anti–mouse FITC. Cells were then blocked with 1% mouse serum in PBS for 20 minutes at 4°C, prior to clustering of rafts with anti–β-CTX (1:300; Calbiochem) at 37°C for 20 minutes. Lipid rafts and FasL cell surface staining were visualized using confocal microscopy (image acquisition using a Zeiss LSM 510 microscope, a Zeiss Plan Apochromat 100× /1.4 objective, and Slowfade mounting medium [Molecular Probes]. Raw images were deconvolved using Huygens Essential software (SVI, Hilversum, The Netherlands) and colocalization analyses were performed with Imaris software (Bitplane, Zurich, Switzerland).
Flow cytometry
FasL-transfected 293T cells were stained with anti-FasL (NOK-1.1, 1:300; eBioscience) or isotype control, followed by staining with Alexa 488–labeled goat antimouse (1:3500; Molecular Probes). Staining was analyzed on a FACScan flow cytometer (BD Bioscience, San Jose, CA) using the FlowJo software (FlowJo, Ashland, OR).
Cytotoxicity assay
FasL-mediated cytotoxicity assays were performed as described previously.34 Briefly, FasL-transfected 293T cells or PMA-stimulated (20 ng/mL) and ionomycin-stimulated (200 ng/mL) A1.1 cells or human T-cell blasts were incubated with 3H-thymidine–labeled target cells (L1210-Fas) for 6 hours in 96-well flat-bottom plates at different effector-target (E/T) ratios. Cells were then lysed and unfragmented DNA was harvested on Unifilter-96 GF/C glass fiber plates (Perkin-Elmer, Boston, MA) and counted in a β counter. DNA fragmentation was calculated as follows: % DNA fragmentation = 100 ×(1 – cpm experimental group/cpm control group). In experiments using 293T cells transfected with different FasL mutants, unequal cell surface expression of the mutants17,19 was compensated by dividing % DNA fragmentation by the mean of fluorescence intensity of cell surface FasL staining, as determined by flow cytometry.
Results
Partial localization of FasL in lipid rafts
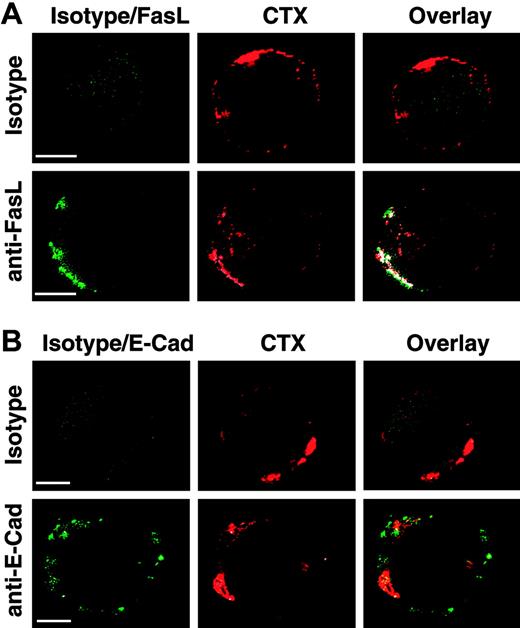
We initially aimed at analyzing whether FasL localization in lipid rafts can be visualized by fluorescence microscopy. Lipid rafts are small in size (< 50 nm)22 ; however, they can be clustered by patching to form large-scale aggregates that can be detected by conventional microscopy. HEK 293T cells were therefore transfected with a full-length FasL expression plasmid and we examined the cell surface localization of FasL by immunostaining. Lipid rafts were visualized by staining the cells with the β subunit of cholera toxin (CTX), which binds to GM1, a ganglioside preferentially localized in lipid rafts. Lipid rafts were then further clustered by cross-linking CTX with anti-CTX antibodies. Figure 1 illustrates that patched lipid rafts form large clusters that were easily detectable. Interestingly, FasL staining showed a high degree of colocalization with CTX (Figure 1A). In contrast, the transmembrane molecule E-cadherin, used as a negative control, showed a completely different staining pattern and did not colocalize with CTX (Figure 1B). Thus, these results suggested that FasL may be localized in lipid rafts.
