Abstract
Bcl-6 protein expression, a marker of germinal center origin, has been associated with a favorable prognosis in diffuse large B-cell lymphoma (DLBCL). To determine the prognostic significance of this marker when rituximab (R) was added to cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) chemotherapy, we prospectively studied Bcl-6 protein expression by immunohistochemical staining of 199 paraffin-embedded specimens from patients enrolled in the US Intergroup phase 3 trial comparing R-CHOP to CHOP with or without maintenance R. In Bcl-6– patients, failure-free survival (FFS) and overall survival (OS) were prolonged for those treated with R-CHOP alone compared to CHOP alone (2-year FFS 76% versus 9%, P < .001; 2-year OS 79% versus 17%, P < .001). In contrast, no differences in FFS and OS were detected between treatment arms for Bcl-6+ cases. In the multivariate analysis, treatment arm (CHOP versus R-CHOP) was the major determinant of both FFS (P < .001) and OS (P < .001) for the Bcl-6– subset, whereas the International Prognostic Index risk group was the only significant predictor of outcome among Bcl-6+ cases. Bcl-2 protein expression was not predictive of outcome in either group. In this study, we observed a reduction in treatment failures and death with the addition of R to CHOP in Bcl-6– DLBCL cases only. Our finding that Bcl-6+ cases did not benefit from the addition of R to CHOP requires independent confirmation.
Introduction
Bcl-6 protein expression, a marker of germinal center derivation, has been identified as one of the strongest predictors of outcome in the diffuse, large B-cell lymphomas (DLBCLs).1 Multiple immunohistochemical studies and analysis using quantitative reverse transcriptase–polymerase chain reaction (RT-PCR) have shown that Bcl-6 protein expression alone or in combination with other germinal center markers predicts for a favorable outcome in DLBCL.2,4 Using DNA microarray techniques, a distinct gene expression profile has been associated with germinal center origin and with longer survival than other forms of DLBCL.5,6 Germinal center origin is a prognostic factor that is independent of the clinically based International Prognostic Index (IPI) risk groups.7 Nearly all studies of prognostic indicators in DLBCL, including the IPI, are based on clinical outcome following treatment with an anthracycline-containing multiagent regimen such as cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP). New strategies, such as the addition of rituximab (R) to combinations such as CHOP, may be associated with different biologic or clinical prognostic indicators compared with conventional chemotherapy.
In an effort to improve outcomes in patients over the age of 60 years with DLBCL, the US Intergroup conducted a prospective phase 3 trial comparing CHOP to R-CHOP, and maintenance R (MR) to observation (OBS) from February 1998 through July 2001.8 A companion trial investigating potential biologic markers of prognosis, including Bcl-6 protein expression, accrued cases concomitantly. The overall objectives of the laboratory study were (1) to identify prognostic indicators in patients aged 60 years or older with DLBCL, (2) to determine if biomarkers such as Bcl-6 retain their prognostic significance in the context of treatment that includes R, and (3) to assess the predictive value of biologic markers in patients receiving CHOP and R-CHOP. We found that the addition of R to CHOP eliminated the prognostic significance of Bcl-6 protein expression. Our analysis shows an improved outcome in the Bcl-6– cases treated with R-CHOP, but not among Bcl-6+ DLBCL patients who have a relatively favorable outcome when treated with conventional CHOP chemotherapy.
Patients and methods
Eligibility
Eastern Cooperative Oncology Group (ECOG) or Southwest Oncology Group (SWOG) patients enrolled in the US Intergroup trial (E4494, C9793, S4494) comparing CHOP versus R-CHOP, with a second randomization to MR versus OBS, were eligible for this prospective study if adequate paraffin-embedded biopsy tissue or unstained slides were submitted. A tissue diagnosis of untreated DLBCL confirmed by central review and classified according to the World Health Organization criteria was required. Cases with any confirmed follicular architecture were not eligible for study. In all cases, a B-cell phenotype was documented by immunohistochemistry or flow cytometry using an anti-CD20 antibody. Transformed lymphomas and HIV-associated lymphomas were excluded from this trial. All participants signed informed consent documents approved by the institutional review board at each participating site, in accordance with the Declaration of Helsinki. This analysis includes all cases for which material was submitted for Bcl-6 staining.
