Abstract
Mouse gene–targeting studies have documented a central role of the p110δ isoform of phosphoinositide 3-kinase (PI3K) in B-cell development and function. A defect in B-cell antigen receptor (BCR) signaling is key to this B-cell phenotype. Here we further characterize this signaling defect and report that a p110δ-selective small molecule inhibitor mirrors the effect of genetic inactivation of p110δ in BCR signaling. p110δ activity is indispensable for BCR-induced DNA synthesis and phosphorylation of Akt/protein kinase B (PKB), forkhead transcription factor/forkhead box O3a (FOXO3a), and p70 S6 kinase (p70 S6K), with modest effects on the phosphorylation of glycogen synthase kinase 3 α/β (GSK3α/β) and extracellular signal-regulated kinase (Erk). The PI3K-dependent component of intracellular calcium mobilization also completely relies on p110δ catalytic activity. Resting B cells with inactive p110δ fail to enter the cell cycle, correlating with an incapacity to up-regulate the expression of cyclins D2, A, and E, and to phosphorylate the retinoblastoma protein (Rb). p110δ is also critical for interleukin 4 (IL-4)–induced phosphorylation of Akt/PKB and FOXO3a, and protection from apoptosis. Taken together, these data show that defects observed in p110δ mutant mice are not merely a consequence of altered B-cell differentiation, and emphasize the potential utility of p110δ as a drug target in autoimmune diseases in which B cells play a crucial role.
Introduction
Signal transduction through the B-cell antigen receptor (BCR) and cytokine receptors regulates multiple biologic functions such as growth, proliferation, differentiation, and survival, depending on the maturation state of the B lymphocyte.1,4 Intracellular signal transduction by the BCR and cytokine receptors is almost invariably dependent on the activity of the phosphoinositide 3-kinase (PI3K) signaling enzymes.4,5
Mammals have 8 distinct isoforms of PI3K that have been divided in 3 classes on the basis of their lipid specificity and structure.6,7 The class I subset of PI3K enzymes generate lipid second messengers that activate a plethora of downstream targets, including protein kinases (such as Akt/protein kinase B [PKB] and Bruton tyrosine kinase [Btk]), guanosine nucleotide exchange factors (such as P-Rex1 and cytohesins), GTPase-activating proteins (such as members of the centaurin family), and adaptor molecules (such as Bam 32).7,9 Class I PI3Ks are heterodimers consisting of a p110 catalytic subunit and a regulatory subunit. These PI3Ks have been further divided in the IA and IB subclasses, which signal in tyrosine kinase–driven and G protein–coupled receptor pathways, respectively.
Genes for 3 class IA p110 isoforms (p110α, p110β, p110δ) and a single class IB PI3K isoform (p110γ) exist. p110γ and p110δ are mainly expressed in leukocytes, in contrast to p110α and p110β, which have a broader tissue distribution. The class IA regulatory subunits have Src-homology region 2 (SH2) domains that recruit the class IA p110 catalytic subunits to phosphotyrosines, generated in a variety of receptors and their adaptor molecules, leading to translocation of the p110 subunits to the membrane where their lipid substrates reside. Mammals have 3 genes encoding class IA regulatory subunits, which give rise to at least 5 gene products: p85α, p55α, and p50α (all splice variants from the PIK3R1 gene), and p85β and p55γ (encoded by the PIK3R2 and PIK3R3 genes, respectively). There appears to be no selectivity of different class IA p110 subunits to interact with these distinct class IA regulatory subunits. p110γ binds p84 or p101 regulatory subunits, which have no homology to class IA regulatory proteins, and which are important for the activation of p110γ by Gβγ subunits.10
The inhibitors LY294002 and wortmannin have been widely used to implicate the PI3K signaling pathway in cellular signaling, including in cells of the immune system.11,13 However, these agents inhibit most isoforms of PI3K to the same extent, and have many off-target effects.7,13,17 In order to delineate the roles of PI3K isoforms in physiology, mouse gene–targeting studies have been used.18,19 Leukocytes are the most widely investigated cell type in such PI3K mutant mice.2,4,19 These studies have revealed an important role for PI3Ks, especially in B cells. Indeed, targeting of the PIK3R1 gene, for example, leads to impaired B-cell activation, reduced numbers of immature and mature B cells, and reduced in vivo immunoglobulin concentrations.20,21 However, it has not been possible to infer the physiologic roles of the distinct p110 catalytic subunits from these gene-targeting studies on regulatory subunits. Indeed, given that there seems to be no selectivity of specific regulatory subunits to interact with specific p110 isoforms, loss of expression of a single class IA regulatory subunit gene is expected to affect the function of all class IA PI3K isoforms indiscriminately. Moreover, loss of expression of a single regulatory subunit often alters expression of the other regulatory subunits as well as of the p110 proteins. For example, deletion of the p85α subunit leads to reduced expression of all class IA PI3K isoforms.21,22 Such deregulation of PI3K subunit expression has complicated interpretation of the molecular mechanisms underlying the observed phenotypes (discussed in Vanhaesebroeck et al18,23 ).
p110α and p110β knock-out (KO) mice are embryonic lethal,24,25 and the roles of these PI3Ks in immune signaling have not been defined. In contrast, mice with loss of function of the p110δ isoform are viable, and show defects in B cells, T cells, and mast cells.26,29 B cells were not affected by deletion of p110γ (p110γ KO), which instead led to altered signaling and function of T cells, macrophages, neutrophils, and mast cells.30,34 Taken together, the studies in p110γ and p110δ mutant mice have identified these isoforms of PI3K as potential targets for therapeutic intervention in inflammation and autoimmunity.35,37 Recent work in the pharmaceutical industry has demonstrated that the p110 catalytic subunits are good targets for small molecule inhibitors, and compounds with preference for each of the p110 isoforms have now been reported.38,41 Validation of these compounds and their derivatives for use in human disease is now critical.
In our previous studies on p110δ, we applied a gene inactivation strategy in which we replaced the wild-type (WT) p110δ gene by a knock-in (KI) allele that encodes a kinase-dead p110 (p110δD910A), mutated in the ATP-binding site.28,29 This approach more faithfully mimics the action of small molecule inhibitors than KO or transgenic approaches. To some extent, such mutation mimics the effect of systemic administration of a selective p110δ inhibitor. Other than assessing the kinase-dependent activities of p110s, this strategy also overcomes the complication seen in previous PI3K KO studies that altering the expression level of a single PI3K subunit often alters expression of the others.18 However, the germline inhibition of p110δ could potentially lead to compensatory signaling mechanisms or alterations during development that could influence the phenotype observed. From a pharmacologic point of view it was thus important to determine whether the acute inhibition afforded by a chemical inhibitor would reflect the phenotype observed as a consequence of a constitutive germline mutation. We therefore set out to directly compare the impact of genetic and pharmacologic inactivation of p110δ in B-cell function and signaling. Such studies were prompted by the realization that small molecule inhibitors often do not phenocopy the corresponding mouse gene knockouts.42
Materials and methods
Mice
Homozygous p110δ KI (p110δD910A/D910A) mice were generated and maintained on the C57/BL6 background as described.28 WT littermates generated from heterozygous (p110δWT/D910A) matings were used as controls. All mice were maintained according to United Kingdom Home Office guidelines.