Clustering of cell surface FasL in lipid rafts. 293T cells were transfected with a full-length FasL or E-cadherin expression plasmid and stained either with isotype control, anti-FasL, or anti-E-cadherin antibody (green). Lipid rafts were detected by staining with cholera toxin β subunit (CTX, red). Lipid raft clustering was induced by incubation with anti-CTX antibody. Confocal microscopy images for FasL(A) and E-cadherin (B) as nonraft-localized negative control are shown. Colocalization between lipid rafts and FasL, respectively. E-cadherin was analyzed using Imaris software and is depicted in white. Original magnification × 1000. Bars indicate 5 μm. A typical experiment of 5 is shown.
Clustering of cell surface FasL in lipid rafts. 293T cells were transfected with a full-length FasL or E-cadherin expression plasmid and stained either with isotype control, anti-FasL, or anti-E-cadherin antibody (green). Lipid rafts were detected by staining with cholera toxin β subunit (CTX, red). Lipid raft clustering was induced by incubation with anti-CTX antibody. Confocal microscopy images for FasL(A) and E-cadherin (B) as nonraft-localized negative control are shown. Colocalization between lipid rafts and FasL, respectively. E-cadherin was analyzed using Imaris software and is depicted in white. Original magnification × 1000. Bars indicate 5 μm. A typical experiment of 5 is shown.
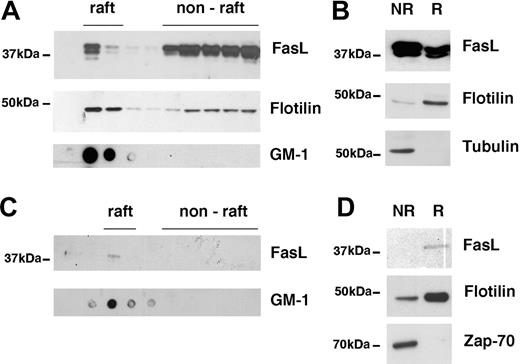
Partial localization of FasL in lipid rafts. (A) 293T cells were transfected with full-length FasL and lipid rafts were isolated using the sucrose gradient method. FasL and flotillin in the raft and nonraft fractions were detected by Western blot, GM1 by dot blot. A typical experiment of 6 is shown. (B) 293T cells were transfected with FasL as described and lipid rafts were isolated using the short method. FasL, flotillin, and tubulin were detected by Western blot. NR indicates nonraft fraction; R, raft fraction. A typical experiment of 20 is shown. (C) Human peripheral blood T-cell blasts were stimulated with PMA and ionomycin, and lipid rafts were isolated using the sucrose density method. FasL in the raft and nonraft fractions was detected by Western blot, GM1 by dot blot. A typical experiment of 3 is shown. (D) Detection of FasL, flotillin, and Zap-70 in nonraft (NR) and raft (R) fraction of human T-cell blasts using the short raft isolation method. A typical experiment of 3 is shown.
Partial localization of FasL in lipid rafts. (A) 293T cells were transfected with full-length FasL and lipid rafts were isolated using the sucrose gradient method. FasL and flotillin in the raft and nonraft fractions were detected by Western blot, GM1 by dot blot. A typical experiment of 6 is shown. (B) 293T cells were transfected with FasL as described and lipid rafts were isolated using the short method. FasL, flotillin, and tubulin were detected by Western blot. NR indicates nonraft fraction; R, raft fraction. A typical experiment of 20 is shown. (C) Human peripheral blood T-cell blasts were stimulated with PMA and ionomycin, and lipid rafts were isolated using the sucrose density method. FasL in the raft and nonraft fractions was detected by Western blot, GM1 by dot blot. A typical experiment of 3 is shown. (D) Detection of FasL, flotillin, and Zap-70 in nonraft (NR) and raft (R) fraction of human T-cell blasts using the short raft isolation method. A typical experiment of 3 is shown.
We next analyzed biochemically whether FasL resides in lipid rafts. Lipid rafts can be separated from cytoplasm and other parts of the cell membrane due to their insolubility in nonionic detergents at 4°C and flotation on a discontinuous sucrose gradient. FasL-transfected 293T cells were thus lysed in buffer containing Triton X-100 and the subcellular components were separated on a sucrose gradient.25 Lipid raft fractions were identified by detection of GM1 (Figure 2A). The quality of these raft preparations was further confirmed by detection of flotillin, a well-known raft-associated protein. Flotillin was primarily detected in the GM1+ fractions confirming its localization in lipid rafts. Interestingly, we observed that a significant proportion of FasL was found in raft fractions, indicating that FasL is constitutively associated with lipid rafts (Figure 2A).