Clinical trial
In this Intergroup trial, patients 60 years of age or older were stratified by the number of IPI risk factors and randomized to treatment with 6 to 8 cycles of CHOP chemotherapy with or without R as previously described.8,9 Induction R was administered at a dose of 375 mg/m2 on days –7 and –3, and 48 to 72 hours before the third, fifth, and seventh cycles. Complete or partial responders (n = 415) were again stratified according to their initial IPI scores and randomized to either OBS or MR administered weekly for 4 consecutive weeks every 6 months for a total of 4 cycles.
Immunohistochemical studies
Sections (5 μm) for routine staining with hematoxylin and eosin were obtained from each tissue specimen to demonstrate the presence of lymphoma within the material to be studied. Immunohistochemical staining of 5-μm tissue sections was performed as previously described using heat-induced antigen retrieval.10 Sections were deparaffinized, dehydrated, and stained with either immune sera or commercially provided monoclonal antibody. Polyclonal antisera (AR-1) raised in rabbits against synthetic peptides corresponding to amino acids 41-54 of the human Bcl-2 protein were used to evaluate Bcl-2 expression.10 Expression of the germinal center–related protein Bcl-6 was evaluated using the commercially available reagent from DakoCytomation (Fort Collins, CO).
Pathology review
To confirm a diagnosis of DLBCL and to exclude cases with follicular components, all cases underwent tertiary review by at least 2 members of the 7-member panel of expert hematopathologists. In the event of discordant opinions, a consensus review by the panel or a third reviewer determined eligibility. Immunohistochemical stains were also reviewed by at least 2 members of the panel, and disagreements were settled by review by the panel or a third reviewer. Cases were scored as entirely negative, 1% to 9%, 10% to 20%, 21% to 50%, and more than 50% positive. Two cut-points were investigated for each marker. For purposes of this analysis, cases in which more than 50% of lymphoma cells stained with anti–Bcl-2 were called “Bcl-2 positive” consistent with the published literature.2,11 For Bcl-6, any definitive staining of large, neoplastic cells was considered positive.
Statistical plan
Patient characteristics were compared between groups using the Fisher exact test and Wilcoxon rank-sum test. Failure-free survival (FFS) was defined as the time from randomization to relapse, nonprotocol treatment, or death. Overall survival (OS) was measured from randomization to death from any cause. FFS and OS were estimated using the Kaplan-Meier method.12 The prognostic value of biomarkers for response (complete response + partial response [CR + PR]), FFS, and OS was evaluated for all patients and by induction and maintenance therapy using univariate (Fisher exact test, log-rank) and multivariate (Cox proportional hazard regression models) analyses. The multivariate analysis controlled for the effect of the IPI (low/low-intermediate versus high-intermediate [HI]/high) and Bcl-2 status. Similar methods were used to compare the outcomes for CHOP versus R-CHOP by Bcl-6 expression to evaluate the predictive value of the markers. Adjustments were made for the evaluation of multiple markers and cut-points in the univariate analyses with P ≤ .0055 considered statistically significant (n = 9).13 For analyses with small numbers of patients, nonsignificance may result from limited power, especially for those who did not express Bcl-6. All P values were based on 2-sided tests.
Analysis of the association between Bcl-6 expression and FFS/OS was complicated by a significant interaction between induction therapy and MR (HR = 2.10, 95% CI [1.01, 4.36], P = .05) in that MR improved the outcome after CHOP but not after R-CHOP.9 To compare induction treatments without the confounding effect of maintenance, analyses cannot simply exclude all MR patients because the proportion of nonresponders relative to the whole population would be higher and therefore underestimate FFS and OS. As in our report of the Intergroup trial,8 an unbiased estimate was achieved by applying an approach (weighted Cox regression) that approximately doubled the information for patients randomized to observation.14,15 As previously described for weighted Cox regression,16 the robust variance estimator provides a proper estimate of the variance of the relative risk estimate in this setting and can be implemented using the S-Plus function coxph. The concept of using a weighted analysis to remove the bias that can result from analyzing only a subset of the patients in 2-stage randomized designs is consistent with previously proposed methods for the missing data problem.17,21 The results from the weighted Cox regression are denoted in this paper as the analyses removing the effect of MR.
