A trifunctional bispecific antibody (BiLu) directed against murine CD3 and human epithelial-cell adhesion molecule (EpCAM) was tested for its ability to improve cell-mediated adoptive immunotherapy in a murine model of B16 melanoma cells transfected with human EpCAM. Intraperitoneal inoculation of naive C57BL/6 (C57) splenocytes induced lethal graft versus host disease (GVHD) in 85% to 97% of sublethally irradiated (BALB/c × C57BL/6) F1 (F1) hosts inoculated intraperitoneally with a sublethal or lethal dose of melanoma cells. BiLu antibodies given intraperitoneally concomitantly with alloreactive C57 cells effectively prevented GVHD-related and tumor-related death in 16 of 25 F1 mice inoculated with a sublethal tumor-cell dose and in 10 of 20 mice inoculated with a lethal tumor-cell dose over a follow-up period of more than 200 days. BiLu treatment also efficiently prevented severe GVHD, which was induced by high doses of BALB/c-derived splenocytes. Trifunctional bispecific antibodies (TbsAbs) capable of cross-linking T lymphocytes, natural killer, and other FcγR-positive effector cells, via their Fc region, to the tumor cells may be applied together with adoptive allogeneic-cell therapy to maximize antitumor responses while acting on GVHD in patients with minimal residual disease.
Introduction
Bispecific antibodies (bsAbs) consisting of 2 different antigenic specificities have been developed as immunotherapeutic reagents for targeting immune cells to tumor tissue.1-5 This strategy is based on the assumption that appropriate effector-target cell interaction via a physical contact between immune cells and tumor cells activates cytotoxic mechanisms that lead to an efficient eradication of tumor cells. Many different bispecific antibody formats have been created over the last 20 years with varying quality as to production, in vitro and in vivo efficacy, and recruitment of effector cells including the creation of additional T-cell stimuli.6-10 Several of these constructs have reached the clinical phase with minor to moderate effects.11-15 Trifunctional bispecific antibodies (TbsAbs) are artificially engineered immunoglobulins with a unique composition of heavy chains of mouse IgG2a and rat IgG2b representing highly homologous Ig subclasses. Both isotypes are very potent in terms of immunologic effector functions, such as complement-dependent cytotoxicity (CDC) and antibody-dependent cell-mediated cytotoxicity (ADCC). It is noteworthy that the Fc region composed of these 2 subclasses effectively binds to human FcγI and FcγIII receptors on accessory cells (eg, macrophages, dendritic cells, and natural killer cells) but not to the inhibitory Fcγ receptor type II expressed, for example, on B cells.16 As a consequence, TbsAbs can not only redirect T cells to tumor cells but also induce recruitment and activation of accessory cells through their Fc region. The simultaneous activation of different mechanisms at the tumor site such as phagocytosis, perforin-mediated lysis, and cytokine release results in a particularly efficient destruction of tumor cells.17,18 Remarkably, apoptosis-resistant tumor cells also can be eliminated by this process.17 As a further consequence of the so induced uptake of tumor material by the antigen-presenting system, even a long-lasting protective antitumor immunity could be established as already demonstrated in 2 immunocompetent murine tumor models.19 While most bsAb treatments aiming to achieve antitumor response have been combined with syngeneic/autologous-derived cells, we and others7,20-24 have used an immunotherapeutic strategy based on the allogeneic reaction of major histocompatibility-mismatched cells, known in clinical practice as donor lymphocyte infusion (DLI), following hematopoietic stem cell transplantation (SCT). Unfortunately, the use of allogeneic-cell therapy (alloCT) in the context of currently applicable protocols in experimental models and in clinical practice is frequently accompanied by life-threatening acute and chronic graft versus host disease (GVHD) that is difficult to control effectively with currently available treatments.25-30 Because bispecific antibodies allow the immune cells to target tumor cells, it might be useful to combine bsAbs with alloCT to direct alloreactive cells to tumor tissue rather than to normal host cells, thereby allowing development of more efficient graft versus tumor (GVT) effect as well as to minimize the risk of GVHD. In the following study, F1 recipients with melanoma cells expressing EpCAM as the target molecule were treated with TbsAbs (CD3 × EpCAM) in conjunction with naive parental antihost-reactive lymphocytes to assess their capability to selectively eliminate tumor cells while sparing normal host cells, thereby avoiding lethal GVHD.