The localization of FasL in lipid rafts was further confirmed using a different raft isolation protocol.25 This method allows the precipitation of nonionic detergent-insoluble lipid rafts and associated proteins by ultracentrifugation. Figure 2B shows a similar distribution pattern of FasL in the raft versus nonraft fraction. As a control, the raft marker flotillin was predominantly found in the raft fraction, whereas the cytoplasmic protein tubulin was exclusively detected in the nonraft fraction. We conclude that in transiently transfected 293T cells FasL localizes partially to membrane lipid rafts.
To exclude that raft localization in FasL-transfected 293T cells is an overexpression artifact, we also investigated FasL raft localization in normal human T cells. Peripheral blood mononuclear cells were stimulated with phytohemagglutinin, T cells were expanded with IL-2 for 7 days, and then shortly restimulated with PMA and ionomycin. This treatment leads to a significant induction of FasL protein expression and cell surface expression.35 Figure 2C shows that although FasL was expressed in normal T cells at lower levels than in FasL-transfected 293T cells, FasL was clearly detected in the raft (GM1+) fraction. This finding was further confirmed with the second raft isolation protocol, demonstrating that FasL was predominately detected in the raft fraction of activated T-cell blasts (Figure 2D). In contrast, the nonraft-associating protein Zap-70 was only found in the nonraft fraction. These data confirm that FasL is localized in lipid rafts of activated human T cells and FasL transfectants.
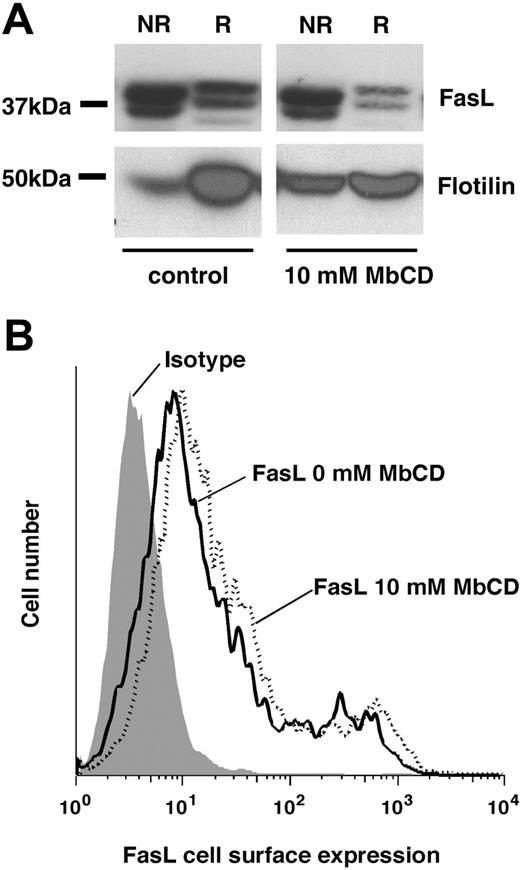
Methyl-β-cyclodextrin extrudes FasL from lipid rafts. (A) FasL-transfected 293T cells were treated for 30 minutes with control or 10 mM MbCD and rested for 30 minutes in medium, and lipid rafts were isolated. FasL and flotillin were detected by Western blot. NR indicates nonraft fraction; R, raft fraction. Typical experiments of 5 are shown. (B) FasL-transfected 293T cells were treated with MbCD as described and FasL cell surface expression was detected by flow cytometry. A typical experiment of 3 is shown.
Methyl-β-cyclodextrin extrudes FasL from lipid rafts. (A) FasL-transfected 293T cells were treated for 30 minutes with control or 10 mM MbCD and rested for 30 minutes in medium, and lipid rafts were isolated. FasL and flotillin were detected by Western blot. NR indicates nonraft fraction; R, raft fraction. Typical experiments of 5 are shown. (B) FasL-transfected 293T cells were treated with MbCD as described and FasL cell surface expression was detected by flow cytometry. A typical experiment of 3 is shown.