Results
Bcl-6 protein expression and baseline characteristics
The Intergroup clinical trial accrued 632 patients including 544 with complete IPI data. Of the 387 cases from the participating cooperative groups (ECOG and SWOG), 211 were eligible for this prospective correlative study. All had a confirmed diagnosis of DLBCL with adequate material for study, provided consent for this correlative study, and had satisfied the eligibility criteria of the Intergroup protocol. Insufficient material was the major reason for exclusions; either material was not submitted for this correlative trial or was insufficient when reviewed by our pathology panel. Twelve additional cases were excluded because staining for Bcl-6 was not interpretable, leaving 199 patients in the final analysis. Bcl-6 was scored as positive in 154 (77%) of the 199 cases. In 45% of cases, more than half of the large neoplastic cells stained positively for Bcl-6. A minority (16%) of cases stained for Bcl-6 in 20% or less of the malignant lymphocytes.
Patient characteristics in this correlative study were representative of the larger Intergroup clinical trial population (Table 1). The only difference was that a somewhat greater proportion of patients in the laboratory study population had stage III-IV disease relative to the larger group (80% versus 74%, P = .05). When the Bcl-6+ and Bcl-6– subgroups were compared, no differences were detected in the distribution of patient characteristics including IPI score (63% versus 64% HI/high IPI, P = .9; 55% versus 58% HI/high age-adjusted IPI, P = .4), performance status (PS; 15% versus 13% with PS > 1, P = .9), stage of disease (81% versus 78% with stage III-IV disease, P = .7), lactate dehydrogenase (LDH; 60% versus 71% with elevated LDH level, P = .2), and number of extranodal sites (31% versus 24% with > 1 extranodal site involved, P = 0.5).
Similar percentages of patients in the CHOP (74%) and R-CHOP (80%) arms were Bcl-6+. Patient characteristics including IPI scores were balanced between the treatment arms (Table 1). Similarly, no significant differences in patient characteristics were observed according to treatment with R-CHOP or CHOP by Bcl-6 status. Although a higher, but not statistically significant, percentage of Bcl-6– patients treated with CHOP had HI/high IPI scores relative to those treated with R-CHOP, the age-adjusted IPI scores were distributed equally.
Bcl-6 expression and clinical outcome
With a median follow-up of 3.4 years, the estimated clinical outcomes at 2 years favored Bcl-6+ patients. The 2-year FFS estimates (± SE) were 63% ± 4% for Bcl-6+ versus 54% ± 8% for Bcl-6– patients (P = .05). For OS, the estimates were 74% ± 4% for Bcl-6+ versus 58% ± 7% for Bcl-6– patients (P = .04). Overall response rates (CR/PR) were not different according to Bcl-6+ and Bcl-6– status (79% versus 82%, P = .68).
Table 2 shows outcome according to Bcl-6 expression and induction treatment with CHOP and R-CHOP. Response rates, according to Bcl-6+ and Bcl-6– status, were similar within the CHOP subgroup (75% versus 79%, P = .8) and within the R-CHOP subgroup (81% versus 86%, P = .8). In contrast, the prognostic value of Bcl-6 expression for FFS and OS differed by induction treatment. For patients treated with CHOP, the 2-year estimated FFS and OS rates were superior for Bcl-6+ patients relative to Bcl-6– patients (FFS, 61% versus 38%, P = .004; and OS, 73% versus 42%, P = .001). For patients who received R-CHOP, the 2-year estimates for FFS and OS were not influenced by Bcl-6 status (FFS, 64% versus 75%, P = .6; OS, 74% versus 76%, P = .7).
