Abstract
We have shown previously that EM011, a synthetic compound, binds tubulin with a higher affinity than the founding compound, noscapine, without changing total microtubule polymer mass. Now we show that EM011 is potently effective against vinblastine-resistant human lymphoblastoid line CEM/VLB100 and its parental vinblastine-sensitive line CEM. The cytotoxicity is mediated by cell cycle arrest at G2/M phase and subsequent apoptosis, as indicated by altered plasma membrane asymmetry, loss of mitochondrial transmembrane potential, activation of caspase-3, and increased DNA fragmentation. Furthermore, oral EM011 treatment of nude mice bearing human lymphoma xenografts results in pronounced tumor regression by triggering apoptosis and significantly lengthens the survival time of mice. EM011 treatment does not have obvious side effects in tissues with frequently dividing cells, such as the spleen and duodenum. In addition, EM011 does not show any toxicity in the liver, lung, heart, brain, and sciatic nerve. More importantly, EM011 does not affect hematopoiesis as determined by complete blood count profiles. These findings suggest that EM011 may be a safe and effective chemotherapeutic agent for oral treatment of drug-resistant human lymphomas. (Blood. 2006;107:2486-2492)
Introduction
The microtubule cytoskeleton plays a critical role in a variety of cellular processes, including cell shaping, cell migration, and intracellular transport. In addition, microtubules form the spindle apparatus during mitosis that carries out the physical separation of sister chromatids. Microtubules are intrinsically dynamic polymers, and this dynamic property is crucial for many of their cellular functions, especially for successful mitosis. Alteration of microtubule dynamics with small molecules can halt cell cycle progression at mitosis by activating a cellular surveillance mechanism called the spindle checkpoint.1,2
The critical role that dynamic microtubules play in mitosis makes them a suitable target for the development of chemotherapeutic drugs against the rapidly dividing cancer cells. The effectiveness of microtubule-interfering agents in cancer therapy has been validated by the use of taxanes and vinca alkaloids for the treatment of many cancer types.1-4 Unfortunately, because of the gross effects of taxanes and vinca alkaloids on microtubule arrays at therapeutic doses, these drugs produce various side effects, notably neurologic and hematologic toxicities.2 In addition, drug resistance develops after prolonged treatment due to overexpression of drug efflux pumps on the plasma membrane, mutations in the drug target, microtubules/tubulin, and many other mechanisms.5-7 It is, therefore, of great significance to discover and develop novel antimicrotubule agents that can overcome drug resistance and have improved pharmacologic profiles.
We have recently discovered that noscapine, a naturally occurring alkaloid, binds tubulin with a 1:1 stoichiometry.8 Noscapine binding to tubulin alters the dynamic property of microtubules, primarily by increasing the time that microtubules spend in a pause state.9,10 Noscapine does not significantly affect microtubule polymer mass and organization; however, it efficiently activates the spindle checkpoint and halts cell cycle progression at mitosis.10 Noscapine-arrested normal cells are able to resume cell cycle progression on drug metabolism or clearance of the drug.9 However, many cancer cells, owing to mutational lesions, prematurely inactivate the spindle checkpoint and undergo multiple rounds of DNA synthesis without cytokinesis, accumulating a genotoxic amount of DNA and ultimately leading to apoptosis.9 Noscapine has shown a potent anticancer property both in cultured cancer cells and in mouse models of various human cancer types.8,9,11-13 This agent is currently in phase 1/2 clinical trials for non-Hodgkin lymphoma or chronic lymphocytic leukemia refractory to chemotherapy.
In this study, we report that EM011, a 10- to 15-fold more potent noscapine analog,14 effectively inhibits vinblastine-resistant as well as vinblastine-sensitive human T-cell lymphoid tumor xenografts in nude mice, thereby markedly increasing longevity. In contrast, although vinblastine inhibits tumors derived from vinblastine-sensitive lymphoid cells, it causes excessive body weight loss and morbidity of mice. We further show that unlike vinblastine, EM011 is orally available and does not cause any toxicity in the duodenum, liver, spleen, kidney, lung, heart, brain, and sciatic nerve. Moreover, EM011 does not alter the cell counts in blood or any signatures of hematologic pathologies such as the levels of alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase (ALK PHOS), and blood urea nitrogen (BUN). These results distinguish EM011 from microtubule-interfering agents currently used in the clinic, which severely compromise the hematopoietic system and cause various organassociated toxicities.
Materials and methods
Chemicals, antibodies, and cell lines
Noscapine and vinblastine were purchased from Sigma (St Louis, MO). Rabbit polyclonal antibodies against cleaved caspase-3 and cleaved poly-(ADP-ribose) polymerase (PARP) were from Cell Signaling Technology (Beverly, MA), and horseradish peroxidase-conjugated antirabbit secondary antibody was from Jackson ImmunoResearch (Bar Harbor, ME). The human lymphoblastoid cell line CEM and its vinblastine-resistant variant, CEM/VLB100, were kindly provided by Dr William T. Beck (University of Illinois at Chicago). CEM/VLB100 cells were demonstrated to be multidrug resistant (MDR) due to P-glycoprotein (Pgp) overexpression.15,16 Both CEM and CEM/VLB100 cells were grown in RPMI 1640 medium (Cellgro, Herndon, VA) supplemented with 10% fetal bovine serum and 1% penicillin/streptomycin.