Cells
B cells were purified from the spleens of 8- to 10-week-old mice as described.28 In brief, spleen cell suspensions were depleted of erythrocytes by osmotic lysis, and B cells purified by negative selection by a magnetic activated cell-sorting (MACS) separation column (MACS system; Miltenyi Biotec, Bergisch Gladbach, Germany) using anti-CD43 monoclonal antibody (mAb)–coated magnetic microbeads (Miltenyi Biotec). Splenic B cells and the WEHI-231 B-cell lymphoma cell line were cultured in RPMI 1640 medium (Gibco Invitrogen, Paisley, United Kingdom) supplemented (complete RPMI 1640 medium) with 2 mM glutamine (Gibco Invitrogen), 5 × 10–5 M β-mercaptoethanol (Sigma, St Louis, MO), 100 U/mL penicillin (Gibco Invitrogen), 100 μg/mL streptomycin (Gibco Invitrogen), and 10% heat-inactivated fetal calf serum (Gibco Invitrogen).
Antibodies and reagents
The isoform-selective antibodies to p110α, p110β, and p110δ have been described previously.28,29 Commercial sources of antibodies were as follows: antibodies to p110γ were from Alexis Biochemicals (Nottingham, United Kingdom); P-Akt/PKB S473, P-Akt/PKB T308, Akt/PKB, P-GSKα/β S32/9, P-p70 S6K T389, P-p70 S6K T421/S424, P-IκBα S32, P-STAT6 Y641, STAT6, and P-Rb S807/811 were purchased from Cell Signaling Technology (Beverly, MA); IRS-1, IRS-2, P-FOXO3a T32, P-tyrosine (clone 4G10), and p85 (06-195) were purchased from Upstate Biotechnology (Lake Placid, NY); P-Erk Y204 (E-4), p110β (S-19), cyclin D2 (M-20), cyclin E (M-20), cyclin A (C-19), p21 (M-19), p27 (C-19), p107 (C-18), and E2F-1 (C-20) were from Santa Cruz Biotechnology (Santa Cruz, CA); p130-Rb2 (clone 10) was from BD Transduction Laboratories (San Diego, CA); P-Rb T821 was purchased from Biosource International (Camarillo, CA); and GAPDH (6C5) was purchased from Abcam (Cambridge, United Kingdom). Horseradish peroxidase (HRP)–conjugated secondary antibody, γ-[32P]ATP (22 200 × 1010 Bq/mmol [6000 Ci/mmol]) and [3H]-thymidine (18.5 × 1010 Bq/mmol [5.0 Ci/mmol]) were purchased from Amersham Biosciences (Buckinghamshire, United Kingdom). LY294002 was from Calbiochem (La Jolla, CA). F(ab′)2 goat anti–mouse immunoglobulin M (IgM) was purchased from Jackson ImmunoResearch Laboratories (West Grove, PA). Interleukin 4 (IL-4) was purchased from R&D Systems (Minneapolis, MN). Anti-CD40 was purchased from BD PharMingen (San Diego, CA). LY294002, IC87114, and AS-604850 were dissolved in dimethyl sulfoxide (DMSO; Sigma, St Louis, MO) and stored at –20°C. All other reagents were of chemical grade.
Cell stimulations
For BCR stimulation, cells were treated with 10 μg/mL F(ab′)2 goat anti–mouse IgM for the indicated times. For IL-4 stimulation, purified splenic B cells were starved for 1 hour in serum-free RPMI 1640 medium, followed by stimulation with 20 ng/mL IL-4 in the same starvation medium for the indicated times. In all experiments where pharmacologic inhibitors were used, an equivalent volume of DMSO carrier was added to the cells. Pharmacologic agents were added to the cells 30 minutes prior to stimulations.
Proliferation assay
Purified splenic B cells (105/well) were left unstimulated or stimulated with 10 μg/mL F(ab′)2 goat anti–mouse IgM in triplicate using 250 μL of complete RPMI 1640 medium in 96-well plates in the presence of 0.0185 MBq/well (0.5 μCi/well) [3H]-thymidine from the start of the culture, followed by measurement of incorporated radioactivity by scintillation counting. Similar conditions were used for WEHI-231 cells, except that cells were not stimulated with anti-IgM or IL-4, and cultured at 103/well with addition of 0.0185 MBq/well (0.5 μCi/well) [3H]-thymidine during the last 7 hours of the culture.
In vitro PI3K assays
Assays on PI3K isolated from cells were performed as follows. Protein (500 μg) of total cell lysate was immunoprecipitated using either phosphotyrosine peptide matrix YPVPMLG (where YP is phosphotyrosine—the YPVPMLG peptide contains the consensus binding sequence for the SH2 domain of the class IA PI3K regulatory subunits) or antibodies to insulin receptor substrate (IRS)–1 or IRS-2. In the latter case, immunoprecipitations using rabbit anti–mouse IgG were used as controls. Kinase assays were performed using phosphatidylinositol-4,5-bisphosphate (PtdIns(4,5)P2; Lipid Products, Redhill, Surrey, United Kingdom) as a substrate as previously described.43 Radioactivity in the phosphatidylinositol-3,4,5-trisphosphate (PtdIns(3,4,5)P3) spot was measured by a Molecular PhosphorImager FX (Bio-Rad, Hercules, CA) and expressed as arbitrary units. The activity present in the control IgG immunoprecipitates was subtracted from that found in IRS immunoprecipitates. Assays on recombinant p110γ were performed as follows. N-terminally truncated human p110γ (amino acids 39 to 1102; GenBank entry AF327656) in fusion with an N-terminal GST-tag was incubated at room temperature with kinase buffer (10 mM MgCl2, 1 mM β-glycerophosphate, 1 mM DTT, 0.1 mM Na3VO4, 0.1% Na cholate, and 15 μM ATP/3.7 × 103Bq (100 nCi) γ-[33P]ATP; final concentrations) and lipid vesicles containing 18 μM phosphatidylinositol (PtdIns; Sigma) and 250 μM of phosphatidylserine (PtdSer; Fluka, Buchs, Switzerland) (final concentrations), in the presence of inhibitors or the equivalent amount of DMSO. Kinase reactions were stopped by addition of 250 μgof neomycin-coated Scintillation Proximity Assay (SPA) beads (Amersham Biosciences) and processed as described.44 IC50 (50% inhibitory concentration) determinations with full-length GST-tagged human p110α (GenBank entry U79143), GST-human p110β (GenBank entry S67334), and GST-human p110δ (GenBank entry U86453), copurified from TN5 insect cells with human p85α (GenBank entry M61906) to evaluate inhibitor selectivity, were performed as indicated for human p110γ with the exception that ATP and PtdIns concentrations were adjusted to their corresponding Km values obtained for each isoform.41
Immunoblotting
Whole-cell lysates were prepared essentially as described.28 In brief, after the indicated stimulation the cells were collected, washed 2 times with ice-cold phosphate-buffered saline (PBS), and lysed in ice-cold lysis buffer. The insoluble fraction was cleared by centrifugation and the supernatant was adjusted to equal protein concentration (Bio-Rad protein assay kit) for each stimulation condition and then the proteins were reduced with 1 volume of 2 × Laemmli sample buffer. The proteins were resolved by 10% sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE), transferred to polyvinylidene difluoride membranes (Immobilon-P; Millipore, Bedford, MA) and probed with the indicated antisera, followed by detection of immunoreactive proteins by an enhanced chemiluminescence system (Amersham Biosciences).