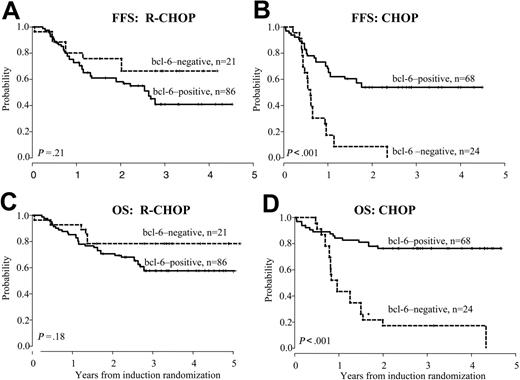
We conducted a secondary analysis (see “Patients and methods”) to eliminate the beneficial effect of MR after CHOP observed in the Intergroup clinical trial. The differences in prognostic significance of Bcl-6 status according to treatment were even more pronounced when MR patients were excluded (Table 2; Figure 1). When outcomes for Bcl-6+ and Bcl-6– cases were compared, significantly longer FFS (54% versus 9%, P < .001) and OS (77% versus 17%, P < .001) were observed for Bcl-6+ patients who received CHOP, whereas neither FFS (58% versus 76%, P = .2) nor OS (71% versus 79%, P = .2) was influenced by Bcl-6 status among patients treated with R-CHOP.
FFS and OS based on Bcl-6 expression. Failure-free survival (A-B) and overall survival (C-D) according to Bcl-6 expression for cases treated on R-CHOP and CHOP induction arms. Analysis excludes patients who were randomized to receive MR.
FFS and OS based on Bcl-6 expression. Failure-free survival (A-B) and overall survival (C-D) according to Bcl-6 expression for cases treated on R-CHOP and CHOP induction arms. Analysis excludes patients who were randomized to receive MR.
Bcl-2 expression was also determined in this correlative laboratory study. Consistent with the literature, 59% of the 189 cases that were analyzed for Bcl-2 were scored as positive (> 50% malignant lymphocytes).10,11 Comparing Bcl-2+ and Bcl-2– subgroups, there were marginal differences in the percentages of patients with elevated LDH levels (57% versus 72%; P = .05), HI/high IPI (60% versus 72%; P = .09) and HI/high age-adjusted IPI (51% versus 64%; P = .07). In patients treated with CHOP or R-CHOP, no significant differences in patient characteristics were observed when analyzed according to Bcl-2 status, with the exception of a marginally higher percentage of Bcl-2– patients with elevated LDH levels among the group treated with CHOP compared with R-CHOP (82% versus 64%, P = .1). More specifically, comparing patients treated with CHOP and R-CHOP according to Bcl-2+ and Bcl-2– status, respectively, there were no differences for the following patient characteristics: median age (P = .9; P = .9), stage III-IV (P = .9; P = .9), PS greater than 1 (P = .2; P = .8), more than one extranodal site (P = .2; P = .2), and bone marrow involvement (P = .9; P = .8). Similarly, comparing patients treated with CHOP and R-CHOP, respectively, there were no differences in distribution across IPI risk groups as follows: HI/high IPI (Bcl-2+, 57% versus 61%, P = .7; Bcl-2–, 79% versus 67%, P = .3), and HI/high age-adjusted IPI (Bcl-2+, 46% versus 54%, P = .5; Bcl-2–, 73% versus 58%, P = .2). No differences in response rates (P = .9), FFS (P = .3), or OS (P = 0.9) were noted according to Bcl-2 expression. Similarly, when analyzed according to treatment arm there were no differences in objective response rates between Bcl-2+ and Bcl-2– cases (CHOP, P = .6; R-CHOP, P = .9). Bcl-2 status did not influence FFS or OS according to treatment arm whether the analysis included or excluded the MR patients (FFS: CHOP, P = .9 and .9, with and without MR, respectively, R-CHOP, P = .1 and .2, respectively; OS: CHOP, P = .4 and .6, respectively, R-CHOP, P = .3 and .3, respectively). Nonetheless, Bcl-2 expression was included in the multivariate analyses based on observations in other studies.
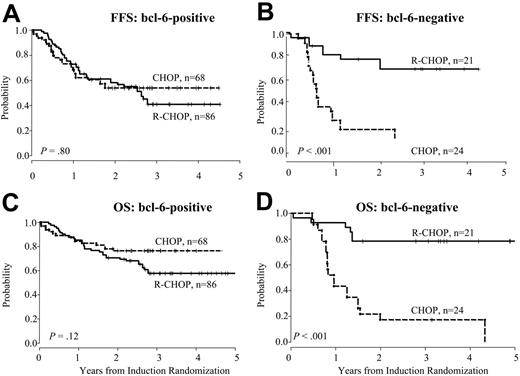
FFS and OS according to induction treatment. Failure-free survival (A-B) and overall survival (C-D) according to induction treatment for Bcl-6+ and Bcl-6– cases. Analysis excludes patients who were randomized to receive MR.