Disruption of lipid rafts does not alter FasL cell surface expression
We next attempted to identify the role of lipid rafts in the regulation of FasL expression and activity. Lipid rafts were thus destroyed by treatment of FasL-transfected 293T cells with the cholesterol-depleting agent methyl-β-cyclodextrin (MbCD).25 This treatment resulted in a significant shift of the lipid raft marker flotillin from the raft fraction to the nonraft fraction, indicating the efficacy of the lipid raft disruption (Figure 3A). Accordingly, we also observed a shift of FasL from the raft to the nonraft fraction, demonstrating that MbCD can induce the redistribution of FasL within the plasma membrane.
To exclude that MbCD alters the levels of FasL cell surface expression FasL-transfected and MbCD-treated 293T cells were stained with anti-FasL and analyzed by flow cytometry. Figure 3B clearly illustrates that lipid raft disruption by MbCD treatment did not alter the overall cell surface expression of FasL. Similarly, treatment of 293T cells with 2.5 to 10 mM MbCD for 20 minutes did not affect the cellular viability over the next 18 hours, as measured by annexin V. Under these experimental conditions we never observed an MbCD-induced increase in cell death more than 8% over background levels.
Disruption of lipid rafts inhibits FasL-mediated cytotoxicity
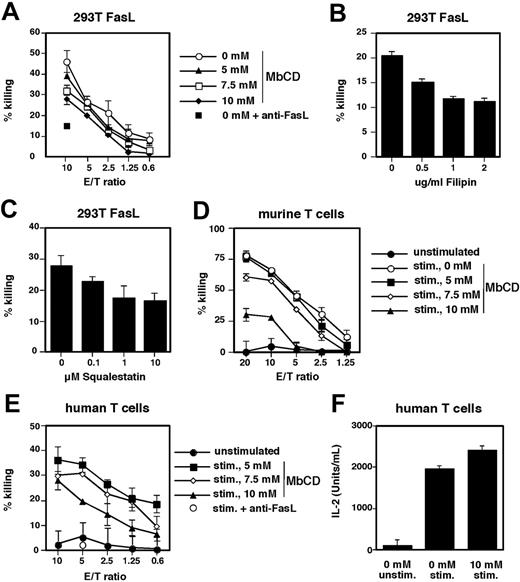
These data indicated that FasL is at least partially localized in lipid rafts and that MbCD treatment can exclude FasL from the raft fraction. We therefore investigated whether association with lipid rafts is required for FasL-mediated cytotoxicity and whether disruption of lipid rafts affects its cytotoxic activity. FasL-transfected 293T cells were treated with increasing concentrations of MbCD and then cocultured with Fas-sensitive target cells. Untreated FasL-expressing effector cells potently induced target cell killing and the specificity of the FasL-mediated killing was confirmed by neutralization with an anti-FasL antibody (Figure 4A). Interestingly, pretreatment of effector cells with MbCD dose dependently inhibited FasL activity and thereby target cell killing, indicating that lipid raft association is important for the apoptosis-inducing activity of FasL.
To exclude that MbCD may nonspecifically affect FasL activity, we further investigated the effect of lipid raft disruption using 2 different methods. Both the treatment of FasL-transfected 293T cells with filipin (sequestering cholesterol)36 and squalestatin (inhibition of cholesterol synthesis)32 significantly inhibited the killing of Fas-expressing target cells (Figure 4B-C). Thus, these data support an important role for lipid rafts in the regulation of FasL activity.
To assess the relevance of raft association for endogenous FasL we also investigated the effect of MbCD on FasL-mediated cytotoxicity in the murine T-cell hybridoma cell line A1.15,34 (Figure 4D) and human T-cell blasts (Figure 4E). Both human and murine T cells expressed FasL on activation and killed Fas-expressing target cells efficiently. In agreement with our finding that MbCD treatment destroys lipid rafts and abrogates FasL-mediated killing in 293T cells, we also observed a dose-dependent inhibition of FasL-mediated killing in human and murine T cells (Figure 4D-E). The reduced killing was evidently due to disruption of lipid rafts and reduced apoptosis-inducing activity of FasL and not due to toxic effects of MbCD on T cells as MbCD, at concentrations used and during the time frame of the assay, did not induce cell death in the effector T cells (data not shown). Importantly, IL-2 production in response to activation with PMA and ionomycin was normal in MbCD-treated human and murine T cells, indicating that the effect of MbCD-mediated lipid rafts disruption and inhibition of FasL-induced killing was specific and did not affect the overall activation or survival of the T cells (Figure 4F).