Calcium flux measurements
The release of Ca2+ into the cytoplasm was measured essentially as described.45 Purified splenic B cells were loaded with 3 μM of the Ca2+-sensitive dye fluo-4 acetoxymethyl ester (Fluo-4 am; Molecular Probes, Eugene, OR) in PBS with 0.5% bovine serum albumin (BSA; Sigma). The cells were washed and resuspended in PBS with 0.5% BSA containing 1 mM CaCl2. After a further incubation of 30 minutes to allow complete de-esterification of intracellular Fluo-4 am ester, the variations in absorbance were measured using a Perkin Elmer (Wellesley, MA) LS55 luminescence spectrometer. The cells were incubated with the inhibitors or DMSO alone for 5 minutes before stimulation. The fold increase in fluorescent intensity of stimulated cells relative to baseline measurements is shown.
B-cell apoptosis assay
Splenic B cells purified as described were stimulated in 24-well plates in complete medium with or without 5 ng/mL IL-4. Twenty-four hours later, the cells were stained with fluorescein isothiocyanate (FITC)–conjugated antibody anti-B220 (BD PharMingen) and 2 μg/mL 7-amino-actinomycin D (7-AAD; BD PharMingen) and analyzed on a FACScalibur flow cytometer (BD Biosciences, San Jose, CA) using the CellQuest software (BD Biosciences) for data acquisition and analysis. Apoptotic cells were identified based on 7-AAD staining and reduced size (FSC intensity). Similar results were obtained using Annexin V (BD PharMingen) as an alternative indicator of apoptosis (data not shown).
Results
Effect of constitutive p110δ inactivation on PI3K subunit expression and PI3K activity in primary B cells
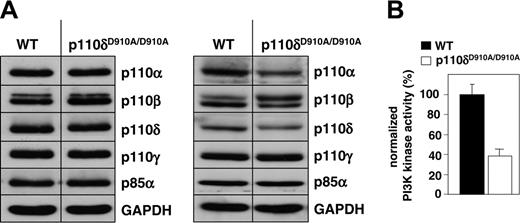
Similar to what was observed in primary T cells and mast cells from p110δD910A/D910A KI mice,28,29 the expression levels of the class I PI3K subunits in p110δD910A/D910A primary splenic B cells were unaltered compared with those in WT B cells (Figure 1A, left panel). However, minor alterations in PI3K subunits expression were occasionally observed (an example is shown in the right panel of Figure 1A). The reason for such altered expression of PI3K subunits is not clear but may be related to the phenotypes resulting from the KI mutation, which may also vary with age. Indeed, we previously documented that p110δD910A/D910A B cells in the spleen appear to have undergone normal differentiation to follicular B cells as assessed by expression of B200, sIgM, and sIgD markers.28 However, p110δD910A/D910A mice lack marginal zone B cells and have a 50% or greater reduction in follicular B cells in the spleen, and hence we cannot exclude that subtle alterations in differentiation or other features in the remaining p110δD910A/D910A B cells affect the characteristics and signaling of the remaining B cells and their functional responses.
We next assessed the impact of the p110δ D910A mutation on the overall class IA PI3K activity in p110δD910A/D910A primary B cells. This was done by measuring the in vitro PI3K activity precipitated in complex with class IA regulatory subunits from total cell extracts.43 As can be seen from Figure 1B, the overall class IA PI3K activity was reduced by approximately 60% in p110δD910A/D910A B cells. The remaining 40% of PI3K activity recovered from p110δD910A/D910A B cells in these experiments most likely represents the contribution of the p110α and/or p110β PI3K isoforms.
p110δ activity is required for progression of B cells into the cell cycle upon BCR stimulation
As previously reported,28 homozygous p110δ inactivation (p110δD910A/D910A) almost completely blocked in vitro proliferation of BCR-stimulated B cells (Figure 2A). Interestingly, B cells heterozygous for the p110δ inactivation (p110δWT/D910A) showed an intermediate proliferative defect (Figure 2A), indicative for a dose-dependent activity of p110δ in BCR signaling that cannot be effectively compensated for by other PI3Ks.
Effect of p110δ inactivation on PI3K expression and activity in primary splenic B cells. (A) Expression of class I PI3K isoforms in total cell lysates (50 μg/lane), as assessed by immunoblotting. Left and right panels are from 2 independent analyses, carried on pooled lysates from 3 mice each. (B) Class IA-associated lipid kinase activity present in primary splenic B cells. Cell extracts were purified using YpVPMLG peptide complexes as described in “Materials and methods,” and assayed by an in vitro PI3K assay using PtdIns(4,5)P2 as substrate. PI3K activity is presented relative to that found in WT cells. Error bars indicate SD.
Effect of p110δ inactivation on PI3K expression and activity in primary splenic B cells. (A) Expression of class I PI3K isoforms in total cell lysates (50 μg/lane), as assessed by immunoblotting. Left and right panels are from 2 independent analyses, carried on pooled lysates from 3 mice each. (B) Class IA-associated lipid kinase activity present in primary splenic B cells. Cell extracts were purified using YpVPMLG peptide complexes as described in “Materials and methods,” and assayed by an in vitro PI3K assay using PtdIns(4,5)P2 as substrate. PI3K activity is presented relative to that found in WT cells. Error bars indicate SD.