FFS and OS according to induction treatment. Failure-free survival (A-B) and overall survival (C-D) according to induction treatment for Bcl-6+ and Bcl-6– cases. Analysis excludes patients who were randomized to receive MR.
Table 3 shows the relative risk (RR) estimates from the multivariate analysis by treatment. A significant interaction between Bcl-6 expression and treatment was observed after adjusting for IPI and Bcl-2 expression (FFS P = .001, OS P < .001). Among patients treated with CHOP, Bcl-6 status was the major determinant of FFS (RR = 0.2, P < .001) and death (RR = 0.2, P < .001). In contrast, for R-CHOP patients, Bcl-6 status was not a determinant of outcome, whereas IPI significantly influenced the risks for FFS (RR = 7.1, P < .001) and OS (RR = 26, P = .002).
To investigate the predictive value of Bcl-6 protein expression, we looked at outcomes for Bcl-6+ and Bcl-6– cases according to induction therapy as described. Bcl-6+ patients had similar outcomes after CHOP compared to R-CHOP (P = .7 for FFS and P = .4 for OS), whereas Bcl-6– patients had significantly inferior outcomes with CHOP alone (P = .02 for FFS and P = .03 for OS). When MR patients were excluded using the weighted analysis, there was no difference in outcomes between induction arms for Bcl-6+ cases (P = .8 for FFS and P = .1 for OS). In contrast, the effect of adding R to induction therapy for Bcl-6– cases was more pronounced in the weighted analysis excluding MR patients (Figure 2). For Bcl-6– patients, estimated 2-year FFS was 9% ± 6% after CHOP compared with 76% ± 9% after R-CHOP, P < .001 and OS was 17% ± 8% after CHOP compared with 79% ± 8% after R-CHOP, P < .001. In sum, the adverse effect of Bcl-6– status for patients treated with CHOP was not seen with R-CHOP, suggesting that rituximab prevented treatment failures in this subgroup.
Because this study was stratified by IPI and not by Bcl-6 status, a multivariate analysis was also performed to evaluate the effect of treatment controlling for Bcl-6 expression, Bcl-2 expression, and IPI. Relative risk estimates by Bcl-6 status are shown in Table 4. Among Bcl-6+ patients, the estimated relative risks for FFS and OS do not differ (P = .68 and .11, respectively) by treatment but IPI is highly significant (P = .004 and .002, respectively). Among Bcl-6– patients, the risks of failure (RR = 0.1, P < .001) and death (RR = 0.1, P < .001) are significantly lower for R-CHOP patients.
Outcomes in this study according to the second randomization (MR versus OBS) and Bcl-6 expression were also evaluated but the power to detect differences was limited by small numbers with just 14 MR and 20 OBS patients in the Bcl-6– subgroup (Table 5). At 2 years from maintenance randomization, no differences were observed in either FFS or OS according to Bcl-6 status in either the MR or OBS subgroups. When further evaluated by induction treatment, significant differences according to Bcl-6 status were detected in these analyses with limited power only among the cases treated with CHOP followed by observation. In patients treated with CHOP and then randomized to observation, Bcl-6– status (n = 8) conferred an inferior estimated FFS (P < .001) and OS (P < .001) compared to Bcl-6+ cases (n = 22).
Discussion
Consistent with prior reports, we found that Bcl-6 protein expression is a powerful predictor of outcome in patients with DLBCL treated with CHOP chemotherapy. In contrast, Bcl-6 status was not a prognostic marker among patients treated with R-CHOP in this US Intergroup trial. The addition of R to conventional CHOP chemotherapy, either as induction or maintenance, has now been shown to improve outcome in patients with DLBCL.8,22,23 Our findings suggest that this improvement in outcome may be primarily due to the beneficial effect of R added to CHOP in the Bcl-6– subset of DLBCL.