Disruption of lipid rafts inhibits FasL activity. (A) FasL-transfected 293T cells were treated with increasing concentrations of MbCD and cocultured at different effector-target ratios (E/T ratios) with Fas-sensitive target cells. After 6 hours target cell killing was assessed by the induction of DNA fragmentation. Specificity was confirmed by neutralization with anti-FasL. A typical experiment of 5 is shown. (B) FasL-transfected 293T cells were treated with increasing concentrations of filipin and cocultured with Fas-sensitive target cells at an E/T ratio of 2:1. Target cell killing was assessed by the induction of DNA fragmentation. A typical experiment of 2 is shown.(C) FasL-transfected 293T cells were treated with increasing concentrations of squalestatin and target cell killing was assessed as described (E/T ratio, 2:1). A typical experiment of 2 is shown. (D) The murine T-cell hybridoma A1.1 was treated with increasing concentrations of MbCD and then stimulated with PMA and ionomycin to induce FasL expression. Cells were then cocultured at different E/T ratios with Fas-sensitive target cells, and DNA fragmentation was measured. A typical experiment of 3 is shown. (E) Human T-cell blasts were treated as described in panel D and target cell killing was assessed. A typical experiment of 4 is shown. (F) Human T-cell blasts were treated with control or 10 mM MbCD for 20 minutes and stimulated with PMA and ionomycin overnight; IL-2 production was measured by enzyme-linked immunosorbent assay (ELISA). A typical experiment of 3 is shown.
Disruption of lipid rafts inhibits FasL activity. (A) FasL-transfected 293T cells were treated with increasing concentrations of MbCD and cocultured at different effector-target ratios (E/T ratios) with Fas-sensitive target cells. After 6 hours target cell killing was assessed by the induction of DNA fragmentation. Specificity was confirmed by neutralization with anti-FasL. A typical experiment of 5 is shown. (B) FasL-transfected 293T cells were treated with increasing concentrations of filipin and cocultured with Fas-sensitive target cells at an E/T ratio of 2:1. Target cell killing was assessed by the induction of DNA fragmentation. A typical experiment of 2 is shown.(C) FasL-transfected 293T cells were treated with increasing concentrations of squalestatin and target cell killing was assessed as described (E/T ratio, 2:1). A typical experiment of 2 is shown. (D) The murine T-cell hybridoma A1.1 was treated with increasing concentrations of MbCD and then stimulated with PMA and ionomycin to induce FasL expression. Cells were then cocultured at different E/T ratios with Fas-sensitive target cells, and DNA fragmentation was measured. A typical experiment of 3 is shown. (E) Human T-cell blasts were treated as described in panel D and target cell killing was assessed. A typical experiment of 4 is shown. (F) Human T-cell blasts were treated with control or 10 mM MbCD for 20 minutes and stimulated with PMA and ionomycin overnight; IL-2 production was measured by enzyme-linked immunosorbent assay (ELISA). A typical experiment of 3 is shown.
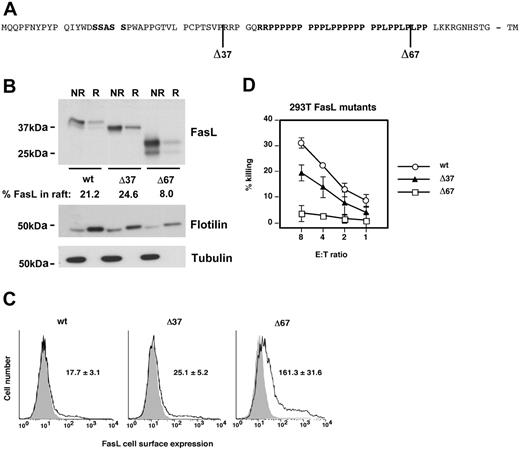
Role of proline-rich domain in FasL lipid raft localization. (A) Schematic organization of the FasL cytoplasmic tail. Positions of FasL cytoplasmic mutants are indicated. Putative signaling domains are in bold. TM indicates transmembrane domain. (B) 293T cells were transfected with full-length or different FasL cytoplasmic deletion mutants (Δ37, Δ67) and lipid rafts were isolated using the short method. FasL, flotillin, and tubulin were detected by Western blot. NR indicates nonraft fraction; R, raft fraction. The percentage of FasL in lipid rafts was determined densitometrically. A typical experiment of 5 is shown. (C) 293T cells were transfected with full-length FasL or deletion mutants and cell surface FasL expression was detected by flow cytometry. Shaded histograms, isotype control; black lines, anti-FasL staining. Numbers indicate mean fluorescence intensities of triplicates ± SD. (D) FasL activity was measured by induction of target cell killing. Target cell killing was normalized for unequal cell surface expression of full-length and mutant FasL constructs (C) by dividing percent DNA fragmentation with the mean fluorescence intensity of FasL cell surface expression. A typical experiment of 4 is shown.