Genetic inactivation of p110δ impairs in vitro B-cell proliferation and induction of cell-cycle regulators. (A) Purified splenic B cells were cultured with or without anti-IgM for 48 hours, followed by measurement of [3H]-thymidine incorporation. Error bars indicate SD. (B-C) Cell lysates were prepared from WT and p110δD910A/D910A B cells at the indicated time points following anti-IgM stimulation, followed by assessment of expression of cell-cycle regulators by immunoblotting.
Genetic inactivation of p110δ impairs in vitro B-cell proliferation and induction of cell-cycle regulators. (A) Purified splenic B cells were cultured with or without anti-IgM for 48 hours, followed by measurement of [3H]-thymidine incorporation. Error bars indicate SD. (B-C) Cell lysates were prepared from WT and p110δD910A/D910A B cells at the indicated time points following anti-IgM stimulation, followed by assessment of expression of cell-cycle regulators by immunoblotting.
We next assessed the effect of p110δ inactivation on changes in components of the cell-cycle machinery following BCR crosslinking. As can be seen from Figures 2B and 2C, BCR-induced expression of cyclins D2, A, and E, all known to correlate with cell cycle progression, did not occur upon genetic inactivation of p110δ (no cyclins or other cell-cycle regulators were detected in p110δD910A/D910A B cells after 48 hours; data not shown). This correlated with a lack of phosphorylation of the retinoblastoma protein (Rb) on sites (S807/811 and T821) that are substrates for cyclin-dependent kinase (CDK) 4 and CDK6 during early G1 (when cyclin D2 is in complex with CDK4/6) and CDK2 (in complex with cyclins E and A) later in G1. p107 and p130, pocket proteins related to Rb, were found to be differentially regulated upon BCR engagement in WT B cells, in agreement with previous observations.46,48 Indeed, whereas p130 expression was found to be unaltered during cell cycle, p107 levels were found to be increased and most likely to be hyperphosphorylated (as evidenced by the increase in size on the SDS-PAGE gel). This BCR-dependent induction and phosphorylation of p107 were not observed in p110δD910A/D910A B cells. The levels of E2F1, the downstream target of Rb, were also found to be increased in WT B cells, in line with the knowledge that this protein can induce its own transcription.49,55 Again, this was only observed in WT B cells (Figure 2B; data for 48-hour culture of p110δD910A/D910A B cells are not shown).
Effect of PI3K inhibitors on anti-IgM-induced B-cell proliferation. Primary splenic B cells were cultured with anti-IgM in presence or in absence of the indicated doses of PI3K inhibitors, followed by measurement of [3H]-thymidine incorporation after 48 hours. Results were expressed as percentage of control response of cells that were not treated with PI3K inhibitors. Error bars indicate SD.
Effect of PI3K inhibitors on anti-IgM-induced B-cell proliferation. Primary splenic B cells were cultured with anti-IgM in presence or in absence of the indicated doses of PI3K inhibitors, followed by measurement of [3H]-thymidine incorporation after 48 hours. Results were expressed as percentage of control response of cells that were not treated with PI3K inhibitors. Error bars indicate SD.
The expression level of p27, a CDK inhibitor, was gradually reduced upon BCR engagement in WT, but not in p110δD910A/D910A B cells (Figure 2B). p21, a CDK inhibitor which has the additional capacity to function as an assembly factor for cyclin D1/CDK4 complexes,56 was low in unstimulated cells and only induced in WT B cells (Figure 2B).
Taken together, these results show that loss of p110δ activity results in a complete inability of resting B cells to enter the cell cycle upon BCR stimulation.
Effect of p110δ-selective small molecule inhibitors on BCR-induced proliferation
We next investigated whether the B-cell phenotype of the p110δD910A/D910A KI mice can be mimicked by pharmacologic inhibition of p110δ in WT B cells. For these studies, we used IC87114, a small molecule inhibitor with selectivity for p110δ (Sadhu et al57 ; Table 1). The effect of this compound was compared to that of LY294002, which inhibits all PI3K isoforms, and of AS-604850, a p110γ-specific inhibitor.41 As can be seen from Figure 3 and Table 2, IC87114 was extremely potent at blocking BCR-induced B-cell proliferation, with an IC50 of 0.04-0.14 μM over a 24- to 72-hour time period. The higher potency of this compound to inhibit BCR-induced B-cell proliferation compared with LY294002 correlates with the more potent in vitro inhibitory effect of IC87114 on p110δ activity (Table 1). At higher doses, IC87114 can inhibit p110γ and p110β (Sadhu et al57 ; Table 1). However, treatment with the p110γ inhibitor AS-604850 did not inhibit BCR-stimulated B-cell proliferation over a broad dose-range, but on the contrary, resulted in a modest (20%-30%) increase of [3H]-thymidine incorporation (Figure 3). The reason for this stimulatory effect is unknown at the moment. When tested on its own, AS-604850 did not increase B-cell proliferation (data not shown). In addition, B cells from p110γ KO mice have a normal phenotype, and do not show increased BCR-stimulated proliferation.30 Taken together, pharmacologic intervention with p110δ activity mimics the effect of genetic inactivation of this PI3K on BCR-induced proliferation, in an inhibitor- and gene-dosage–dependent manner (Figures 2A, 3).
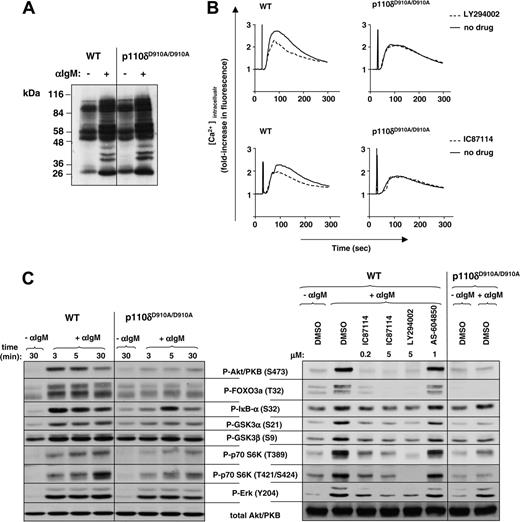
Effect of p110δ inactivation on BCR-driven early signaling in primary splenic B cells. (A) Primary splenic B cells were cultured with or without anti-IgM for 5 minutes, followed by cell lysis and immunoblot analysis of whole-cell lysates for the presence of phosphotyrosine. (B) Calcium flux was determined in presence or absence of PI3K inhibitors in response to stimulation with 10 μg/mL anti-IgM in presence or absence of LY294002 (5 μM) or IC87114 (0.1 μM). (C) lysates from control or anti-IgM–treated B cells were analyzed by immunoblot for the indicated signaling molecules. In the right panel, cells were treated with various PI3K inhibitors prior to 5-minute stimulation with anti-IgM.