The BCL6 proto-oncogene encodes a transcriptional repressor that is required for germinal center formation. Emerging data suggest that the normal function of BCL6 in germinal center B (GCB) cells is to facilitate somatic hypermutation, a process that generates antibody diversity and increases antigen affinity by suppression of p53-mediated apoptosis in response to double-stranded DNA breakage.24,25 Down-regulation of BCL6 may be necessary for normal GCB to differentiate to memory B cells or plasma cells.26 Constitutive expression of BCL6 as a result of translocation, mutation, or other mechanisms may contribute to lymphomagenesis through maturation arrest and a pathologic expansion of GCB.
Several groups have reported that Bcl-6 protein expression alone or in combination with other germinal center markers predicts for a favorable outcome in DLBCL treated with anthracycline-containing chemotherapy.1,3 The frequency of Bcl-6+ cases differs in reported series; the frequency in this trial (77%) is higher than that reported previously by Hans et al (56%) and is more consistent with the frequency reported by Colomo et al (72%) and Lossos et al (63%).1,3,27 Such differences may represent the underlying patient population as well as technical factors related to staining, interpretation, and scoring of positive results. The apparently less favorable outcome of Bcl-6– patients treated with CHOP in our series, when compared with those of Lossos and Hans, can be explained by the more advanced age of our patients and the greater percentage of cases with advanced stage and high-intermediate or high-risk disease by the IPI. Unlike previous studies investigating the prognostic significance of Bcl-6 protein expression, the current analysis was a planned prospective study performed in older patients participating in a large study of standard chemotherapy with or without R. In the context of the US Intergroup trial, Bcl-6+ and Bcl-6– cases had similar outcomes if R was incorporated into the treatment regimen. This finding underscores the need to re-evaluate previously established prognostic markers in the setting of new therapies.
Gene expression profiling results are consistent with the immunohistochemical studies identifying Bcl-6 as a prognostic factor.5,6,28 Using DNA microarray techniques to examine the expression of thousands of genes, investigators have recently distinguished at least 2 molecularly distinct subgroups of DLBCL—one that appears to be derived from GCB cells and another that has characteristics of activated peripheral blood B cells (ABCs).5,6 Relative to ABC cases, GCB lymphomas treated with chemotherapy have favorable outcomes independent of IPI risk groups. The GCB cell signature is based on the expression of a large number of key genes including BCL6.6 In a subsequent study, a panel of just 6 genes including BCL6 was sufficient to assign prognosis in patients treated with standard chemotherapy.4 However, it is important to recognize that Bcl-6 protein expression alone does not identify a DLBCL as GCB-like.3 In the study of Hans et al, an algorithm for GCB-like and non–GCB-like subgroups of DLBCL was based on protein expression of Bcl-6, CD10, and MUM-1.3 Not all Bcl-6+ cases were assigned to the GCB-like category.
Consistent with our results, Bcl-6 expression, but not Bcl-2 expression, was prognostic in the Hans series.3 However, other studies have reported the prognostic significance of Bcl-2 in DLBCL.11,29,32 Although different cut-points have been used for defining Bcl-2 positivity in prior trials, the percentage of positive cases (45%-66%) reported in most of the literature is similar to ours (59%).11,29,32 Of interest, the Groupe d'Etude des Lymphomes de l'Adulte (GELA) reported that Bcl-2 expression was prognostic in patients with DLBCL treated with CHOP but not with R-CHOP.11 If so, the fact that Bcl-2 lacked prognostic significance in patients treated with CHOP in our study could be related to sample size (the majority of patients received R as induction or maintenance or both) or to the use of a polyclonal antibody reagent rather than the monoclonal antibody used by the GELA group, although both antibody reagents are directed against residues 41-54 of the human Bcl-2 protein.10,11
The multivariate analysis of prognostic factors presented in Tables 3 and 4 highlights the continued importance of the IPI scoring system. The data indicate that although Bcl-6 protein expression subsumes the significance of IPI in patients treated with CHOP, IPI is the dominant variable predictive of outcome after R-CHOP (Table 3) and the most important variable among Bcl-6+ cases (Table 4). These findings indicate that important heterogeneity, for which IPI is a surrogate, remains to be understood in DLBCL. In addition to molecular signatures based on cell of origin (GC versus ABC), robust gene expression profiles associated with host immune response and other features have been recently identified, underscoring the complexity of pathogenesis in DLBCL while defining possible new treatment targets.33 The results of the current study indicate that patients with higher IPI risk scores require new therapeutic initiatives. Attention to both gene expression patterns and IPI scores in the evaluation of novel therapies is therefore justified.
The mechanism by which the addition of R to CHOP improved outcomes selectively in the Bcl-6– cases is unknown but may represent a direct cytotoxic effect of R alone (antibody-dependent cellular cytotoxicity, complement-mediated cytotoxicity, and induction of apoptosis34 ) or an as yet uncharacterized effect of either R alone or in combination with CHOP on cell survival mechanisms unique to Bcl-6– DLBCL. Genes in the NF-κB pathway are overexpressed in ABC-like DLBCL and inhibitors of NF-κB are preferentially effective in ABC-like cell lines.35,36 Jazirehi and colleagues reported that R inhibits the constitutive NFκB signaling pathway in selected non-Hodgkin lymphoma (NHL) B-cell lines, leading to increased sensitivity to chemotherapy.37 Perturbation of other pathways such as the ERK1/2 pathway by R may lead to the down-regulation of Bcl-XL and enhanced sensitivity to chemotherapy as suggested by preclinical work in B-cell lines.38
Our study results demonstrate why it is imperative that prognostic indicators or models be reevaluated in the context of each new therapeutic strategy. If confirmed, our findings could lead to the selective use of R with CHOP in only the Bcl-6– subset of DLBCL, based on standardized assessment of Bcl-6 expression. In the future, different therapeutic strategies may be designed to specifically target Bcl-6+ and Bcl-6– DLBCL based on differences in their underlying cell survival mechanisms and sensitivity to chemotherapy and now R. For example, some Bcl-6+, GCB-like lymphomas are thought to result from deregulated expression of BCL6 and may be amenable to inhibitors of histone deacetylase or more specific BCL6 interference, whereas NF-κB inhibitors may be selectively active in ABC-like lymphomas.37,39,40 The heterogeneity of DLBCL continues to challenge laboratory and clinical investigators. Prospective correlative studies paired with large informative data sets from clinical trials provide valuable resources to further tailor and increase the efficacy of DLBCL treatment.
Prepublished online as Blood First Edition Paper, January 31, 2006; DOI 10.1182/blood-2005-10-4222.
This study was conducted by the Eastern Cooperative Oncology Group (Robert L. Comis, Chair) and was supported by grants CA23318, CA66636, CA32291, CA38926, CA32102, CA04919, CA11083, CA13650, CA17145, and CA21115 from the Public Health Service, the National Cancer Institute of the National Institutes of Health, and the Department of Health and Human Services and Genentech.
J.N.W. designed the study, supervised all aspects of the research and analysis, and wrote the manuscript; E.A.W. performed all statistical analyses and assisted in writing the manuscript; S.J.H. assisted in the design of the study, analysis of data, and writing of the manuscript, and served as the Eastern Cooperative Oncology Group (ECOG) Lymphoma Chair; M.K. assisted in the design of the study and supervised all immunohistochemical staining; D.V., P.J.K., W.R.M., M.C., R.E.F., E.D.H., and L.J.M. were responsible for verifying histology and scoring immunohistochemical stains; T.M.H. and J.K.W. were chair and cochair of the clinical trial and assisted in the writing of the manuscript; R.I.F. assisted in analysis of data and the writing of the manuscript, and served as the Southwest Oncology Group (SWOG) Lymphoma Chair; J.C.R. assisted in design of the trial, provided the anti–Bcl-2 reagent, supervised immunohistochemical staining, and assisted in the writing of the manuscript; and R.D.G. supervised and participated in the pathology review and immunohistochemical scoring and assisted in analysis of data and the writing of the manuscript.
Presented at the 45th annual meeting of the American Society of Hematology, San Diego, CA, December, 2003.13
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Kristen Burton, Ollistine Jude, and Martin Bast for administrative assistance and Ren-Wei Guo and Adekunle Raji and the staff of the ECOG Pathology Coordinating Office for technical assistance. In addition, we are grateful to the investigators, nurses, and data managers of ECOG and SWOG for their participation. The contents of this publication are solely the responsibility of the authors and do not necessarily represent the official views of the National Cancer Institute.