Role of proline-rich domain in FasL lipid raft localization. (A) Schematic organization of the FasL cytoplasmic tail. Positions of FasL cytoplasmic mutants are indicated. Putative signaling domains are in bold. TM indicates transmembrane domain. (B) 293T cells were transfected with full-length or different FasL cytoplasmic deletion mutants (Δ37, Δ67) and lipid rafts were isolated using the short method. FasL, flotillin, and tubulin were detected by Western blot. NR indicates nonraft fraction; R, raft fraction. The percentage of FasL in lipid rafts was determined densitometrically. A typical experiment of 5 is shown. (C) 293T cells were transfected with full-length FasL or deletion mutants and cell surface FasL expression was detected by flow cytometry. Shaded histograms, isotype control; black lines, anti-FasL staining. Numbers indicate mean fluorescence intensities of triplicates ± SD. (D) FasL activity was measured by induction of target cell killing. Target cell killing was normalized for unequal cell surface expression of full-length and mutant FasL constructs (C) by dividing percent DNA fragmentation with the mean fluorescence intensity of FasL cell surface expression. A typical experiment of 4 is shown.
FasL lipid rafts localization is regulated by its cytoplasmic tail
To investigate which domain of FasL mediates its association with lipid rafts we used expression vectors with different truncations of the FasL cytoplasmic tail.17,19 Of major importance in this respect was the analysis of 2 domains in the cytoplasmic tail that have been previously implicated in the interaction with other proteins. These include the SxxS-motif,37 a putative casein kinase I substrate, and a characteristic proline-rich domain with multiple putative Src homology 3 (SH3)–binding motifs (PxxP)17,19 (Figure 5A). Interestingly, full-length wild-type FasL was significantly less expressed in transfected 293T cells than the different truncation mutants. This reduced protein expression is in agreement with a recently published role of the N-terminal end in FasL translation and stabilization.38 Importantly, however, the Δ37 mutant, which lacks the SxxS motif (Figure 5A), showed a similar degree of FasL raft localization, excluding an important role of this domain in raft recruitment (Figure 5B). In marked contrast, deletion of the proline-rich stretch (Δ67) substantially reduced FasL raft localization (Figure 5B).
The importance of this proline-rich domain in raft recruitment and regulation of FasL activity was also confirmed in a FasL killing assay. Whereas the Δ37 mutant showed only a slightly reduced apoptosis-inducing capacity in Fas-positive target cells than wild-type Fas ligand, this killing activity was substantially attenuated in the Δ67 (Figure 5C).
Role of FasL-interacting proteins on FasL lipid raft localization
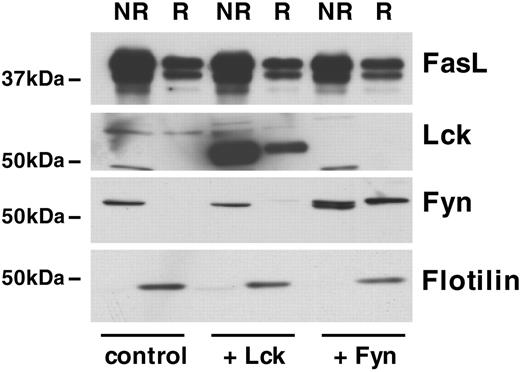
The use of the different FasL truncations suggested an important role of the proline-rich domain in the recruitment of FasL to lipid rafts and regulation of FasL activity. Different proteins have been described to interact with this domain and thereby may potentially regulate the recruitment of FasL to lipid rafts.20,39,40 For example, the Src-like kinases Fyn and Lck, which contain SH3 domains, bind to the proline-rich domain of FasL20,39,40 and also localize to lipid rafts. We thus tested whether coexpression of FasL with Fyn, respectively, Lck, would affect the raft localization of FasL. Figure 6 shows that a significant proportion of Lck and even more pronounced for Fyn was detected in the raft fraction. However, coexpression of FasL and Fyn or Lck did not result in a detectable increase of raft-associated FasL. Because Fyn and Lck overexpression did not significantly alter FasL raft association and FasL was detected in the raft fraction even in the absence of Fyn and Lck expression, these data suggest that other FasL-interacting molecules or the cytoplasmic tail of FasL by itself may be responsible for localization to lipid rafts.