Materials and methods
Mice
Female BALB/c H-2d (BALB), C57BL/6 H-2b (C57), and (BALB × C57BL/6)F1 H-2d/b (F1) mice aged 10 to 12 weeks and weighing 22 to 24 g were used in this study. All mice were purchased from Harlan, Jerusalem, Israel, and maintained in the animal facility of the Hadassah University Hospital with sterilized food and water ad libitum, in full compliance with the Israeli regulations and US Public Health Service policy on humane care and use of laboratory animals for the protection of animal rights.
Tumor cells
A murine model of melanoma cell line (B16) transfected with human EpCAM was used in the experiments.19 The tumor cells were maintained in RPMI 1640 medium supplemented with 5% fetal bovine serum (FBS) (GIBCO, Grand Island, NY), 2 mM l-glutamine, 1% nonessential amino acids, 1 mM sodium pyruvate, 0.5 mg/mL Geneticin (GIBCO), 100 U/mL penicillin, and 100 μg/mL streptomycin. All culture supplements (except FBS and Geneticin) were purchased from Biological Industries, Beit Haemek, Israel. Cells were kept at 37°C in a humidified 5% CO2 air incubator. B16-EpCAM cells were harvested by 0.25% trypsin in 0.05% EDTA, washed with RPMI 1640, and resuspended for intraperitoneal inoculation (0.25 mL per mouse).
Spleen cells
Splenocytes were prepared by teasing spleen cells over a metal mesh, filtering them through nylon mesh, and suspending them in phosphate-buffered saline (PBS) for intraperitoneal injection (30 × 106 per mouse).
Radiation therapy
Sublethal total body irradiatation (TBI) was administered using a 6MEV linear accelerator at a dose rate of 1.9 Gy/min (4 Gy) to prevent rejection of donor spleen cells used for induction of GVHD and GVT effects.
Antibody treatment
The trifunctional bispecific antibody (BiLu) containing specific antigen-binding sites directed against murine CD3 and human EpCAM was used in the experiments. It was prepared as previously described.19 Briefly, the BiLu consists of the 2 parental antibodies 17A2-specific for murine CD331 and C215 directed to human EpCAM (kindly provided by Mr Dohlsten, Pharmacia UpJohn, Uppsala, Sweden). It is an intact bsAb with the IgG subclass combination of rat IgG2b × mouse IgG2a, produced by quadroma technology.32 Purification of BiLu was performed using protein A and cation exchange chromatography as described.6 BiLu was given intraperitoneally either alone or in conjunction with cell therapy (10 μg per mouse).
Experimental design
Recipient F1 mice were conditioned with nonlethal TBI of 4 Gy. Twenty-four hours later, 5 × 103 or 5 × 104 B16-EpCAM tumor cells were inoculated intraperitoneally. On the following day, 30 × 106 naive spleen cells derived from C57 or BALB donors were incubated with BiLu antibodies for 10 minutes on ice, and then the mixture was inoculated intraperitoneally.
Follow-up
In all experiments, mice were checked daily for the appearance of signs and symptoms of GVHD such as hunched posture, ruffled fur, diarrhea, and cachexia as assessed by weight. Body weight was measured on a weekly basis. Survival was monitored, and GVHD-related death was determined if mice were showing GVHD symptoms. In addition, mice were investigated postmortem for detection of the presence of tumor metastases in the peritoneum to determine tumor-related death.
Flow cytometry analysis
B16-EpCAM tumor cells (4 × 105) were incubated with 0.5 μg BiLu antibody in staining buffer (PBS containing 1% bovine serum albumin and 0.03% sodium azide) for 30 minutes on ice, washed, and incubated with FITC F(ab′)2 fragment mouse anti-rat IgG (H+L) (Jackson ImmunoResearch Labs, West Grove, PA) for 30 minutes on ice. Tumor cells were analyzed by fluorescence-activated cell sorting (FACS) (FACStar plus; Becton Dickinson, San Jose, CA), and the percentage of tumor cells expressing EpCAM determinant was measured. In all tests more than 88% of B16-EpCAM cells stained positive (data not shown). These studies confirmed that the BiLu antibody was capable of recognizing tumor cells most efficiently.
Statistical analysis
Body weights were presented as mean plus or minus standard error (SE). The Kaplan-Meier method was used to calculate the probability of survival as a function of time after tumor inoculation.33 The statistical significance of survival between pairs of Kaplan-Meier curves was evaluated by the log-rank test.34 Statistical significance of differences in body weights of control versus BiLu-treated mice was evaluated by standard 2-tailed, unpaired Student t test. A value of P below .05 was considered statistically significant.
Results
The effect of BiLu treatment on GVHD induction in tumor-free mice compared with mice inoculated with B16-EpCAM tumor cells
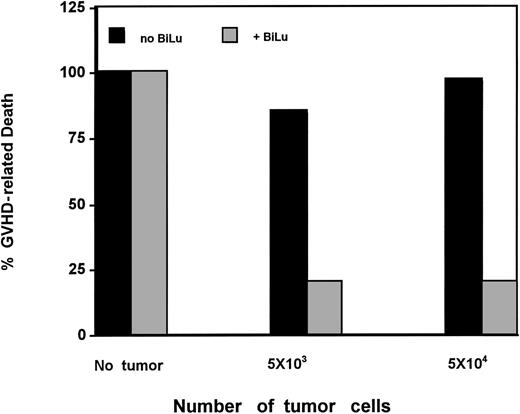
BiLu treatment was tested for its effect on GVHD induction in tumor-free mice as compared with tumor-bearing mice. Results presented in Figure 1 show that injection of haploidentically mismatched C57 splenocytes into tumor-free F1 mice induced lethal GVHD in 18 of 18 animals. Pretreatment of naive C57 cells with BiLu did not prevent GVHD-related death in 5 of 5 tumor-free mice. The median survival of these mice was 19 and 24 days, respectively. As seen in Figure 1, pretreatment of naive C57 cells with BiLu prevented GVHD induction in 80% of tumor-bearing mice. Five of 25 mice and 4 of 20 mice inoculated with 5 × 103 or 5 × 104 tumor cells and treated with BiLu died of GVHD after a median survival of 76 and 30 days, respectively. Statistical analysis revealed that pretreatment of the GVHD induction inoculum (allogeneic C57BL/6 cells) with BiLu produced a significantly better anti-GVHD effect in tumor-bearing mice than in similarly treated tumor-free recipients inoculated with BiLu-pretreated C57 cells (P < .001).
Susceptibility of tumor-free F1 mice versus F1 mice inoculated with B16-EpCAM melanoma cells to lethal GVHD induced by C57 splenocytes given intraperitoneally with or without BiLu.
Susceptibility of tumor-free F1 mice versus F1 mice inoculated with B16-EpCAM melanoma cells to lethal GVHD induced by C57 splenocytes given intraperitoneally with or without BiLu.
The effect of C57 splenocytes given with or without BiLu on the development of GVHD assessed by measurement of body weight of F1 mice inoculated intraperitoneally with 5 × 104 B16-EpCAM melanoma cells.
The effect of C57 splenocytes given with or without BiLu on the development of GVHD assessed by measurement of body weight of F1 mice inoculated intraperitoneally with 5 × 104 B16-EpCAM melanoma cells.
The effect of BiLu treatment on GVHD symptoms
GVHD was induced by intraperitoneal inoculation of naive splenocytes derived from C57 donors in sublethally irradiated F1 mice that had been inoculated intraperitoneally with 5 × 103 B16-EpCAM tumor cells. A high percentage of mice inoculated with naive C57 splenocytes (23 of 27) displayed GVHD symptoms such as hunched posture, ruffled fur, diarrhea, and loss of 2 g of body weight over 18 days (Figure 2). On the other hand, only 5 of 25 mice inoculated with BiLu-pretreated C57 splenocytes displayed GVHD symptoms. Most of the mice inoculated with C57 cells pretreated with BiLu gained 2 to 3 g in weight over 18 days (Figure 2) and maintained a normal healthy appearance for more than 200 days. Statistically significant differences (P < .05) in body weight following inoculation of untreated as compared with BiLu-pretreated naive C57 splenocytes were observed on days 10 and 18. One injection of naive BALB splenocytes into F1 mice inoculated with B16-EpCAM tumor cells (5 × 103) caused mild GVHD symptoms, such as hunched posture and ruffled fur, and most of the mice finally recovered. After a second administration of BALB splenocytes these symptoms were more prominent, leading to lethal GVHD in 7 of 27 mice. In contrast, mice inoculated with BALB splenocytes pretreated with BiLu antibodies did not display any signs or symptoms of GVHD, and all of them remained disease-free survivors (10 of 10) during a follow-up period of more than 200 days (Table 1).
The effect of host semiallogeneic lymphocytes and trifunctional bispecific antibody on survival
C57 splenocytes, which are syngeneic to the B16-EpCAM tumor cells but haploidentically mismatched to the host cells, were inoculated into sublethally irradiated F1 mice with or without BiLu pretreatment. Control F1 mice that received injections of 5 × 103 tumor cells and were inoculated with C57-derived naive splenocytes died (26 of 27) after a median of 20 days, while only 9 of 25 F1 mice treated with BiLu-pretreated naive C57 splenocytes died; 16 of 25 mice remained tumor free with no signs of GVHD for a median of more than 212 days (Table 2). BiLu treatment without cell therapy had an antitumor effect on mice inoculated with a low tumor-cell dose of 5 × 103. Its efficacy in preventing GVHD in tumor-bearing mice, supposedly by targeting the donor T cells to the tumor, encouraged us to test its effect in mice inoculated with a higher tumor-cell dose (5 × 104) and proved lethal in 100% of untreated mice (68 of 68) with a median survival of 21 days. Although BiLu treatment without cell therapy also had a substantial antitumor effect on the high tumor-cell dose (10 of 17 mice remained tumor free), BiLu treatment given concomitantly with AlloCT using C57 naive splenocytes resulted in 10 of 20 healthy-appearing mice with no GVHD and no evidence of tumor for more than 220 days. Treatment with BiLu protected recipients of alloreactive C57 spleen cells, as GVHD-related death was observed in only 4 of 20 mice inoculated with naive C57 cells, whereas 31 of 32 untreated control mice inoculated with untreated naive cells died of GVHD after a median of 19 days (Table 3).
The effect of BiLu treatment on tumor AlloCT
BALB splenocytes, which are fully mismatched to B16-EpCAM tumor cells but haploidentically mismatched to host alloantigens, were inoculated into sublethally irradiated (4 Gy) F1 mice with or without prior pretreatment with BiLu. One dose of BALB splenocytes in mice inoculated with 5 × 103 tumor cells neither produced a GVT effect (18 of 32 mice died of tumor) nor caused lethal GVHD (only 1 of 32 mice died of GVHD) (Table 4). Two doses of BALB splenocytes (a total of 60 × 106) cells, however, induced GVT effect accompanied by severe GVHD, resulting—in 27 mice—in 6 tumor-related deaths, 7 GVHD-related deaths, and 14 disease-free survivors. Mice inoculated with 5 × 103 tumor cells and treated with BiLu and 1 or 2 doses of BALB splenocytes led to 19 of 20 and 10 of 10 tumor- and GVHD-free mice, respectively, after a follow-up period of more than 212 days (Table 4). Notably, naive BALB splenocytes given to mice inoculated with higher doses of tumor cells (5 × 104) did not cause GVHD but postponed tumor-related death for a median of 52 days, in contrast to the median of survival of 21 days for untreated control mice (Table 5). Treatment with BiLu given concomitantly with BALB splenocytes resulted in disease-free survival in 13 of 22, which was not statistically significantly different (P = .414) from BiLu treatment alone given without BALB splenocytes in mice inoculated with 5 × 104 tumor cells (10 of 17 mice survived; Table 5).
Discussion
The trifunctional bispecific antibody, BiLu, directed against the human tumor antigen (EpCAM) and murine CD3, given with lymphocytes alloreactive against the host, successfully prevented lethal GVHD while exerting an efficient antitumor effect, leading to disease-free survival of more than 200 days in mice inoculated with EpCAM-transfected B16 melanoma. The anti-GVHD effect of BiLu was especially evident in sublethally irradiated F1 mice inoculated with parental C57 splenocytes. Because the intensity of GVHD induced by a similar dose of parental naive BALB splenocytes was far less dramatic, as has been discussed elsewhere,35 the anti-GVHD effect of BiLu in this setting could not be evaluated. However, when 2 doses of BALB-derived splenocytes were given and more severe GVHD developed, the anti-GVHD effect of BiLu was also clearly evident in this setting.
A major goal of our study was to determine the possible role of tumor existence on targeting the alloreactive lymphocytes by BiLu to the tumor to achieve the most efficient GVT effect while controlling or minimizing GVHD development by diverting the alloreactive lymphocytes away from healthy tissues.
Remarkably, the anti-GVHD effect of BiLu clearly depended on the presence of tumor cells bearing the EpCAM antigen in the recipient. While even a low dose of tumor cells was sufficient to confer significant protection from lethal GVHD in 80% of the mice, BiLu did not prevent GVHD induction in naive tumor-free F1 mice inoculated with C57 splenocytes. This result suggests that prevention of GVHD by BiLu was not mediated via depletion of an alloreactive-cell subset that plays a major role in the GVH reaction but was due, rather, to the fact that in the presence of EpCAM-expressing target cells the donor lymphocytes were diverted to the tumor, away from GVHD-sensitive organs in the host. The fact that the BiLu antibody was bound to more than 88% of the tumor cells, as tested by us using FACS analysis, supports our assumption that in the tumor-bearing host a preferential affinity to tumor cells was helpful in preventing GVHD. Direct binding and retargeting of Fcγ receptor type I- and III-positive cells (eg, NK cells and macrophages) and CD3+ T cells to the tumor by TbsAbs was visualized as previously documented.7,16,18,19,36 In view of another report showing that in the absence of tumor target, BsAb treatment by itself does not activate systemic autochthonous stimulation of T cells,37 one may assume that the GVHD that occurred in nontumor-bearing F1 hosts was mediated primarily by the inoculated spleen cells themselves without the risk of engagement of BiLu-activated host cells.
As shown here and as reported previously,19 BiLu treatment alone given after inoculation of a low dose of tumor cells can provide an efficient antitumor effect.
The antitumor effect mediated by C57 splenocytes alone could not be evaluated due to the incidence of severe and lethal GVHD. In contrast, some beneficial GVT effect (13 of 32 mice survived; Table 4) could be observed after treatment with naive BALB splenocytes without BiLu, because GVHD was mild and nonlethal. This antitumor effect was further amplified (14 of 27 mice survived; Table 4) by inoculation of 2 consecutive doses of BALB splenocytes (P ≤ .006 for comparison with 1 dose of BALB or with a control of tumor alone). The use of alloCT by itself has previously been shown to be an effective immunotherapeutic strategy for achieving graft versus leukemia (GVL)/GVT effects in experimental animal models of leukemia and mammary carcinoma27,28 as well as in patients with hematologic malignancies and metastatic solid tumors.20-26 In the present study, GVT effects against tumor cells were dramatically improved by the addition of BiLu treatment (10 of 10 mice survived; Table 4), which enhanced the targeting of alloreactive effector cells while diverting such cells away from host tissues susceptible to GVHD. Remarkably, no mouse was lost due to GVHD in the BiLu group whereas 7 of 27 mice died of GVHD and 6 of 27 of tumor growth after 2 splenocyte infusions in the non-BiLu group.
Due to the trifunctional properties of the BiLu (CD3 × EpCAM) used in our study, it was feasible to target naive splenocytes containing CD3+ T cells and FcγR-positive natural killer (NK) cells as well as monocytes and antigen-presenting dendritic cells, all of which may contribute to the achievement of a most effective antitumor response.
Immunocytochemical and computerized videomicroscopic techniques applied previously in tumor models of prostate carcinoma showed that the antitumor activity of the BiLu antibody is mediated through nonapoptotic signals that induce necrotic killing via pore-forming proteins released by various cell subsets that are attracted by the BiLu antibody in the local environment of the tumor.18
Because in many primary tumor cells, and more frequently in metastatic cells, the expression of class I major histocompatibility complex (MHC) antigens is either lost or suppressed,38 the combination of T-cell-dependent and the non-MHC-restricted killing activity exerted by accessory cells may circumvent aberrant down-regulated MHC expression by tumor cells.
In our study, the BiLu antibody and the tumor cells were coinjected intraperitoneally to afford the best opportunity for interaction and recruitment of relevant cell subsets in the tumor area, as previously demonstrated in the B16 melanoma model.19 Furthermore, our previous experience with alloCT in another tumor model showed that it was important to administer the effector cells intravenously rather than intradermally or subcutaneously when the tumor cells were inoculated intradermally or subcutaneously.28
The trifunctional BiLu antibody directed to CD3 and EpCAM antigens may serve as a relevant model for a variety of human epithelial tumors overexpressing, for example, EpCAM or Her2/neu or other malignancies expressing tumor-associated antigens as target antigens on their surface.10,13,14,16-18,39,40
Although in principle trifunctional antibodies can be used alone through activation of the patient's own cells, the combination of such constructs with alloCT would make it possible to benefit from different anticancer effector mechanisms to achieve maximal targeting of anticancer effector cells to the tumor site, while in parallel avoiding or minimizing the risk of uncontrolled GVHD.
Prepublished online as Blood First Edition Paper, October 18, 2005; DOI 10.1182/blood-2005-07-2738.
One of the authors (H.L.) has declared a financial interest in a company whose potential product was studied in the present work. We have recently submitted a patent application for the use of trifunctional bispecific antibodies to maximize anticancer effects while avoiding GVHD.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank the Danny Cunniff Leukemia Research Laboratory, the Gabrielle Rich Leukemia Research Foundation, the Novotny Trust, the Fig Tree Foundation, and Ronne and Donald Hess for their continuous support of our ongoing basic and clinical research. We also thank Peter Ruf of TRION Research for kindly providing the BiLu antibody.