Cytotoxicity assay and cell cycle analysis
Cells grown in 96-well plates were treated with gradient concentrations of EM011 for 48 hours. Sulforhodamine B assay was used to evaluate the cytotoxicity of EM011 and to determine the lethal dose 50 (LD50), the drug dose needed to achieve 50% cell kill. Flow cytometric analysis of the cell cycle was performed as described previously.14 Briefly, 2 × 106 cells were washed twice with ice-cold PBS and fixed in 70% ethanol for 24 hours. The pellets were washed twice with PBS and stained with propidium iodide (PI) and RNase A for 45 minutes in dark. Samples were then analyzed on a FACSCalibur flow cytometer (Beckman Coulter, Fullerton, CA).
Annexin V staining
Cells were stained with Alexa-Fluor 488-conjugated annexin V and PI using the Vybrant apoptosis assay kit from Molecular Probes (Eugene, OR) following the manufacturer's protocol. Two-color flow cytometric analysis was performed on a FACSCalibur equipped with a single argon-ion laser. Annexin-stained cells were also visualized with a Zeiss 510 confocal microscope (Carl Zeiss, Thornwood, NJ) using a 63× objective with a numerical aperture of 1.4 (Figure 2E, 2F).
Evaluation of mitochondrial transmembrane potential
The ampholytic cationic fluorescent probe dihexylocarbocyanine iodide (DiOC6) was used to monitor drug-induced changes in mitochondrial transmembrane potential. Briefly, cells were loaded with DiOC6 (50 nM) for 30 minutes at 37°C before flow cytometric analysis. The supernatant was removed, and cells were harvested and resuspended in PBS. Measurement of the retained DiOC6 was performed in a FACSCalibur flow cytometer.
Determination of caspase-3 activity
Cells were incubated with 10 μM EM011 for 0, 24, 48, and 72 hours. Caspase-3 activity was examined by measuring the luminescence resulting from the cleavage of Z-DEVD-aminoluciferin (Promega, Madison, WI). The luminescent signal, which is directly proportional to the level of caspase-3 activity, was measured with a luminescence plate reader.
Immunoblot analysis
Proteins were resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred onto PVDF membranes (Millipore, New Bedford, MA). The membranes were blocked for 2 hours in Tris-buffered saline containing 0.2% Tween 20 and 5% fat-free dry milk, and then incubated first with primary antibodies and then horseradish peroxidase-conjugated secondary antibodies for 2 hours and 1 hour, respectively. Specific proteins were visualized with enhanced chemiluminescence detection reagent according to the manufacturer's instructions (Pierce Biotechnology, Rockford, IL).
Immunofluorescence microscopy
Cells grown on poly-l-lysine/fibronectin coated coverslips were treated with 10 μM EM011 for 72 hours, fixed in methanol for 5 minutes at -20°C, washed with PBS, and then blocked with 2% BSA/PBS for 30 minutes. Cells were incubated with a mouse monoclonal anti-α-tubulin antibody (DM1A, Sigma) for 1 hour at 37°C, washed in PBS, and then incubated with a FITC-conjugated secondary antibody for 1 hour at 37°C. Cells were then stained with PI prior to mounting and observed with a Zeiss 510 confocal microscope (using a 63× objective with a numerical aperture of 1.4 (Figure 3A-D).
Terminal deoxynucleotidyl-transferase-mediated TUNEL assay
DNA strand breaks were identified using the APO-BrdU dUTP nick-end labeling (TUNEL) assay kit following the manufacturer's instructions (Molecular Probes). This assay was run on a flow cytometer equipped with a 488-nm argon laser as the light source. PI and Alexa-Fluor 488 were the 2 dyes used. Single and dual parameter displays were created using the CellQuest data acquisition software (Becton Dickinson, Mountain View, CA). The gating display was the standard dual parameter DNA doublet discrimination display with the DNA area signal on the y-axis and the DNA width signal on the x-axis. From this display, a gate was generated around the nonclumped cells and the second gated dual parameter display was generated with the DNA (linear red fluorescence) on the x-axis and the Alexa-Fluor 488 (log green fluorescence) on the y-axis. Apoptotic cells were subsequently counted as those expressing high Alexa-Fluor 488 fluorescence.
Animals and implantation of cancer cells
Eight- to 10-week-old female BALB/c athymic (nu/nu) nude mice (HarlanSprague Labs, Indianapolis, IN) were housed in the Emory University Animal Care Facility and were injected subcutaneously with 106 cells (CEM or CEM/VLB100) per mouse. Treatment was initiated 8 to 12 days later when tumors were palpable and measurable (∼100 mm3). Three axes of tumors were measured every day with vernier calipers, and tumor volume was calculated as 1/2 × length × width2 in mm3. In control groups of each experiment, the rapidly growing lymphoma tumors required that animals be humanely killed when tumor volumes were 4000 mm3 or greater or became ulcerated and the mice showed morbidity (Institutional Animal Care and Use Committee [IACUC] guidelines of Emory University). This served as an end point for these experiments. For survival studies, the treated groups were observed for about 100 days prior to being humanely killed.
Drug treatment
Tumor-bearing mice were randomly grouped (6 mice/group). EM011 was administered (300 mg/kg) in deionized water (pH 4.0) by daily gavage. Untreated mice received equal volumes of deionized water (pH 4.0) only. As a control, mice were treated with 10 mg/kg vinblastine intravenously, and untreated animals received the vehicle solution intravenously. Body weight was monitored every day.
Histopathologic analysis
At the end of the experiment, blood was collected from anesthetized animals of treated and untreated groups for complete blood count (CBC) analysis (CDC Technologies, Oxford, CT). Duodenum, liver, kidney, spleen, lung, heart, brain, and tumors were formalin-fixed, paraffin-embedded, and 5 μm-sections were stained with hematoxylin and eosin.12 The sciatic nerve sections were stained using Luxol fast blue/periodic acid-Schiff stain. Microscopic evaluation was performed by 2 pathologists.
Immunohistochemical analysis
For TUNEL staining of tissue sections, paraffin-embedded tissues were dewaxed at 60°C for 15 minutes, washed in xylene, and then rehydrated through a graded series of ethanol and distilled water. The resulting sections were incubated with proteinase K for 20 minutes, incubated with blocking solution (0.3% H2O2 in methanol) for 30 minutes, and then incubated in permeability solution (0.1% Triton X-100/0.1% sodium citrate) on ice for 2 minutes. The slides were incubated with 50 μL TUNEL reaction mixture for 60 minutes at 37°C in a humidified chamber, incubated with 50 μL of streptavidin HRP solution for 30 minutes, and then incubated with 60 μL 3,3′-diaminobenzidine solution for 10 minutes. Activated caspase-3 was detected after immunostaining of tumor sections using a specific monoclonal antibody (Dako, Carpenteria, CA) followed by HRP-coupled antimouse IgG staining. Coverslips were mounted and analyzed with a Zeiss Axiovert light microscope (Figure 5).
Results
EM011 effectively kills human lymphoma cells and a vinblastine-resistant variant in vitro
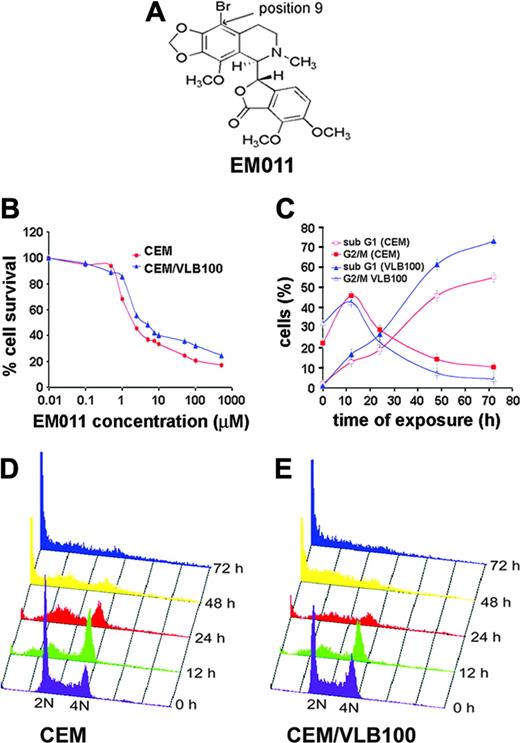
EM011 was derived from our founding anticancer compound, noscapine, which is in clinical trials. Essentially, noscapine contains isoquinoline and benzofuranone flat ring systems attached at an angle by a C-C bond including 2 chiral centers. However, only one stereoisomer of noscapine is biologically active.8 Unlike noscapine, EM011 possesses a bromine atom replacing an acidic proton at position 9 (Figure 1A). We have shown previously that EM011 binds tubulin with a higher affinity than noscapine and is 10- to 15-fold more effective than noscapine in killing cancer cells.14 Like noscapine, EM011 does not perturb microtubule polymer mass.14 Microtubule-interfering drugs such as taxanes and vinca alkaloids are active against many tumor types. However, these agents fail to manage the drug resistant phenotypes of recurrent tumors.1,2 CEM/VLB100 cells are 270-fold more resistant to vinblastine as compared to the parental CEM cells and exhibit Pgp-associated MDR phenotype.15,16 With sulforhodamine B-based cytotoxicity assay, we found that EM011 was potently active toward both the parental CEM cells and the drug resistant CEM/VLB100 cells (Figure 1B). The LD50 values of EM011 for CEM and CEM/VLB100 cells were 1.9 μM and 3.9 μM, respectively (Figure 1B).
EM011 induces G 2/M phase arrest in CEM and CEM/VLB100 cells
We next examined the cell cycle progression of EM011-treated CEM and CEM/VLB100 cells. As shown in Tables 1-2 and Figure 1C, EM011 treatment resulted in a time-dependent accumulation of CEM and CEM/VLB100 cells in the G2/M phase, as revealed by an increasing population of cells with 4N DNA content. The maximum percentage of G2/M arrest was about 45% for CEM cells and about 47% for CEM/VLB100 cells at 12 hours of EM011 treatment (Tables 1-2; Figure 1C). The G2/M cell population then decreased and the sub-G1 population, which has less than 2N DNA content indicative of apoptosis, increased. The sub-G1 population reached a peak at 72 hours of EM011 treatment (∼57% for CEM and ∼75% for CEM/VLB100). Figure 1D-E displays 3-dimensional cell cycle progression profiles over the time of EM011 treatment in CEM and CEM/VLB100 cells, respectively. Together, these results indicate that EM011-treated cells arrest in G2/M phase prior to apoptosis.
EM011 effectively kills CEM and CEM/VLB100 cells and induces G2/M arrest. (A) Molecular structure of EM011. An acidic proton on position 9 of the isoquinoline ring system of the founding compound, noscapine, is substituted with a bromine group in EM011. (B) Plot shows the percentage of cell survival versus EM011 concentration. CEM and CEM/VLB100 cells were treated with gradient concentrations of EM011 for 48 hours, and the percentage of surviving cells at indicated drug concentrations was measured. (C) Quantitative representation of apoptotic index and mitotic index as a function of time of treatment with 10 μM EM011 in CEM and CEM/VLB100 cells. Panels D and E show the time effects of EM011 on the cell cycle progression of CEM and CEM/VLB100 cells, respectively. Cells were harvested for analysis at the indicated times, stained with PI, and analyzed by flow cytometry. The x-axis shows the intensity of PI fluorescence, which indicates cellular DNA content in different cell cycle phases. The y-axis represents the cell counts, and the z-axis shows the time of EM011 treatment. Results are representative of 3 experiments performed in triplicate.
EM011 effectively kills CEM and CEM/VLB100 cells and induces G2/M arrest. (A) Molecular structure of EM011. An acidic proton on position 9 of the isoquinoline ring system of the founding compound, noscapine, is substituted with a bromine group in EM011. (B) Plot shows the percentage of cell survival versus EM011 concentration. CEM and CEM/VLB100 cells were treated with gradient concentrations of EM011 for 48 hours, and the percentage of surviving cells at indicated drug concentrations was measured. (C) Quantitative representation of apoptotic index and mitotic index as a function of time of treatment with 10 μM EM011 in CEM and CEM/VLB100 cells. Panels D and E show the time effects of EM011 on the cell cycle progression of CEM and CEM/VLB100 cells, respectively. Cells were harvested for analysis at the indicated times, stained with PI, and analyzed by flow cytometry. The x-axis shows the intensity of PI fluorescence, which indicates cellular DNA content in different cell cycle phases. The y-axis represents the cell counts, and the z-axis shows the time of EM011 treatment. Results are representative of 3 experiments performed in triplicate.
EM011 induces loss of plasma membrane asymmetry and apoptosis
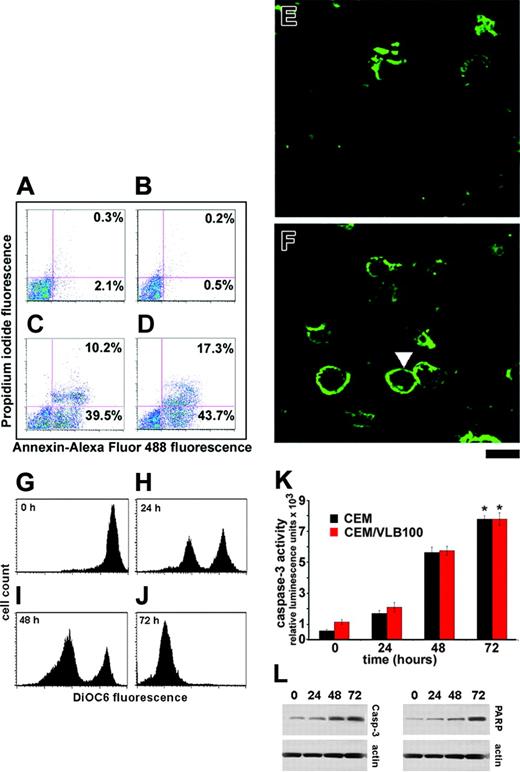
We investigated further if indeed apoptosis followed mitotic arrest in EM011-treated cells. Biochemically, the early apoptotic process is characterized by loss of lipid asymmetry between the 2 leaflets of plasma membrane, in that phosphatidylserine becomes abnormally externalized from the inner leaflet and gets displayed on the outer leaflet. To quantify both early and late apoptotic cells, we performed annexin V staining along with an impermeant DNA binding dye, PI, and analyzed with flow cytometry. Figure 2A-B shows the density plots of PI versus annexin Alexa-Fluor 488 fluorescence obtained from untreated control CEM and CEM/VLB100 cells, respectively. The untreated cell cultures contained very few apoptotic cells (∼2%), which we assigned as the background cell death. CEM cells treated with EM011 for 48 hours showed both early and late apoptosis (39.5% and 10.2%, respectively), suggesting continued initiation and execution of apoptosis (Figure 2C). Interestingly, CEM/VLB100 cells also showed 43.7% of early apoptotic and 17.3% of late apoptotic cells after 48 hours of EM011 treatment (Figure 2D). Consistent with these results, confocal microscopy revealed early apoptotic cells on EM011 treatment, as shown by the green staining of phosphatidylserine on the outer cell boundary (Figure 2F, solid white arrowhead). In contrast, the untreated control cells did not display such a staining pattern and showed only nonspecific background staining (Figure 2E).
EM011-induced apoptosis is mediated through the mitochondrial pathway
One mechanism by which apoptosis occurs involves loss of mitochondrial membrane integrity and transmembrane potential (ie, collapse of Δψm). This collapse of Δψm can be monitored by a reduction in the uptake of the fluorochrome, DiOC6. As shown in Figure 2G-J, EM011 caused a substantial reduction in the uptake of DiOC6 in CEM/VLB100 cells. The percentage of cells with reduced mitochondrial potential increased with the time of EM011 treatment, being 49% at 24 hours (Figure 2H) and reaching a maximum of about 99% at 72 hours (Figure 2J). Similarly, we found a time-dependent reduction of Δψm in CEM cells following EM011 treatment (data not shown).
The collapse of mitochondrial Δψm causes an uncoupling of the respiratory chain and an efflux of small proapoptotic factors, ultimately activating the key executioner caspase, caspase-3. The activation of caspase-3 is caused by upstream caspases and involves the cleavage of the inactive proenzyme into an active form. The active form can be monitored using a small peptide substrate that becomes luminogenic on cleavage. As shown in Figure 2K, we observed a time-dependent activation of caspase-3 in both CEM and CEM/VLB100 cells. Immunoblot analysis further confirmed the activation of caspase-3. As shown in Figure 2L, 10 μM EM011 caused an increase in cleaved caspase-3 and cleaved PARP at 48 and 72 hours of treatment, indicating extensive apoptosis.
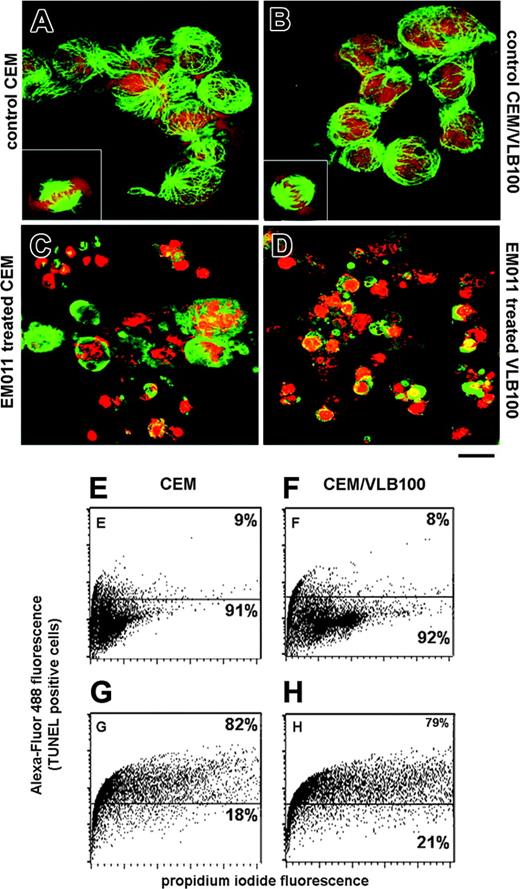
Typical morphologic changes in late apoptotic cells include membrane blebbing, formation of apoptotic bodies, disruption of the cytoskeleton, and hypercondensation and fragmentation of the chromatin. To visualize EM011-induced morphologic changes, cells treated with EM011 for 72 hours were stained with a tubulin-specific antibody and a DNA-binding dye. As shown in Figure 3A-B, untreated control CEM and CEM/VLB100 cells show normal microtubule arrays and nuclei in interphase and display bipolar spindles in mitosis. Prolonged EM011 treatment caused devastating morphologic changes and induced the formation of apoptotic bodies (Figure 3C-D). We also quantified the increase in the concentration of 3′ ends due to DNA fragmentation, using a flow cytometry-based TUNEL assay. As shown in Figure 3G-H, about 80% of CEM and CEM/VLB100 cells were TUNEL+ after 72 hours of EM011 treatment. In contrast, only a small population of untreated control cells was TUNEL+ (Figure 3E-F).
Efficacy of oral EM011 treatment in xenograft nude mice models
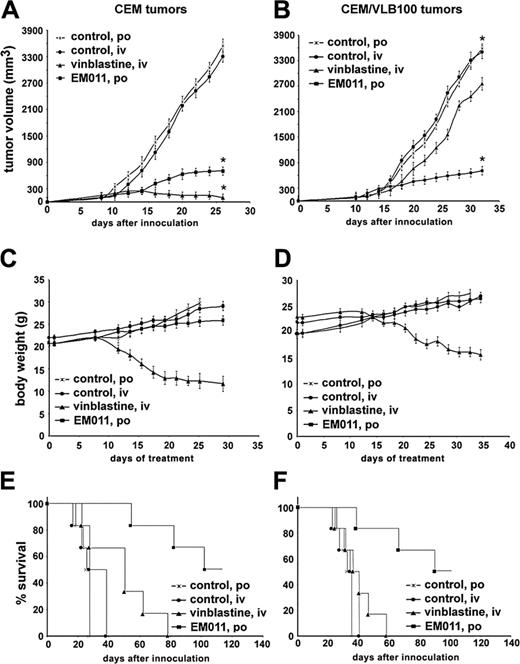
We next asked if EM011 was effective against human tumors implanted subcutaneously in nude mice. When xenografts were palpable with tumor size of about100 mm3, we grouped the mice randomly into control and treatment groups of 6 animals each. The treatment groups received individually therapeutic dosages of EM011 (300 mg/kg orally) and vinblastine (10 mg/kg intravenously), since vinblastine is clinically effective intravenously, whereas EM011 is available for oral use. We included matched control groups that received vehicle orally as well as intravenously in our study. As shown in Figure 4A, EM011 treatment significantly reduced tumor volume in CEM xenografts. On day 26 of treatment, EM011 reduced tumor volume by about 80% as compared to the vehicle control (from 3545 ± 154 mm3 to 702 ± 96 mm3, average tumor volume ± SE, P < .01). We found that EM011 treatment did not cause any apparent body weight loss in mice (Figure 4C). Kaplan-Meier analysis revealed that the median animal survival increased by about 4-fold by EM011 treatment as compared to the vehicle control (Figure 4E). In contrast, although vinblastine therapy showed better results in tumor regression (3310 ± 131 mm3 and 103 ± 85 mm3, average tumor volume ± SE, for control untreated and vinblastine-treated groups, respectively, day 26, P < .01; Figure 4A), mice in this group suffered a tremendous body weight loss and death (Figure 4C,E).
A positive correlation between Pgp/MDR1 expression and poor survival or response has been established in leukemias and myelomas.17 It has also been shown that patients whose tumors overexpress Pgp/MDR1 are 3 times more likely to fail to respond to chemotherapy than patients who are negative for Pgp/MDR1.18 To test if MDR xenografts also respond to EM011 treatment, we monitored tumor volume in mice bearing CEM/VLB100 xenografts. We also compared EM011-treated groups with vinblastine-treated groups. As shown in Figure 4B, in CEM/VLB100 xenografts, mice did not show significant reduction in tumor volume by vinblastine treatment (2758 ± 154 mm3, day 32) compared with matched controls (3513 ± 145 mm3, day 32). In contrast, EM011 treatment of CEM/VLB100 xenografts showed a remarkable reduction in tumor volume by about 81% after 32 days of oral treatment (3585 ± 111 mm3 and 720 ± 102 mm3, average tumor volume ± SE, for control and EM011-treated groups, respectively, P < .01). The median animal survival increased by about 3-fold on EM011 treatment as compared to control (Figure 4F). Furthermore, mice in this group maintained normal weight gain (Figure 4D) and showed no signs of discomfort during the course of treatment.
EM011 induces apoptosis. Panels A-D show flow cytometric analysis of phosphatidylserine (PS) exposure in untreated CEM (A), untreated CEM/VLB100 (B), EM011-treated CEM (C), and EM011-treated CEM/VLB100 (D) cells. Alexa-Fluor 488 conjugate of annexin V was used in combination with PI to distinguish among 3 subpopulations: PI- and Alexa-Fluor 488- population indicates viable cells (bottom left quadrant); PI- and Alexa-Fluor 488+ population indicates early apoptotic cells (lower right quadrant); PI+ and Alexa-Fluor 488+ population indicates late apoptotic cells (top right quadrant). Representative annexin V-stained cells are shown in panel F (EM011-treated) as green rings surrounding early apoptotic cells (solid arrowhead) as opposed to the control untreated cells in panel E, where green rings are absent. However, some nonspecific staining is evident. Scale bar = 20 μm. (G-J) EM011 reduces mitochondrial transmembrane potential in a time-dependent manner. CEM/VLB100 cells were treated with EM011 for 0, 24, 48, and 72 hours, incubated with 50 nM DiOC6, and then analyzed by flow cytometry. The x-axis represents the DiOC6 fluorescence intensity, and the y-axis represents the number of cells. (K) Quantitation of caspase-3 activity in EM011-treated CEM and CEM/VLB100 cells. Cells were treated with 10 μM EM011 for 0, 24, 48, and 72 hours, and caspase-3 activity was analyzed using the luminogenic substrate Z-DEVD-aminoluciferin. *P < .01. (L) Representative immunoblot of cleaved caspase-3 and cleaved PARP along with the loading control, actin. Results are representative of 3 independent experiments performed.
EM011 induces apoptosis. Panels A-D show flow cytometric analysis of phosphatidylserine (PS) exposure in untreated CEM (A), untreated CEM/VLB100 (B), EM011-treated CEM (C), and EM011-treated CEM/VLB100 (D) cells. Alexa-Fluor 488 conjugate of annexin V was used in combination with PI to distinguish among 3 subpopulations: PI- and Alexa-Fluor 488- population indicates viable cells (bottom left quadrant); PI- and Alexa-Fluor 488+ population indicates early apoptotic cells (lower right quadrant); PI+ and Alexa-Fluor 488+ population indicates late apoptotic cells (top right quadrant). Representative annexin V-stained cells are shown in panel F (EM011-treated) as green rings surrounding early apoptotic cells (solid arrowhead) as opposed to the control untreated cells in panel E, where green rings are absent. However, some nonspecific staining is evident. Scale bar = 20 μm. (G-J) EM011 reduces mitochondrial transmembrane potential in a time-dependent manner. CEM/VLB100 cells were treated with EM011 for 0, 24, 48, and 72 hours, incubated with 50 nM DiOC6, and then analyzed by flow cytometry. The x-axis represents the DiOC6 fluorescence intensity, and the y-axis represents the number of cells. (K) Quantitation of caspase-3 activity in EM011-treated CEM and CEM/VLB100 cells. Cells were treated with 10 μM EM011 for 0, 24, 48, and 72 hours, and caspase-3 activity was analyzed using the luminogenic substrate Z-DEVD-aminoluciferin. *P < .01. (L) Representative immunoblot of cleaved caspase-3 and cleaved PARP along with the loading control, actin. Results are representative of 3 independent experiments performed.
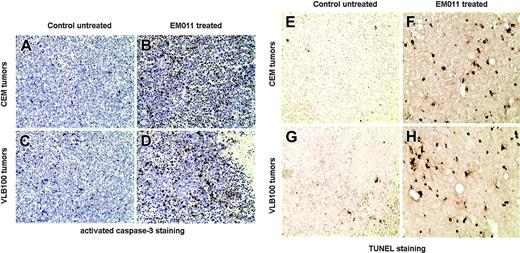
We next asked whether EM011 caused tumor regression in the xenografts by triggering apoptosis. Consistent with our results achieved from caspase-3 activation and TUNEL assays in cultured cells, we observed a widespread staining of activated caspase-3 (Figure 5B-D) and TUNEL+ cells (Figure 5F,H) in the regressed tumors of EM011-treated CEM and CEM/VLB100 xenografts.
These results indicate that EM011 causes tumor regression by inducing apoptosis.
EM011 causes morphologic changes in cells and increases TUNEL+ staining. Untreated CEM and CEM/VLB100 cells (A-B) show normal microtubule arrays and nuclei in interphase and display bipolar spindles in mitosis (insets). Prolonged EM011 treatment causes devastating morphologic changes and induces the formation of apoptotic bodies (C-D). (E-H) Quantitation of apoptosis by TUNEL analysis in untreated control cells (E-F) and EM011-treated cells (G-H). Scale bar = 40 μm. Results are representative of 2 independent experiments performed in triplicate.
EM011 causes morphologic changes in cells and increases TUNEL+ staining. Untreated CEM and CEM/VLB100 cells (A-B) show normal microtubule arrays and nuclei in interphase and display bipolar spindles in mitosis (insets). Prolonged EM011 treatment causes devastating morphologic changes and induces the formation of apoptotic bodies (C-D). (E-H) Quantitation of apoptosis by TUNEL analysis in untreated control cells (E-F) and EM011-treated cells (G-H). Scale bar = 40 μm. Results are representative of 2 independent experiments performed in triplicate.
Histologic evaluation of EM011 therapy
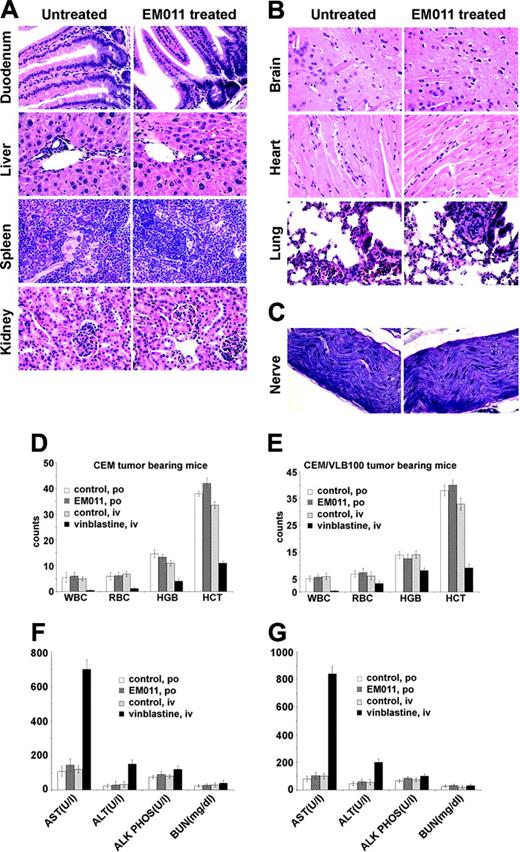
Toxicity is a serious concern for the management of human cancers by chemotherapeutic agents, including the microtubule-interfering agents taxanes and vinca alkaloids.2,4,19-21 To determine whether EM011 treatment results in toxicities to normal tissues, we examined tissue sections of the duodenum, liver, spleen, kidney, heart, brain, lung, and sciatic nerve of tumor bearing mice by hematoxylin and eosin staining (Figure 6A-C). Our results showed that EM011 did not cause any detectable pathologic abnormalities in the mice. There was a complete absence of metastatic lesions in these organs in EM011-treated groups. As shown in Figure 6A, the duodenum showed normal mucosa, submucosa, and muscularis mucosa; the liver showed normal hepatic lobular architecture and intact portal tracts; the splenic follicles and vascular sinusoids were indistinguishable between the EM011-treated and untreated control groups; and the kidneys showed normal glomeruli, tubules, interstitium, and blood vessels. Microsections of the brain did not reveal any infarcted areas, and the cerebral cortex and gray and white matters were normal in the EM011-treated group (Figure 6B). The cardiac myocytes and interstitium also appeared normal, and the lung tissue showed normal alveoli and bronchioli (Figure 6B). In addition, EM011 did not cause degeneration of the long sciatic nerve, as shown by Luxol fast blue/periodic acid-Schiff staining (Figure 6C).
EM011 is effective against human lymphoma tumor xenografts. Both EM011 and vinblastine reduce tumor volume in mice bearing CEM tumors (A). However, only EM011 is effective against CEM/VLB100 tumors (B). Panels C and D show that EM011 treatment is well-tolerated and mice do not suffer from body weight loss, whereas vinblastine reduces body weight significantly and causes morbidity. (E-F) Kaplan-Meier analysis reveals that EM011 confers an apparent survival advantage over vinblastine. Tumor volume is shown as mm3± SE; *P < .01.
EM011 is effective against human lymphoma tumor xenografts. Both EM011 and vinblastine reduce tumor volume in mice bearing CEM tumors (A). However, only EM011 is effective against CEM/VLB100 tumors (B). Panels C and D show that EM011 treatment is well-tolerated and mice do not suffer from body weight loss, whereas vinblastine reduces body weight significantly and causes morbidity. (E-F) Kaplan-Meier analysis reveals that EM011 confers an apparent survival advantage over vinblastine. Tumor volume is shown as mm3± SE; *P < .01.
EM011 inhibits tumor growth by triggering apoptosis. (A-D) Activated (cleaved) caspase-3 was examined by immunohistochemical staining of paraffin-embedded tumor sections from untreated control (A,C) and EM011-treated (B,D) mice. (E-H) Representative TUNEL-stained micrographs of tumor sections from untreated control (E,G) and EM011-treated (F,H) mice. Original magnification, × 200.
EM011 inhibits tumor growth by triggering apoptosis. (A-D) Activated (cleaved) caspase-3 was examined by immunohistochemical staining of paraffin-embedded tumor sections from untreated control (A,C) and EM011-treated (B,D) mice. (E-H) Representative TUNEL-stained micrographs of tumor sections from untreated control (E,G) and EM011-treated (F,H) mice. Original magnification, × 200.
EM011 does not cause obvious pathologic abnormalities in normal tissues. (A-C) Hematoxylin and eosin staining of paraffin-embedded sections of the duodenum, liver, spleen, kidney (A), brain, heart, lung (B), and sciatic nerve (C). Original magnification, × 400 (objective, 40 ×/0.65 NA). Panels D and E show the white blood cells (WBC) and red blood cells (RBC) counts, and hemoglobin (HGB) concentrations, and hematocrit (HCT) values of mice bearing tumor xenografts; P < .05. Panels F and G show the levels of alanine transaminase (ALT), aspartate aminotransferase (AST), alkaline phosphatase (ALK PHOS), and blood urea nitrogen (BUN) of mice bearing tumor xenografts.
EM011 does not cause obvious pathologic abnormalities in normal tissues. (A-C) Hematoxylin and eosin staining of paraffin-embedded sections of the duodenum, liver, spleen, kidney (A), brain, heart, lung (B), and sciatic nerve (C). Original magnification, × 400 (objective, 40 ×/0.65 NA). Panels D and E show the white blood cells (WBC) and red blood cells (RBC) counts, and hemoglobin (HGB) concentrations, and hematocrit (HCT) values of mice bearing tumor xenografts; P < .05. Panels F and G show the levels of alanine transaminase (ALT), aspartate aminotransferase (AST), alkaline phosphatase (ALK PHOS), and blood urea nitrogen (BUN) of mice bearing tumor xenografts.
Hematologic evaluation of EM011 therapy
We also performed a CBC analysis and found that EM011 did not alter the counts of white blood cells (WBCs) or red blood cells (RBCs) nor hemoglobin and hematocrit levels in animals bearing CEM and CEM/VLB100 xenografts (Figure 6D-6E). In contrast, vinblastine-treated animals suffered a marked reduction in the RBC and WBC counts and hemoglobin and hematocrit levels (Figure 6D-E). We also evaluated the organassociated toxicity in untreated, vinblastine-treated, and EM011-treated groups of mice (Figure 6F-G). We found that the ALT and AST, which are indicative of hepatic dysfunction, were comparable between EM011-treated and untreated mice (Figure 6F-G). However, vinblastine-treated groups showed increased ALT and AST levels, suggesting hepatoxicity.
Discussion
As our knowledge of microtubule-interfering drugs is advancing, we are recognizing that the mechanisms underlying the anticancer activity of these drugs may primarily lie in their inhibitory effects on microtubule dynamics, rather than in their effects on microtubule polymer mass. It is becoming appreciated that the maximal tolerated dose is not a better criteria than maximum therapeutic dose.1 Thus, chemical compounds that weakly bind to tubulin and suppress microtubule dynamics without significantly affecting microtubule polymer mass, such as estramustine,22 noscapine,10 and dicoumarol,23 are expected to display anticancer activity without causing deleterious toxicity to normal tissues, offering better therapeutic outcomes. Because EM011 belongs to the noscapinoid class of antimicrotubule agents, its nontoxic histologic as well as hematologic profile may be attributed to its subtle effects on microtubule dynamics without altering the total microtubule polymer mass.10,14
Drug resistance is another serious factor that limits the applicability of microtubule-interfering drugs for cancer therapy. Multidrug resistance, mainly due to Pgp overexpression, makes successful chemotherapy more complex and difficult to achieve. In this study, we have shown that EM011 is not only effective against the CEM human lymphoblastoid cell line, but is also effective against the vinblastine-resistant derivative line that exhibits multidrug resistance, CEM/VLB100, both in cultured cells and in the mouse model. EM011 is clearly promising for the therapy of vinblastine refractory tumors and, more importantly, for the therapy of MDR tumors. Mutations in the drug-binding sites on tubulin have also been implicated in the emergence of drug resistance.5,6,24 Because the microtubule is a validated target of anticancer drugs,1,2,25 it is necessary that we take advantage of other drug-binding sites that can evade drug resistance arising from mutations in the existing sites. Interestingly, noscapinoids appear to bind tubulin at a site different from the sites for taxanes and vinca alkaloids.25 This offers a unique opportunity to further exploit the potential of the microtubule as a target for cancer chemotherapy.
Taken together, our data provide compelling evidence that EM011 is effective against MDR human tumor cells as well as tumor xenografts by inducing apoptosis. In addition, EM011 is not toxic to normal tissues at the doses effective for tumor regression. Therefore, we believe that EM011 is a safe and effective anticancer drug with a potential for the oral treatment of drug-resistant lymphomas and holds great promise for further clinical studies.
Prepublished online as Blood First Edition Paper, November 10, 2005; DOI 10.1182/blood-2005-08-3516.
Supported by a grant from the National Institutes of Health (H.C.J.) and a startup fund from Nankai University (J.Z.).
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We wish to thank Dr William T. Beck for providing cell lines and members of the Joshi Laboratory for helpful discussions. We are grateful to Dr Meenakshi Gupta for blindly evaluating animal tissue sections.