Role of the FasL-interacting proteins Lck and Fyn in lipid raft recruitment. 293T cells were cotransfected with full-length FasL and vector control, Lck, or Fyn. Lipid rafts were isolated, and FasL, Lck, Fyn, and flotillin were detected by Western blot. NR indicates nonraft fraction; R, raft fraction. A typical experiment of 5 is shown.
Role of the FasL-interacting proteins Lck and Fyn in lipid raft recruitment. 293T cells were cotransfected with full-length FasL and vector control, Lck, or Fyn. Lipid rafts were isolated, and FasL, Lck, Fyn, and flotillin were detected by Western blot. NR indicates nonraft fraction; R, raft fraction. A typical experiment of 5 is shown.
Discussion
Transmembrane FasL expressed on effector T and natural killer (NK) cells is an important mechanism of apoptosis induction in target cells, for example, virus-infected cells, activated T and B cells, etc, but is also responsible for tissue destruction in many immunopathologic diseases, such as hepatitis and graft-versus-host disease (for a review, see Brunner et al13 ). Hence, the expression and activity of FasL in cytotoxic effector cells has to be tightly regulated. Much of the attention on FasL regulation has been focusing on its transcriptional control.14 Here we now demonstrate a novel mechanism of FasL regulation and propose that posttranscriptional modification of FasL by clustering in lipid rafts is equally important in the fine-tuning of its proapoptotic activity. Although this is to our knowledge the first report on the role of lipid rafts in the regulation of FasL activity in T cells, our finding is supported by 2 recent publications showing that ectopic expression of FasL in HeLa cells results in its clustering in lipid raft,41 and that dehydrodidemnin B (Aplidin)–mediated chemotherapy of Jurkat cells induces translocation of endogenous Fas and FasL to lipid rafts.42 However, these reports did not provide any information on whether FasL raft association also occurs in normal untransformed T cells and whether raft localization affects its biologic activity. Our data clearly support an important role of FasL recruitment to lipid rafts in the regulation of its biologic activity in transfectants as well as in normal T cells.
An important question that evolves from this observation is why FasL is regulated at both a transcriptional as well as posttranscriptional level, that is, by recruitment to lipid rafts. Although transcriptional control of FasL expression is crucial to define in which cell type and at which differentiation stage FasL is expressed—for example, naive, resting, and activated T cells—it cannot control the polarized expression of FasL protein in the immunologic synapse between T cell and target cell. Uniformly expressed FasL, throughout the entire plasma membrane, may not only result in inefficient killing of the T cell–activating target cell due to limiting local concentrations, but possibly also causes substantial bystander killing of “innocent” normal tissue cells. Clustering of FasL in the immunologic synapse through recruitment to lipid rafts, however, may ensure that FasL is expressed at a high local concentration and with high activity at the contact site between effector cell and target cell. It is thus interesting to note that Henkler et al41 demonstrated the formation of supramolecular Fas/FasL clusters with high stability in the contact site between FasL- and Fas-expressing cells. It is tempting to speculate that these large clusters are at least in part mediated by aggregation of FasL in lipid rafts. Although this report also demonstrated the presence of FasL in membrane rafts, it remained undefined whether lipid rafts are required for the biologic activity of FasL.