Effect of p110δ inactivation on BCR-driven early signaling in primary splenic B cells. (A) Primary splenic B cells were cultured with or without anti-IgM for 5 minutes, followed by cell lysis and immunoblot analysis of whole-cell lysates for the presence of phosphotyrosine. (B) Calcium flux was determined in presence or absence of PI3K inhibitors in response to stimulation with 10 μg/mL anti-IgM in presence or absence of LY294002 (5 μM) or IC87114 (0.1 μM). (C) lysates from control or anti-IgM–treated B cells were analyzed by immunoblot for the indicated signaling molecules. In the right panel, cells were treated with various PI3K inhibitors prior to 5-minute stimulation with anti-IgM.
p110δ activity controls early signaling pathways leading to cell-cycle progression
We next compared the effect of genetic and pharmacologic inhibition of p110δ on intracellular signaling pathways leading to cell-cycle progression. Early BCR-stimulated global tyrosine phosphorylation was found to be intact in p110δD910A/D910A B cells (Figure 4A), indicating that these early signaling pathways are intact in these cells.
Mobilization of intracellular Ca2+ in p110δD910A/D910A B cells in response to anti-IgM crosslinking was reduced (Figure 4B, right panels; Okkenhaug et al28 ). The pan-PI3K inhibitor LY294002 did not lead to a further decrease in intracellular Ca2+ flux in p110δD910A/D910A B cells (Figure 4B, top panel), implying that PI3K isoforms other than p110δ do not significantly participate in this response. The residual Ca2+ flux that is present upon LY924002 treatment of WT and p110δD910A/D910A B cells is indicative of PI3K-independent components of Ca2+ flux triggered by BCR ligation. That p110δ largely controls the PI3K-dependent fraction of the BCR-induced Ca2+ flux is further documented by the observation that IC87114 has an equivalent impact to LY294002 on Ca2+ mobilization in WT B cells (Figure 4B, lower panels).
As previously reported,28 BCR-stimulated activation of Akt/PKB was inhibited by greater than 95% in p110δD910A/D910A B cells (Figure 4C, left panel). Inhibition of phosphorylation of downstream targets of Akt/PKB was often almost complete such as in the case of forkhead transcription factor/forkhead box O3a (FOXO3a) and glycogen synthase kinase 3α (GSK3α) (Figure 4C, left panel). Phosphorylation of p70 S6 kinase (p70 S6K) on sites which are indirectly controlled by Akt/PKB activation (T389 and T421/S42458,61 ) was also severely attenuated (Figure 4C, left panel). Phosphorylation of IκB (inhibitor of nuclear factor [NF]–κB) was inhibited at some but not all time points (Figure 4C, left panel), which suggests that other PI3K isoforms in addition to p110δ and/or PI3K-independent signaling pathways contribute to NF-κB signaling downstream of the BCR.62,63
In contrast to the substantial inhibitory effect of p110δ inactivation on Akt/PKB phosphorylation, blockade of the extracellular signal-regulated kinase (Erk) pathway was modest (Figure 4C, left panel), as previously reported.28 Over the course of many experiments, we have found the effect of genetic inactivation of p110δ on Erk phosphorylation to be variable (data not shown), most likely related to subtle variations in the strength of the stimulus and differing responsiveness of the B cells in different experiments.64,67
Pharmacologic inhibition of p110δ using IC87114 on WT B cells mirrored the effect of genetic inactivation of p110δ, apart from the inhibition of Erk and GSK3 phosphorylation which appeared to be somewhat stronger when using IC87114 (Figure 4C, right panel). The p110γ inhibitor AS-604850 did not affect signaling downstream of the BCR (Figure 4C, right panel).
p110δ activity is required for IL-4–mediated signaling leading to B-cell survival
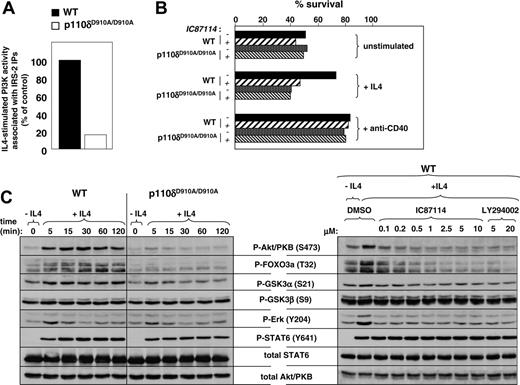
IL-4 is another stimulus known to induce PI3K activity in primary B cells.68,69 This occurs via induction of tyrosine phosphorylation of the adaptor molecule IRS-2 on PI3K-binding motifs, leading to recruitment of class IA PI3Ks.69,70 Interestingly, IL-4–induced PI3K activity associated with IRS-2 was almost completely abrogated in p110δD910A/D910A B cells (Figure 5A; no PI3K activity was found associated with IRS-1; data not shown). These results indicate that p110δ might play an important role in PI3K signaling downstream of the IL-4 receptor.
We therefore investigated the effect of p110δ inactivation on IL-4–induced protection from apoptosis.71 Splenic B cells from WT and p110δD910A/D910A KI mice were incubated with or without IL-4, in the presence or absence of IC87114, followed by measurement of apoptosis 24 hours later. As expected, only a fraction of WT and p110δD910A/D910A B cells survive upon culture without IL-4 (Figure 5B). In the presence of IL-4, WT but not p110δD910A/D910A B cells were protected from apoptosis. IC87114 blocked the IL-4–mediated protection from cell death in WT B cells (Figure 5B) but did not alter the percentage of surviving p110δD910A/D910A B cells. Interestingly, protection provided by ligation of the CD40 receptors was not sensitive to IC87114 treatment (Figure 5B). These results are in line with the knowledge that this pathway is regulated by the tumor necrosis factor receptor–associated factors (TRAFs) without the need for PI3K activity.72,74
We next investigated the effect of genetic and pharmacologic inactivation of p110δ on early signaling events induced by IL-4. Phosphorylation of Akt/PKB on S473 was similarly abolished in p110δD910A/D910A and IC87114-treated WT B cells (Figure 5C). Phosphorylation of FOXO3a was more substantially inhibited than other Akt/PKB targets such as GSK3. As seen after BCR ligation, stimulation of Erk phosphorylation by IL-4 was only modestly inhibited by genetic inactivation of p110δ, yet fully blocked by the p110δ inhibitor IC87114 (Figure 5C). As expected, STAT6, which mediates many of the biologic effects of IL-4, was phosphorylated upon IL-4. This phosphorylation was largely independent of p110δ activity (Figure 5C).
Taken together, these data indicate that p110δ plays a key role in the regulation of survival signaling downstream of the IL-4 receptor.
Effect of p110δ inactivation on IL-4 signaling in primary splenic B cells. (A) Cells were stimulated with or without IL-4 for 15 minutes, followed by assay of PI3K activity in IRS-2 immunoprecipitates. (B) Percent of viable cells in a culture of B cells treated with or without IL-4 or anti-CD40 for 24 hours. (C) Left panel: Cells were stimulated with IL-4 at different time points, followed by analysis of signaling proteins in total-cell lysates. Right panel: WT B cells were stimulated with IL-4 for 15 minutes with or without the indicated PI3K inhibitors.
Effect of p110δ inactivation on IL-4 signaling in primary splenic B cells. (A) Cells were stimulated with or without IL-4 for 15 minutes, followed by assay of PI3K activity in IRS-2 immunoprecipitates. (B) Percent of viable cells in a culture of B cells treated with or without IL-4 or anti-CD40 for 24 hours. (C) Left panel: Cells were stimulated with IL-4 at different time points, followed by analysis of signaling proteins in total-cell lysates. Right panel: WT B cells were stimulated with IL-4 for 15 minutes with or without the indicated PI3K inhibitors.
Effect of PI3K inhibitors on BCR signaling in transformed B cells
We next tested whether p110δ remains of importance in signaling downstream of the BCR in transformed B cells. For these studies, we used the WEHI-231 cell line, which is derived from immature B cells and which has been used widely as a model system for BCR signaling.75 BCR stimulation of this pre–B cell lymphoma cell line induces G0/G1 cell-cycle arrest, followed by apoptosis.76
In contrast to primary B cells, which need stimuli such as BCR triggering to proliferate, WEHI-231 cells survive and proliferate independently of such stimuli. Indeed, whereas BCR signaling in primary B cells leading to cell-cycle progression is p110δ dependent, proliferation of WEHI-231 cells appears to be largely p110δ independent (Figure 6A). Also, the p110γ inhibitor AS-604850 did not inhibit WEHI-231 proliferation (Figure 6A). However, DNA synthesis in these cells can be fully blocked by the pan-PI3K inhibitor LY294002 (Figure 6A), indicating an important role for PI3K isoforms other than p110δ and p110γ in the proliferation of these cells and/or that several PI3K isoforms act in concert. Alternatively, these effects of LY294002 may be related to inhibition of non-PI3K targets of this inhibitor, such as mTOR and other protein kinases.7,13,17
In contrast to primary B cells, WEHI-231 cells have a constitutive activation of PI3K, as evidenced by the presence of basal phosphorylation of Akt/PKB on S473 and T308 (Figure 6B). Anti-IgM–stimulated Akt/PKB phosphorylation in these cells could not be completely blocked with IC87114, and residual phosphorylation on the Akt/PKB target, FOXO3a, was also observed (Figure 6B). These data indicate that while p110δ remains a major PI3K isoform downstream of the BCR in WEHI-231 cells, other isoforms also contribute significantly. The pan-PI3K inhibitor LY294002 completely blocked BCR-induced Akt/PKB phosphorylation, whereas the p110γ inhibitor AS-604850 had no effect (Figure 6B). Hence, p110α and p110β may play a greater role in WEHI-231 cells compared with primary, untransformed mature B cells. Consistent with this notion, phosphorylation of p70 S6K, which was p110δ dependent in primary B cells (Figure 4C), was not blocked by IC87114 in the WEHI-231 cells (Figure 6B). The fact that this phosphorylation is still LY294002 dependent implies a role for p110α and/or p110β upstream of p70 S6K in WEHI-231 cells. Phosphorylation of IκBα and GSK3 was largely PI3K independent in WEHI-231 cells, as evidenced by the lack of inhibition of their phosphorylation by LY294002 (Figure 6B). Whether the reduced dominance of p110δ in WEHI-321 cells relative to mature B cells is a consequence of the different stages of differentiation or of the mutations that contributed to the immortalization of the WEHI-231 cell line remains to be determined.
Effect of PI3K inhibitors on proliferation and BCR-induced early signaling in the WEHI-231 B-cell lymphoma cell line. (A) Effect of different PI3K inhibitors on proliferation of WEHI-231 cells. Proliferation was measured by [3H]-thymidine incorporation after a 48-hour culture. (B) Effect of PI3K inhibitors on early signaling induced by anti-IgM treament in WEHI-231 cells. Left panel: WEHI-231 cells were stimulated with anti-IgM for 5 minutes in the absence or presence of different PI3K inhibitors. Total-cell lysates were immunoblotted using the indicated antibodies. Right panel: WEHI-231 cells were stimulated with anti-IgM for the indicated time points with or without IC87114. Error bars indicate SD.
Effect of PI3K inhibitors on proliferation and BCR-induced early signaling in the WEHI-231 B-cell lymphoma cell line. (A) Effect of different PI3K inhibitors on proliferation of WEHI-231 cells. Proliferation was measured by [3H]-thymidine incorporation after a 48-hour culture. (B) Effect of PI3K inhibitors on early signaling induced by anti-IgM treament in WEHI-231 cells. Left panel: WEHI-231 cells were stimulated with anti-IgM for 5 minutes in the absence or presence of different PI3K inhibitors. Total-cell lysates were immunoblotted using the indicated antibodies. Right panel: WEHI-231 cells were stimulated with anti-IgM for the indicated time points with or without IC87114. Error bars indicate SD.
Discussion
Using mouse gene targeting, we and others have previously reported that the p110δ isoform of PI3K has a critical function downstream of the BCR.26,28 In the current study we further characterized the role of p110δ in BCR signaling using the p110δ-selective inhibitor IC8711457 on WT B cells, and compared its impact with the altered BCR signaling observed in B cells from p110δD910A/D910A mice. The use of these genetic and pharmacologic tools has additionally allowed us to reveal a key role for p110δ in IL-4–mediated signaling leading to cell survival. Pharmacologic intervention with p110γ function was found to have no effect on early BCR signaling in B cells, in line with the lack of a B-cell phenotype in p110γ KO mice.30
Total class IA PI3K activity was found to be reduced by 60% in p110δD910A/D910A B cells (Figure 1B). Apparently, the remaining residual PI3K activity (most likely composed of p110α and p110β) does not suffice for proper BCR signaling, even upon inactivation of only half of the p110δ molecules in heterozygous p110δWT/D910A KI mice (Figure 2A). This extreme dependency on p110δ function for BCR signaling is further substantiated by the observation that very low doses of the p110δ-selective compound IC87114 have a major impact on BCR-stimulated DNA synthesis (Figure 3 and Table 2) and intracellular signaling (Figure 4B-C). Several potential explanations for this apparent important role of p110δ in BCR signaling can be put forward. The first is that BCR signaling may be exquisitely dependent on PI3K activity, and requires all PI3K activity available in the cell for efficient signaling leading to biologic responsiveness. This would mean that minor reductions in the activity of any class IA PI3K would have a strong negative effect on BCR signaling. It will be possible to gain insight into this by testing the effect on BCR signaling of small molecule inhibitors against class IA PI3K isoforms other than p110δ, and by B-cell–restricted genetic inactivation of p110α and p110β in conditional PI3K mutant mice. A further explanation for a critical role for p110δ in B cells may relate to quantitative or qualitative differences between p110δ and other PI3K isoforms. A higher expression level of p110δ relative to that of other PI3K isoforms may be a key parameter. Indeed, based on the notion that the PI3K system is thought to depend to a large extent on recruitment of PI3Ks to phosphotyrosine binding sites, it can be envisaged that the PI3K isoform with the highest relative expression will most efficiently couple to the available PI3K docking sites in the cell. Qualitative differences between PI3K isoforms that could be of importance in isoform-selective signaling include differences in intrinsic protein kinase activity, differential interaction with Ras, and differential regulation of activity in receptor complexes. Evidence for all these features has been previously documented,43,77,79 and it is possible that such characteristics contribute to an important role for p110δ in B cells, and allow this isoform to selectively couple to signaling components downstream of the BCR. The molecular details of such selective coupling are unclear the moment, and require further investigation.
In primary splenic B cells, overall early tyrosine phosphorylation stimulated by BCR triggering is not affected by p110δ inactivation (Figure 4A), indicating that early tyrosine kinase signaling pathways are largely intact. However, activation of Akt/PKB leading to phosphorylation of the transcription factor FOXO3a is almost entirely dependent on p110δ, as are further downstream targets such as GSK3α and p70 S6K (Figure 4C). The PI3K-dependent fraction of early intracellular Ca2+ flux is also completely dependent on p110δ catalytic activity (Figure 4B). Most likely, these functions of p110δ derive from the stimulatory effects of its PIP3 lipid product on the activation of phospholipase C80 (leading to the generation of diacylglycerol and IP3, which induces the release of intracellular Ca2+) and on PDK1, the master upstream kinase for the AGC family of Ser/Thr kinases (which include, among others, Akt/PKB and PKC isoforms81 ). Again, such a key role for p110δ is remarkable given the high level of residual class IA PI3K activity in p110δD910A/D910A cells (Figure 1B). Taken together, it is clear that p110δ has influence over the activation of an array of essential signaling intermediates downstream of the BCR, with as a consequence a complete block from entering the cell cycle, with defects in all key mediators of cell-cycle progression (Figure 2B-C).
Our data show that genetic inactivation of p110δ largely mimics the effect of a small molecule inhibitor against this enzyme, with sometimes stronger inhibition by acute inhibition compared with genetic inactivation of p110δ (as is the case for Erk phosphorylation induced by BCR or IL-4 stimulation). Most likely, this relates to compensatory mechanisms in the remaining B cells to overcome constitutive genetic inactivation of p110δ. Alternatively, IC87114 may target additional enzyme(s) upstream of Erk. Of all immune cells investigated, B cells appear to be most sensitive to p110δ inactivation. The observation that pharmacologic inhibition of p110δ causes similar or sometimes even stronger inhibition of B-cell responsiveness to antigen and cytokines suggests that pharmacologic inhibition of p110δ in vivo may also preferentially affect the B-cell compartment. B cells influence the pathogenesis of many autoimmune diseases, including lupus erythematosus and rheumatoid arthritis.82,86 Recently, B-lymphocyte depletion therapy using anti-CD20 (rituximab) mAbs has shown great promise in rheumatoid arthritis.87 At present, no orally available drugs that preferentially target the B-cell compartment are available. Our observations described here further corroborate p110δ as an attractive target for therapeutic interference, and predict that a selective p110δ-specific inhibitor has potential to alleviate autoimmune diseases in which B cells play a pathologic role. Further validation of small molecule inhibitors against p110δ in in vivo disease models is now warranted.
Prepublished online as Blood First Edition Paper, September 22, 2005; DOI 10.1182/blood-2005-07-3041.
Supported in part by a European Union FP5 Programme QLG1-2001-02171 (A.B. and K.O.). K.O. was supported by a Biotechnology and Biological Science Research Council (BBSRC) David Phillips Fellowship (ref. JF19128). Additional funding was by the Ludwig Institute for Cancer Research and the UK BBSRC (grant no. 31/S15153).
Several of the authors (M.C., T.R., C.R.) are employed by a company (Serono Pharmaceutical Research Institute) whose potential product (AS-604850) was studied in the present work.
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Wayne Pearce and Emma Peskett for excellent technical support, and Khaled Ali for critically reading the manuscript.


![Figure 2. Genetic inactivation of p110δ impairs in vitro B-cell proliferation and induction of cell-cycle regulators. (A) Purified splenic B cells were cultured with or without anti-IgM for 48 hours, followed by measurement of [3H]-thymidine incorporation. Error bars indicate SD. (B-C) Cell lysates were prepared from WT and p110δD910A/D910A B cells at the indicated time points following anti-IgM stimulation, followed by assessment of expression of cell-cycle regulators by immunoblotting.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/107/2/10.1182_blood-2005-07-3041/4/m_zh80020689410002.jpeg?Expires=1767751156&Signature=feEfppe~ueWIf3XXyOfkDgltaTyKDaIiqwg3LLS4m3JZJHN1~VnbaCfunwMhk7nsSDlfNw2QCNrcU5BNqJ7xulnAI2WwJ5iYkfQZLUeuD2KSReWKjvzm~eD7~dQeE50~k18HtnO1FXJDs9lb7CewF~0gxj77vmi4XfIUbUpARz3BVPdkYUwdPKD79X8j0Sq2AYveddxvtj-xDkspbtXMLfLCQhn38MLRvVBgPCdbZcR049wk2gkEMZZ1BnsBdpradUCgSTaGeEEdhUhImkVA4R~8bGWOgfJ9kNaSqYu7EwTHK-QBFDr-zpmMpw9IKk3aTEVOlK15q0nfPgeGjnNIaA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 3. Effect of PI3K inhibitors on anti-IgM-induced B-cell proliferation. Primary splenic B cells were cultured with anti-IgM in presence or in absence of the indicated doses of PI3K inhibitors, followed by measurement of [3H]-thymidine incorporation after 48 hours. Results were expressed as percentage of control response of cells that were not treated with PI3K inhibitors. Error bars indicate SD.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/107/2/10.1182_blood-2005-07-3041/4/m_zh80020689410003.jpeg?Expires=1767751156&Signature=z0GLAe4ZwM7QPQwp-vwfgZ6gu7QzmwjMmZ9DtDeWqb8w~dJexyV2YvvRvrNIxSneCp3-Op2XSdzlJknThEqDNiHOIIyzX4XyFzIkKEFojDWs~oqX9EECPFj3klNc7LepPUdBimTk1-6W8d5c6DoF9yaaKuHTV46gyb1shK7~TAdB~WKZhtYmxkWmiuJuzYIe3om7JGNdb02dq5sd0at24NOISZS237pCZfPb5XJTdj8z9rExnZ8XjxQ4LkaeH5jqStUFQjGSUdwbJWWRwMZeSLvk4NABv5Co86aqKLDfaImtkqIvlYmC4j7fH1OaW7ezT1FUO9BMk7WAJY2V2d8EnQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

![Figure 6. Effect of PI3K inhibitors on proliferation and BCR-induced early signaling in the WEHI-231 B-cell lymphoma cell line. (A) Effect of different PI3K inhibitors on proliferation of WEHI-231 cells. Proliferation was measured by [3H]-thymidine incorporation after a 48-hour culture. (B) Effect of PI3K inhibitors on early signaling induced by anti-IgM treament in WEHI-231 cells. Left panel: WEHI-231 cells were stimulated with anti-IgM for 5 minutes in the absence or presence of different PI3K inhibitors. Total-cell lysates were immunoblotted using the indicated antibodies. Right panel: WEHI-231 cells were stimulated with anti-IgM for the indicated time points with or without IC87114. Error bars indicate SD.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/107/2/10.1182_blood-2005-07-3041/4/m_zh80020689410006.jpeg?Expires=1767751156&Signature=i7cwts4yR8imJs4FXX~4QjmYCuVmIeQZGbOQ~j4bexTPJuOG3jsOZwC-AxGrXKDhEpS4ut0m68s2cCUIWTdC9OJNB55nFeMXJQcFL2yBjtgqVSDcSGb1XqiFgr-Jd7LB03jo79~KWWPqWiHYG1FmmrvPBWL-4XnPFZ7DeHlnICj9025HzDG0JrPUXYe7cgNgMvxTVPCG58KIbnxhPxntUKZcGtuDjCMUnmTgVGB26pNbWZwNpInY47TW6Rk1OAhKLlnKt1h8HOTLDU7pWFdyhOQK1HfwqEc-YjsRrEXcnh2BroWyUhTREyrzzux~AxhUiqXmjnkG0ZxbPdiEaR7JJg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

![Figure 2. Genetic inactivation of p110δ impairs in vitro B-cell proliferation and induction of cell-cycle regulators. (A) Purified splenic B cells were cultured with or without anti-IgM for 48 hours, followed by measurement of [3H]-thymidine incorporation. Error bars indicate SD. (B-C) Cell lysates were prepared from WT and p110δD910A/D910A B cells at the indicated time points following anti-IgM stimulation, followed by assessment of expression of cell-cycle regulators by immunoblotting.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/107/2/10.1182_blood-2005-07-3041/4/m_zh80020689410002.jpeg?Expires=1767751157&Signature=d6b46aGX90kQ9Z2UJtnYTkFS6P07bUK3Y4xjFR-XrEYrSX6LlcMvawkbj69jJ-nEbqCnBwo2CqoqVcRt31Sz7gzTZAvaeHfAx0hPUdzGfNc5iFyNVtZOc5GOHL5lXZ2qyCH2wfQEFdK1HxngmxmdaiXSp4gV-77ra-5z5MhOQBAFg87KJWMFDuI2avQQaCqe9I19qbrLfL--UjjU5HMUoPEW7XDatvk2AMNvfSx-IRCLYqI5YwYCPNSVXXkD1aO86aSUFpcGtLQzh4j~hygZrEkmP-dvF1hnCNhJhnGHnPo-MuYoh2Md0K5UTs0YRhPHUfzaRSlsGpXJeTSsLGhECA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 3. Effect of PI3K inhibitors on anti-IgM-induced B-cell proliferation. Primary splenic B cells were cultured with anti-IgM in presence or in absence of the indicated doses of PI3K inhibitors, followed by measurement of [3H]-thymidine incorporation after 48 hours. Results were expressed as percentage of control response of cells that were not treated with PI3K inhibitors. Error bars indicate SD.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/107/2/10.1182_blood-2005-07-3041/4/m_zh80020689410003.jpeg?Expires=1767751157&Signature=QaMTm9ut7xTfUqW70WtnGq5wy0KbxbDw1OXhZjt2wesN7LdmlIUVliQo9DQXW1KMC0rjSbXFEmwj~5mnCCdzv-3NPHGQDxVpyh~~RtOLlYAYHWfMxgpBQTSKUz-hNdWFeKP2Tv6JB-JoEotdz~1BzXVA628PhdIoRMfENFoSgRsmnsDgglCzghj-6oXW5SreCn5-kW3C8k2tUvAMUTm3sGmq1xUAraMZuXxjbRfAfoZEn-dqSVLioevarBiawnOE-jP0U0iIovYVpeeG2NAMuj~KnYtkKSsKOd6nSRfkPb~Xxwz3tnweyklv-N4zknJPY7pj8oUoV0PZLBzXYbrL4Q__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

![Figure 6. Effect of PI3K inhibitors on proliferation and BCR-induced early signaling in the WEHI-231 B-cell lymphoma cell line. (A) Effect of different PI3K inhibitors on proliferation of WEHI-231 cells. Proliferation was measured by [3H]-thymidine incorporation after a 48-hour culture. (B) Effect of PI3K inhibitors on early signaling induced by anti-IgM treament in WEHI-231 cells. Left panel: WEHI-231 cells were stimulated with anti-IgM for 5 minutes in the absence or presence of different PI3K inhibitors. Total-cell lysates were immunoblotted using the indicated antibodies. Right panel: WEHI-231 cells were stimulated with anti-IgM for the indicated time points with or without IC87114. Error bars indicate SD.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/107/2/10.1182_blood-2005-07-3041/4/m_zh80020689410006.jpeg?Expires=1767751157&Signature=VaSyicfguy1leNHCa2W3pEeLJFtvwpOoEOnXDOswzxNwJkx1PeRD0354wWMUxZV8n592Hz4Cc6TCY1uPnqS5y4vBfHxHf-sedD13Zffd~B-qj65auxRxhdrcvm7Y3jQbq4FbFZApH77SB2m79ajUtDwXyXg5xI4a8nzTq66y1QLhgcxb7iAq-woD2U08l5-9CBeTppyhwTJe4euQ27dEt7MLRuehLKUdYInHLr1RjHJEFilJMrUS1Wj0i7ulaxuskNNuKTPSAM7Ia8OGqnxxD9UrnxcrXMGnfvqwXdmw6qttI1Gra2IoFIQMbyOYzfBBiPquxWsPhET8UPfsF4-nbA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)