FasL clustering in lipid rafts may not only provide a mechanism to regulate critical local concentrations and activities, but may also be important for efficient Fas receptor clustering and aggregation. Different reports have shown that in certain cell types Fas is constitutively or inducibly associated with lipid rafts and that raft localization is important for activation of downstream signaling events and apoptosis induction.24,27,28,43-45 Thus, lipid raft-aggregated FasLmay permit clustering of the receptor and thereby induce apoptosis more effectively. Our observations are in line with this notion because we have observed that lipid raft destruction in FasL-expressing effector cells abrogates induction of target cell apoptosis. They are further supported by the observation that soluble FasL, devoid of its transmembrane and cytoplasmic portion, is an inefficient trigger of apoptosis and rather acts as an antagonist of transmembrane FasL/Fas interaction.15,46 In contrast, higher order aggregation of soluble FasL, for example, triggered by antibody cross-linking, can restore its biologic activity. Similarly, bivalent anti-Fas antibodies fail to induce apoptosis, whereas IgM and IgG3 isotypes efficiently promote Fas-induced cell death (reviewed by Schneider47 ). Accordingly, FasL clustering in lipid rafts may similarly provide a mechanism for the formation of higher order FasL aggregation, increasing its biologic activity. Interestingly, whereas lipid raft localization of FasL appears to be required for its biologic activity, Fas clustering in lipid rafts may sometimes induce apoptosis even in a ligand-independent manner. Hence, it has been shown that T-cell activation induces redistribution of the Fas receptor into lipid rafts and subsequently apoptosis.28 Similarly, treatment of tumor cells with the ether lipid ET-18-OCH3 induces Fas clustering in lipid rafts and associated apoptosis.36
An important aspect to be addressed in future studies is the mechanism of FasL recruitment to lipid rafts. Our results strongly support an important role for the proline-rich domain in this process. Interestingly, this domain has also recently been described as crucial for the sorting of FasL to secretory lysosomes and transportation to the cell surface.19 Therefore, this proline-rich domain may have a general role in FasL transportation, sorting, and regulation of its activity. It is tempting to speculate that FasL-interacting proteins (at least partially interacting via the proline-rich domain) may be responsible for the recruitment of FasL to lipid rafts. It is thus interesting to note that many of the FasL-interacting proteins are localized or recruited to lipid rafts themselves (eg, Grb2, Fyn, Lck). Similarly, these molecules are also critically involved in T-cell receptor signaling. Because there is a strong correlation between T-cell receptor activation and FasL cell surface expression,18,48 it is reasonable to propose that FasL-interacting molecules may be an important link between T-cell receptor signaling and recruitment of FasL to lipid rafts in the immunologic synapse. However, this connection has to be formally proven. So far, we failed to demonstrate a role for the FasL-interacting molecules Fyn and Lck in the recruitment of FasL to lipid rafts because overexpression did not affect raft localization of FasL. It is though possible that in 293T cells the raft-recruiting role of Fyn and Lck can be compensated by other molecules. Alternatively, endogenous levels of Fyn and Lck in 293T may be sufficient to promote raft localization of FasL. Possibly, FasL recruitment to lipid rafts is also mechanistically different in different cell types. Whereas in 293T cells the majority of FasL protein was intracellular and only a certain proportion was raft-associated, in activated T cells FasL was almost exclusively found in lipid rafts. This observation suggests that differentially expressed FasL-interacting proteins may control FasL sorting to lipid rafts.
In summary, we here provide the first evidence for an important role of lipid rafts in the regulation of FasL-mediated cytotoxicity. We further propose that clustering of death receptors and death ligands in lipid rafts may represent an important general scheme in apoptosis signaling. Finally, the requirement of ligand/receptor aggregation in lipid rafts may also provide an interesting platform for future therapeutic interventions, in particular for the treatment of immune cell–mediated diseases.
Prepublished online as Blood First Edition Paper, December 6, 2005; DOI 10.1182/blood-2005-07-2744.
Supported by research grants from Oncosuisse (OCS-01161-09-2001), EU COST Action 844 (SBF no. C05.0005), and the Swiss National Science Foundation (3100-065021, 310000-110030; T.B.).
D.F.L. is a recipient of a career development award from the Prof Dr Max Cloëtta Foundation.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
The authors would like to thank the members of the Brunner and Müller labs for technical advice and constructive discussions; Gillian Griffiths for the FasL constructs; Gottfried Baier for Fyn and Lck expression plasmids; and Jeremie Rossy, Christoph Oppliger, and Sabine Jakob for reagents and help.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal