Abstract
We have studied cytomegalovirus (CMV) immunity in 17 CMV-positive recipients of T-cell-depleted or T-cell-replete grafts. In recipients of T-cell-replete grafts, the patient's CMV-specific T-cell response was completely ablated. Because primary anti-CMV responses were rare during the first year, immunity depended essentially on the transfer of donor CMV-specific T cells and, therefore, on the CMV positivity of the donor. In the recipients of T-cell-depleted grafts, CMV-specific cytotoxic T cells were of recipient origin in 2 patients who underwent transplantation with CMV-negative donors and in 3 of 8 patients who underwent transplantation with CMV-positive donors, and they were of mixed or donor origin in the other 5 patients studied. Recipient CMV-specific T cells responded vigorously to antigen ex vivo and persisted for several years without replenishment by donor cells. Furthermore, they appeared to have a protective effect because CMV-related complications were absent in the patients with CMV-specific T cells of recipient origin. Clinical outcomes of a cohort of 91 patients corroborated the experimental results. Patients with recipient T cells in their blood were protected regardless of the donor immune status. Hence, when a T-cell depletion protocol is used that favors the survival of recipient T cells, the patient's pretransplantation CMV-specific immunity protects against posttransplantation CMV-related complications.
Introduction
Cytomegalovirus (CMV) is a herpesvirus that infects humans and persists as a latent infection thereafter. Cytotoxic T lymphocytes (CTLs) play a crucial role in the control of the virus. Because cellular immunity is severely affected after hematopoietic stem cell transplantation (HSCT), reactivation of the virus occurs frequently, and CMV-related complications are a major cause of posttransplantation morbidity and mortality.1,2
The central role of CMV-specific T cells in controlling CMV in patients who have undergone transplantation has been documented extensively.3-5 It has been shown that CMV disease develops in more than half the patients lacking detectable anti-CMV T-cell responses.4 Tetramer technology, which allows the direct visualization of virus-specific CTLs, has now replaced the CTL assays with CMV-infected fibroblasts as a target. Recent studies with tetramers have firmly established the presence of CMV-specific CTLs in the blood of patients as the best marker for protection against CMV disease, including patients receiving prophylactic or preemptive antiviral therapy.6-9
Restoration of T-cell immunity after HSCT is a slow process. The rebound of the thymus occurs late,10-12 particularly in adult patients, and the contribution of newly generated T cells during the first 6 months after transplantation may be negligible.13 Therefore, the initial protection against viruses has to come from the donor T cells coinfused with the graft or possibly from recipient T cells that have survived conditioning. This might leave some patients unprotected because no immunity is transferred when a patient receives a transplant of hematopoietic stem cells (HSCs) from a CMV-negative donor; recipient T cells survive only when the graft is thoroughly depleted of donor T cells.14,15
In this study, we monitored the anti-CMV response in 17 patients who received a T-cell-replete or a T-cell-depleted graft from a CMV-positive or -negative donor. We show that distinct combinations of the CMV status of the donor and the type of graft have a significant impact on posttransplantation CMV immunity.
Materials and methods
Monoclonal antibodies, tetramers, and flow cytometry
FACSVantage (Becton Dickinson, Mountain View, CA) was used to sort and analyze cryopreserved mononuclear cells stained with CD8-PE and with allophycocyanin (APC)-labeled tetramers. The synthesis of biotinylated A*0101(A245V)/YSEHPTFTSQY, HLA-A*0201wt/NLVPMVATV, HLA-B*0702wt/TPRVTGGGAM, and HLA-B*0702wt/RPHERNGFTVL using pp65-derived peptides and tetramerization with streptavidin-APC (Molecular Probes, Leiden, The Netherlands) have been previously described.16
Analysis of the recipient/donor origin of tetramer-positive T cells
Polymerase chain reaction (PCR) conditions and fluorochrome-labeled primers used to amplify the short tandem repeat SE33, D11S554, and Penta E have been previously described.17 Analysis was performed on high molecular weight DNA prepared from nuclei from 2 to 5000 fluorescence-activated cell sorter (FACS)-sorted tetramer-positive cells solubilized in buffer (10 mM Tris, pH 7.5, 5 mM MgCl2, 1% Triton-X100, 10% sucrose) and incubated with proteinase K. The ratio of donor to recipient DNA was determined on a 310 Genetic Analyzer (ABI Prism, Foster City, CA) assessing the surface areas of the peaks using the GeneScan program (Applied Biosystems, Foster City, CA).
TCR analysis and CDR3 spectratyping
All procedures and primer sequences for the 21 variable segments of the T-cell receptor beta (TCR-β) chain used in this study have been previously described.12 In brief, total RNA and cDNA were prepared from 2 to 5000 FACS-sorted cells using the RNeasy kits (Qiagen, Hilden, Germany). PCR was performed with 6-FAM, HEX, and TET dye 5′-labeled primers (Amplimmun, Madulain, Switzerland). Data analysis was performed using GeneScan analysis software (Applied Biosystems).
CMV
CMV monitoring was performed weekly until day 100 for patients without CMV reactivation or until day 180 for patients with an early limited period of CMV reactivation. Screening was performed either by detection of the pp65 antigen with monoclonal antibodies (CINAkit; Argène, Biosoft, Varilhes, France) or by PCR using a modified version of the Cobas Amplicor CMV Monitor test platform (Roche Diagnostic Systems, Branchburg, NJ) with a detection threshold of fewer than 20 copies/mL plasma. Both methods have been previously described.18,19 Preemptive ganciclovir therapy was given at a dose of 5 mg/kg intravenously twice a day for 14 days and then 5 mg/kg daily for 2 weeks. In patients with neutropenia, foscarnet at a dose 90 mg/kg intravenously twice a day for 2 weeks and then 90 mg/kg daily 2 weeks was used. In some patients with renal impairment, the doses were adjusted accordingly.
Green fluorescence protein and GFP-pp65-transduced antigen-presenting cells
Antigen-presenting cells transduced with green fluorescence protein (GFP)-pp65 or with GFP alone were obtained by incubation of 0.5 × 106 Epstein-Barr virus (EBV)-transformed B cells in 24-well plates coated with retronectine (Nunc, Roskilde, Denmark) containing 1 mL retrovirus supernatant. The Moloney murine leukemia virus-based retroviral vector LZRS, the packaging cells ϕ-NX-A, and the cloning of GFP and GFP-pp65 into the vector have been described previously.20 After centrifugation for 2 hours at 1350g at 34°C, the cells were incubated overnight at 37°C. Thereafter, the cells were washed and incubated for a further 10 days; this was followed by FACS sorting to obtain the GFP(-pp65)-positive cells.
IFN-γ secretion assay
Production of IFN-γ was measured with the IFN-γ Secretion Assay kit (Miltenyi Biotec, Bergisch Gladbach, Germany) according to the manufacturer's instructions. In brief, 0.5 × 106 mononuclear cells or 0.5 × 106 cloned T cells were incubated overnight with 0.5 × 106 EBV-transformed B-cell lines presenting or not presenting pp65. After 2 washes, the cells were labeled with the IFN-γ catch reagent for 5 minutes on ice and incubated for 40 minutes at 37°C. Thereafter, the membrane-immobilized IFN-γ was measured by FACS after incubation with CD8-PE and the IFN-γ detection antibody labeled with APC with gates on forward scatter/side scatter (FSC/SSC) for viable cells and on fluorescence 1 to exclude the GFP-positive antigen-presenting cells.
Results
Patient/donor characteristics and CMV-specific tetramer-positive T cells
We studied the anti-CMV T-cell response in 17 CMV-positive patients who received a transplant of HSCs from CMV-positive or -negative donors. Criteria for enrollment in the study were CMV positivity of the patient in combination with detectable CMV-specific, tetramer-positive T cells during the month before transplantation and absence of posttransplantation complications that would require chemotherapy, donor lymphocyte infusion, or durable increase of the immunosuppression regimen. Except for patient 13, who received conditioning with busulfan and fludarabine, all patients received conditioning with fractionated total body irradiation (TBI) combined with cyclophosphamide or melphalan as described.21 Table 1 shows age, underlying disease, type of donor, CMV status of the donor, type of graft, graft-versus-host disease (GVHD) prophylaxis, occurrence of GVHD, and reconstitution of CD8+ T cells and of CMV-specific tetramer-positive T cells. Ten patients received grafts that had been T-cell depleted with the rat anti-CD52 IgM monoclonal antibody Campath 1M22 plus complement in vitro. The other patients received either an unmanipulated graft or a graft treated with the humanized anti-CD52 monoclonal antibody Campath 1H (alemtuzumab; Genzyme, Cambridge, MA)23 followed 24 hours later by an infusion of unmanipulated cells. We will refer to the latter 2 graft types as “T-cell-replete grafts.” GVHD prophylaxis consisted of cyclosporin A with a short course of methotrexate for the recipients of T-cell-replete grafts. Engraftment (polymorphonuclear leukocyte [PMN] count greater than 0.5 × 109/L) occurred 15 to 30 days after transplantation. From then on, recipients of T-cell-replete grafts became full donor chimeras. All patients remained in complete remission during the time of the study. Only patient 10 experienced grade 2 GVHD.
With the exception of patients 6 and 14, CD8+ T-cell numbers reached normal or supranormal levels at 2 to 3 months. CMV-specific cells were present (range, 3-65/mL) in all 10 patients who received stem cells from CMV-positive donors, independently of whether they had received T-cell-replete (patients 1, 2) or T-cell-depleted (patients 3-10) grafts. In the patients who received stem cells transplanted from CMV-negative donors (patients 11-17), CMV-specific cells were present in the 2 recipients of T-cell-depleted grafts (patients 16, 17) but only in 1 of 5 recipients of a T-cell-replete graft (patient 13). In spite of the immunosuppression, the percentage of tetramer-positive cells (average in the tetramer-positive patients, 6.7% ± 4.6%; range, 2.3-16) was significantly higher than in the patients before transplantation (1.3% ± 1.6%) or in the CMV-positive donors (0.7% ± 0.7%), a phenomenon that has been described by others.8,24-26
Origin of the posttransplantation CMV-specific T cells is related to graft type and donor CMV status
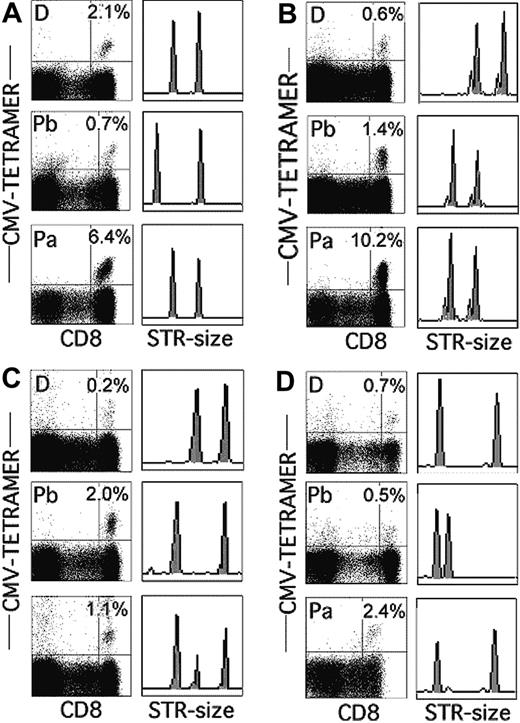
In recipients of T-cell-replete grafts, at least part of the T-cell immunity in the first year after transplantation comes from donor T cells transferred with the graft. T-cell depletion methods, such as the one used in this study, eliminate more than 99% of the donor T cells, allowing residual recipient T cells to repopulate the T-cell compartment.13-15,27 Hence, CMV-specific T cells in the 10 recipients of T-cell-depleted grafts (Table 1) could have been the progeny of a few donor T cells not lysed by the antibody plus complement treatment or the progeny of recipient T cells that had survived the conditioning regimen. Figure 1 shows the analysis of the tetramer-positive T cells in 4 representative recipients of grafts from CMV-positive donors before transplantation and in the donors and patients approximately 3 months after transplantation. In patient 1, the recipient of a T-cell-replete graft, only the short tandem repeat (STR)17 marker of the donor was detected in the samples after transplantation indicating that all CMV-specific lymphocytes were of donor origin (Figure 1A). By contrast, in patients who received T-cell-depleted grafts, the CMV-specific cells could be entirely of patient origin (Figure 1B, patient 3), of mixed origin (Figure 1C, patient 6), or of donor origin (Figure 1D, patient 10). Table 2 shows the percentages of CMV-specific T cells and their origins in sequential samples for all 17 patients studied. Clearly, the presence and origin of tetramer-positive T cells depended almost entirely on the combination of the CMV status of the donor and the type of graft. In the recipients of T-cell-replete grafts of CMV-positive donors (patients 1, 2), all CMV-specific T cells were of donor origin, which is a normal finding in recipients of unmanipulated grafts.26 This loss of pretransplantation, CMV-specific recipient T cells was most likely the result of the allogeneic effect of the high number of donor T cells in T-cell-replete grafts that eradicate all T cells of recipient origin, irrespective of their antigen specificity.27 In recipients of T-cell-depleted grafts, recipient T cells dominated because they were entirely of recipient origin in both patients16,17 who received grafts from CMV-negative donors and in 3 of 8 patients who received grafts from CMV-positive donors (patients 3-5), and they were in the majority in patients 6 to 8. Only in patients 9 and 10 were the CMV-specific cells entirely of donor origin. Interestingly, the donor-recipient ratio did not change significantly with time. CMV-specific cells in patients 3, 4, 5, 16, and 17 remained entirely of recipient origin for the entire period of the study, which for some of the patients was as long as 3 years. Furthermore, the donor-recipient ratio in the patients whose CMV-specific cytotoxic T cells were of mixed origin remained stable throughout the entire period.
Percentages and origins of CMV-specific, tetramer-positive T cells in recipients of HSC grafts from CMV-positive donors 3 months after transplantation. (A) Patient 1. (B) Patient 3. (C) Patient 6. (D) Patient 10. Percentages of tetramer-positive cells in donor (D) and patient before (Pb) and after (Pa) transplantation and the corresponding STR analysis for the loci Penta E (A,D), SE33 (B), and D11S554 (C).
Percentages and origins of CMV-specific, tetramer-positive T cells in recipients of HSC grafts from CMV-positive donors 3 months after transplantation. (A) Patient 1. (B) Patient 3. (C) Patient 6. (D) Patient 10. Percentages of tetramer-positive cells in donor (D) and patient before (Pb) and after (Pa) transplantation and the corresponding STR analysis for the loci Penta E (A,D), SE33 (B), and D11S554 (C).
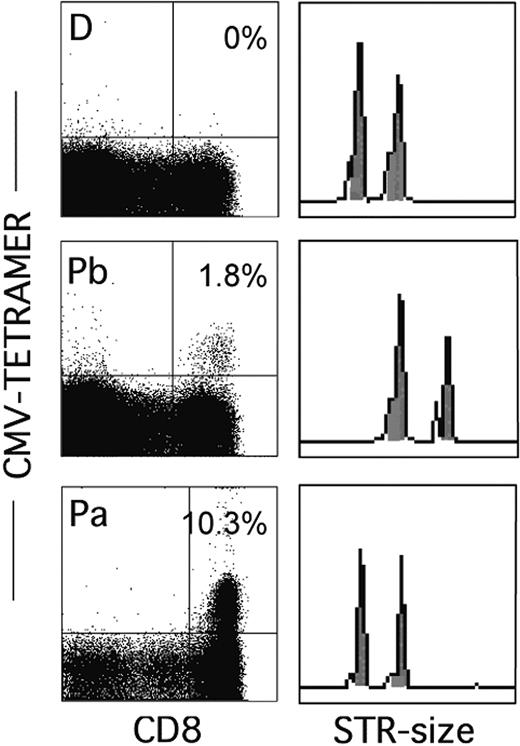
Findings in the 5 recipients of T-cell-replete grafts from CMV-negative donors (patients 11-15) were also uniform. With one exception, no CMV-specific T cells were detected during the first year. Only patient 13 responded from day 36 on, whereas the response was delayed in patient 15 until the second year. Figure 2 shows that although only the patient and not the donor had been tetramer positive before transplantation, all CMV-specific cells in patient 13 were of donor origin (similar results were obtained in patient 15). Apparently, donor T cells transfused with the graft are able to mount a primary anti-CMV response but this only happens in a minority of patients, which means that during the first year tetramer-positive cells are often absent in patients who receive grafts from CMV-negative donors.8,26
Antigen responsiveness of CMV-specific T cells of recipient origin
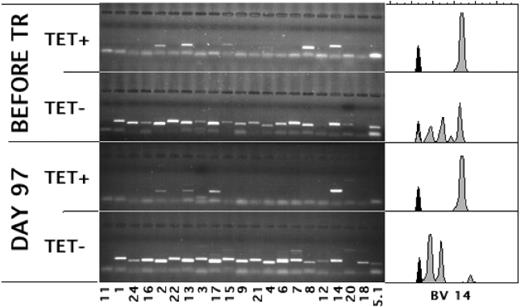
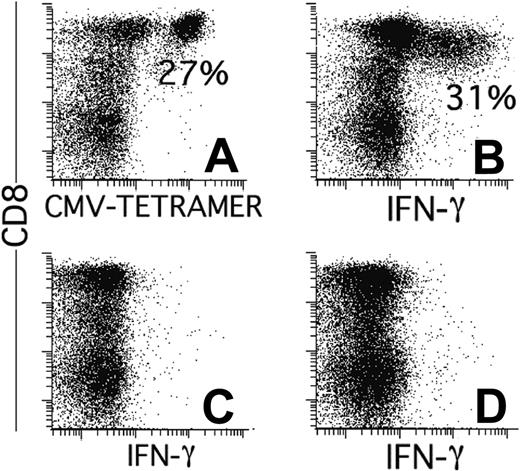
During the first weeks after transplantation, T cells repopulate the host through antigen-driven expansion.28 As a result, the bulk of the T cells may be directed against mismatched histocompatibility antigens29,30 or against viruses31 toward which the T cells confer the initial protective immunity. It is unknown whether, analogous to the donor T cells transfused with the graft, the T cells of recipient origin retain their capacity to proliferate and respond effectively to antigen after the conditioning. Figure 3 (patient 3; similar results were obtained for patient 7) shows that 3 of the TCRs of the anti-CMV T-cell repertoire detected before transplantation (upper panel) were also found after transplantation (lower panel). An estimated 60% to 70% of the tetramer-positive T cells used BV14, which was the same BV14 as that used before transplantation (compare the CDR3 length of the BV14 used by the tetramer-positive cells by the spectratypes27,32 shown in the right panels). Therefore, these cells were the progeny of cells that had survived conditioning. In addition, given that in the first 2 weeks after transplantation the total number of lymphocytes had been lower than 10/mL, they had to have expanded considerably to reach these numbers (58/mL; Table 1). Furthermore, in addition to keeping their proliferative capacity, the patient's CMV-specific cells had retained their capacity to produce IFN-γ in response to antigen. Figure 4 shows the results of one patient with only tetramer-positive cells of patient origin (patient 17; similar results were obtained for patient 5). At day 59 after transplantation, as many as 27% of the CD8+ cells were positive for the HLA-A*0101/YSEHPTFTSQY tetramer (Figure 4A), corresponding to 143/mL CMV-specific CTLs in the blood. Furthermore, more than 30% of the CD8+ cells produced IFN-γ after stimulation with an HLA-A*0101-matched cell line transduced with GFP and pp65 (Figure 4B). This was clearly a response to pp65 because no IFN-γ production was observed without stimulation (Figure 4C) or when the cells were stimulated with the cell line transduced with GFP only (Figure 4D). Because the cells that had been stimulated overnight with pp65 had down-regulated their TCR to an extent that tetramer binding was close to the detection limit, we were unable to show directly that the cells producing IFN-γ were the same as those binding the CMV-specific tetramer-binding cells of recipient origin by simultaneously staining for tetramers and IFN-γ. Therefore, we sorted the IFN-γ-producing cells and cloned them under limiting dilution. All 7 clones obtained after 3 to 4 weeks of culture were of recipient origin, bound tetramers, and produced IFN-γ after stimulation with pp65 (data not shown). Hence, in spite of the TBI in the conditioning regimen, the CMV-specific cells of recipient origin not only retained the capacity to reconstitute the patient; they also retained the potential for the additional 15+ divisions necessary for the in vitro cloning procedure. Furthermore, after expansion in the patient, they retained their capacity to respond to antigen.
CMV-specific, tetramer-positive T cells in patient 13—a recipient of a T-cell-replete graft from a CMV-negative donor—are of donor origin. Data show the percentages of tetramer-positive cells in the donor (D) and the patient before (Pb) and after (Pa) transplantation and the corresponding STR analysis for the locus D11S554.
CMV-specific, tetramer-positive T cells in patient 13—a recipient of a T-cell-replete graft from a CMV-negative donor—are of donor origin. Data show the percentages of tetramer-positive cells in the donor (D) and the patient before (Pb) and after (Pa) transplantation and the corresponding STR analysis for the locus D11S554.
Many of the pretransplantation CMV-specific T-cell clones expanded after transplantation. Lanes show the PCR products of the depicted variable regions of the Vβ-chain (BVs) used by CMV-specific, tetramer-positive (TET+) and CD8+, tetramer-negative (TET-) T cells before and at day 97 after transplantation. Spectratypes of BV14, the BV of the TCR predominantly used by the tetramer-positive T cells after transplantation, are shown on the right.
Many of the pretransplantation CMV-specific T-cell clones expanded after transplantation. Lanes show the PCR products of the depicted variable regions of the Vβ-chain (BVs) used by CMV-specific, tetramer-positive (TET+) and CD8+, tetramer-negative (TET-) T cells before and at day 97 after transplantation. Spectratypes of BV14, the BV of the TCR predominantly used by the tetramer-positive T cells after transplantation, are shown on the right.
CMV-specific T cells of recipient origin produce IFN-γ after stimulation with pp65-transfected, EBV-transformed B-cell lines. (A) Percentages of HLA-A*0101/YSEHPTFTSQY-tetramer positive cells at day 59. IFN-γ production by CD8+ lymphocytes in the same sample cultured overnight with HLA-A*0101-positive EBV cells transduced with GFP and pp65 (B), without stimulator cells (C), or with EBV cells transduced with GFP alone (D). Results are expressed as percentages of CD8+ cells with gates on lymphocytes (scatter) and GFP negativity (fluorescence 1) to exclude the antigen-presenting cells.
CMV-specific T cells of recipient origin produce IFN-γ after stimulation with pp65-transfected, EBV-transformed B-cell lines. (A) Percentages of HLA-A*0101/YSEHPTFTSQY-tetramer positive cells at day 59. IFN-γ production by CD8+ lymphocytes in the same sample cultured overnight with HLA-A*0101-positive EBV cells transduced with GFP and pp65 (B), without stimulator cells (C), or with EBV cells transduced with GFP alone (D). Results are expressed as percentages of CD8+ cells with gates on lymphocytes (scatter) and GFP negativity (fluorescence 1) to exclude the antigen-presenting cells.
CMV-specific T cells of recipient origin protect the patient against CMV-related complications
Table 3 shows the CMV-related complications in 108 patients grouped according to CMV status of the donor and the type of graft. CMV complications of the 17 patients in whom we studied CMV-specific cytotoxic T cells are depicted individually, whereas for the other 91 patients, only the frequency of CMV-related complications per group is shown. These 91 patients underwent transplantation with the same protocols and, like the first 17, were CMV positive and selected for the absence of severe posttransplantation complications. Patients were graded as having no detectable CMV reactivation; CMV reactivation (limited to 1-3 consecutive CMV-positive blood samples during the first 2 months) or clinically relevant complications such as CMV syndrome associated with fever, muscle pain or asthenia, and/or biologic abnormalities such as leukopenia, thrombocytopenia, or abnormal liver function test results; or CMV disease with proven organ involvement.
The data show that the presence of CMV-specific CTLs was associated with protection against CMV. In all but one patient with tetramer-positive cells in the blood, CMV reactivation was absent or limited to short viremia that was easily controlled by 4 weeks of preemptive antiviral therapy with ganciclovir. This was independent of whether the cells were of donor (patients 1, 2, 9, 10), of recipient (patients 3, 4, 5, 16, 17), or of mixed (patients 6, 7, 8) origin. Furthermore, the patients in whom CMV-specific tetramer-positive cells had been studied appeared to be fully representative of patients matched for donor CMV status and graft treatment. CMV-related complications were rare in recipients of a T-cell-depleted graft; only 2 of 29 recipients of a graft from a CMV-positive donor and none of the 7 recipients of a graft from a CMV-negative donor had CMV syndrome or CMV disease. Hence, in spite of the significantly lower transfer of CMV-specific donor T cells in the graft, CMV immunity—some of which must have been provided by the recipient cells—protected most of the patients from CMV. Furthermore, this protection was not less efficient than in the 39 recipients of a T-cell-replete graft from a CMV-positive donor, 5 of whom had CMV syndrome.
The 16 recipients of a T-cell-replete graft from a CMV-negative donor clearly formed a group apart. Consistent with the absence of tetramer-positive cells, the complications in these patients were more severe. Patient 11 had CMV syndrome with fever, leukopenia, thrombopenia, and recurrent and prolonged viremia. Several episodes of CMV reactivation were also observed in patient 12, who died at day 325 of acute respiratory distress syndrome caused by CMV and Stenotrophomonas maltophilia pneumonia. Patient 13 had pneumonia that was limited to the first month after transplantation, whereas patients 14 and 15 remained asymptomatic. Again, the 5 patients studied for the presence of CMV-specific T cells were representative of the entire group. Half of the 16 patients who received T-cell-replete grafts from CMV-negative donors had CMV syndrome or CMV disease.
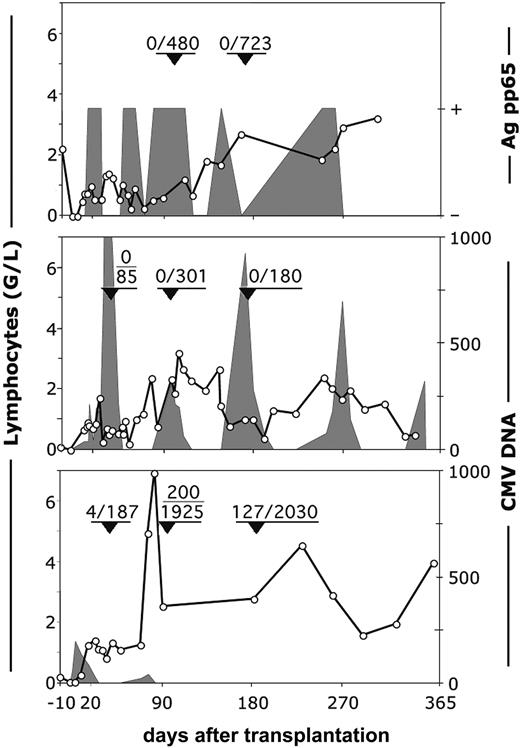
It is important to note that the nature of CMV reactivation in patient 13, the only patient who had tetramer-positive cells in his blood and CMV disease, was very different from that in the 2 patients who had no tetramer-positive cells (patients 11, 12). The latter patients were unable to clear the virus (Figure 5, upper and middle panels) despite intensive antiviral therapy with ganciclovir and foscarnet. In patient 13, T cells reconstituted at a similar pace during the first month, but the difference was that in the first sample tested (day 36), 2% of the CD8+ cells were already tetramer positive (Figure 5, lower panel). Viral DNA detected at 190 copies/mL blood at day 5 decreased from 83 copies/mL blood at day 18 to 23 copies/mL blood at day 25 and remained undetectable until the ganciclovir treatment was discontinued at day 65. Five days later, 2 sequential samples were again PCR positive (20-40 copies/mL); this was followed by a sharp increase in the number of lymphocytes. At day 97, 77% of the lymphocytes were CD8+ T cells, of which 10.3% (200 cells/mL) were tetramer positive. In the meantime, the patient had completely cleared the virus without additional antiviral therapy. High numbers of tetramer-positive cells persisted until the last sample measured at day 181 (128 cells/mL). These data strongly suggest that a primary anti-CMV response from the cotransfused T cells of the CMV-negative donor (Figure 2) is able to protect the patient. However, because these primary responses only occur in a minority of patients, a CMV-positive recipient of an (unmanipulated) graft from a CMV-negative donor is at high risk for CMV-related complications.
Reconstitution of CMV-specific, tetramer-positive cells and the occurrence of CMV-viremia in recipients of HSC grafts from CMV-negative donors. Top panel: patient 11. Middle panel: patient 12. Bottom panel: patient 13. ○ indicates number (G/L) of lymphocytes. Fractions in the graphs show the number per milliliter of tetramer-positive/CD8+ T lymphocytes at the moment indicated by arrowheads. Shaded areas represent the viral load expressed as either positive or negative for the pp65 antigen or as number of viral copies per milliliter of plasma.
Reconstitution of CMV-specific, tetramer-positive cells and the occurrence of CMV-viremia in recipients of HSC grafts from CMV-negative donors. Top panel: patient 11. Middle panel: patient 12. Bottom panel: patient 13. ○ indicates number (G/L) of lymphocytes. Fractions in the graphs show the number per milliliter of tetramer-positive/CD8+ T lymphocytes at the moment indicated by arrowheads. Shaded areas represent the viral load expressed as either positive or negative for the pp65 antigen or as number of viral copies per milliliter of plasma.
Discussion
The use of prophylactic and preemptive antiviral therapies has significantly reduced the incidence of CMV disease in the first months after transplantation. Nevertheless, cellular immunity remains crucial for the control of CMV infection. This is documented most convincingly by the fact that the presence of CMV-specific T cells in the patient's blood is the parameter that correlates best with protection against CMV disease.3-9 The transplantation procedure severely affects T-cell immunity, and many infections in the recipients of HSC transplants who survive long term are the result of impaired restoration of the T-cell compartment.4,33-37 Because T-cell reconstitution through the thymic pathway is slow,10-13,38 the initial immunity has to come from donor T cells coinfused with the graft or possibly from recipient T cells spared by the conditioning. To date, little is known about the protective role of recipient T cells after transplantation because most transplantation protocols eradicate recipient T cells through a combination of the conditioning and an allogeneic effect of the graft. To our knowledge, antigen-specific cells of patient origin have only been described after cord blood transplantation in children who have undergone conditioning regimens without TBI.9 Our study shows that when a T-cell depletion protocol is used that allows recipient T cells to repopulate the T-cell compartment, the T cells maintain their function even after conditioning that includes TBI. Furthermore, T cells appeared to protect the patient from CMV-related complications. Only 2 of 36 patients who underwent transplantation with a T-cell-depleted graft had CMV syndrome/disease, which is remarkably low for a group of patients in whom more than half had tetramer-positive cells of recipient origin.
The low frequency of CMV-related complications in our recipients of T-cell-depleted grafts probably results from the particular T-cell depletion method used in this study. This protocol, which consists of depletion with Campath 1M plus complement in vitro followed by 2 wash steps, spares the recipient T cells,14,15 whereas with most T-cell depletion methods the anti-T-cell antibody is infused into the patient,39-42 eliminating not only donor T cells but also those of the recipient. This would explain why T-cell depletion in other studies39-43 increased the risk for CMV-related complications, whereas our approach was either neutral or, almost paradoxically, even favorable for posttransplantation CMV immunity when a patient received a graft from a CMV-negative donor.
Our study shows strict correlations among the presence and origin of tetramer-positive cells after transplantation, donor CMV status, and type of graft. It may be that our patients were so instructive because we enrolled only patients whose pretransplantation anti-CMV immunity was still intact, which excludes patients at high risk who have undergone multiple cycles of chemotherapy. Furthermore, we excluded patients with posttransplantation complications requiring treatment that could interfere with the development of a cellular anti-CMV response. This selection of best possible patients also explains why, in contrast to previous reports,8 most of our patients already had high numbers of tetramer-positive T cells in the first month after transplantation and why, in contrast to patients treated with steroids,25 all tetramer-binding cells were functional.
Although we did not study patient CMV-specific CD4+ T cells, we think it is likely that CD4+ CMV-specific T cells reconstitute the same way CD8+ cells do. Because the percentage of CD8+ tetramer-binding T cells correlates with the CD4 T-cell response,7,25 it is to be expected that CMV-specific helper T-cell responses occurred in the patients with high numbers of tetramer-positive cells. Many of the CD4+ cells in the recipients of T-cell-depleted grafts in this study have been shown to be of mixed origin.15,27 In the recipients of grafts from CMV-negative donors, patient CD4 cells are the most likely source of CMV-specific T-cell help.
Although posttransplantation conditions were permissive for a memory anti-CMV T-cell response issuing from donor T cells transferred with the graft or from recipient cells that had survived the conditioning, a primary anti-CMV response by the coinfused donor T cells appeared to be more cumbersome. If, as our study and those by others26 suggest, only a minority of patients can mount a primary anti-CMV response in the first year after transplantation, an unmanipulated graft from a CMV-negative donor will only abolish the patient's anti-CMV response without providing an immediate substitute through the infused memory cells. Therefore, the transplantation of grafts from CMV-negative donors must be correlated with an increased frequency of CMV morbidity. Yet, much of the literature on this topic remains controversial.43-45 In a recent analysis of more than 7000 patients, a beneficial effect of receiving a graft from a CMV-positive donor was observed only when the donor was unrelated.2 However, other reports do find significant correlations between the seronegativity of the donor and increased CMV morbidity,42,43,46,47 higher viral load,48 and fewer CMV-specific, tetramer-binding T cells.8
Our study shows a low frequency of CMV-related complications in recipients of T-cell-depleted grafts and a considerable difference between the frequencies of CMV-related complications in recipients of grafts from CMV-positive or CMV-negative donors. Because all recipients of T-cell-depleted grafts had tetramer-positive cells and only 2 of 36 had CMV disease/syndrome, we are confident about our conclusion that a T-cell depletion protocol that spares recipient T cells preserves the patient's anti-CMV immunity. The same can be said concerning the absence of a primary response in recipients of T-cell-replete grafts from CMV-negative donors. All 5 such patients studied matched the group of 39 recipients of T-cell-replete grafts from CMV-positive donors with regard to the intensity of the conditioning and GVHD prophylaxis and to the absence of posttransplantation complications that might have interfered with antiviral immunity. Furthermore, 4 of 5 patients underwent transplantation with grafts from HLA-identical siblings, a proportion similar to the group of patients who underwent transplantation with T-cell-replete grafts from CMV-positive donors in whom 32 of 37 grafts were from HLA-identical siblings. Hence, except for CMV status of the donor, all characteristics of the patients were comparable, strongly suggesting that CMV-related complications occurred because of the difficulty in mounting the primary immune response necessary to protect the patient when the donor is CMV negative and no CMV-specific memory T cells are transferred.
As a whole, the group receiving T-cell-replete grafts from CMV-negative donors differed from the group receiving T-cell-replete grafts from CMV-positive donors. Because there is a relatively low chance that 2 siblings have disparate CMV status, CMV-positive patients who received grafts from CMV-negative donors are most likely to be found among patients receiving grafts from unrelated donors. In fact, in our center, only 9% of the related donor/recipient combinations consisted of a CMV-positive patient and a CMV-negative donor, whereas the percentage for an unrelated combination was as high as 36%. For that reason, it was not possible to enroll mainly HLA-identical sibling pairs to the group who underwent transplantation with CMV-negative donors. In fact, most of the 16 patients in the cohort added for comparison underwent transplantation of stem cells from unrelated donors. However, we do not think this had a major influence on the analysis. It seems likely that the increased frequency of CMV-related complications in patients who received grafts from unrelated donors was attributed to more severe GVHD and to its steroid treatment rather than to other factors associated with unrelated donor transplantation.47 Given that we excluded patients with these complications, we think that factors other than CMV status of the donor had limited impact and that the high frequency of CMV-related complications in the group of 16 patients from CMV-negative donors resulted from lack of transfer of CMV-specific T cells.
In conclusion, we believe that our findings illustrate some general principles of posttransplantation immunity. First, they show that when antigen is present during the initial expansion of the T-cell compartment, the antigen-specific memory T cells expand to reach a considerable clonal size26,29-31 and persist for several years after transplantation. As a result, memory against CMV is preserved in most patients receiving standard conditioning and GVHD prophylaxis. In contrast, primary responses are rare during the first year so that a CMV-positive patient who undergoes transplantation with an unmanipulated graft from a CMV-negative donor will be at high risk for CMV-related complications. Second, our findings show that most of the expanded CMV-specific CTLs may be of patient origin if conditions allow the survival of patient T cells and that such cells are functional and protect the patient from CMV complications. It has been suggested that the modality of T-cell depletion should be tailored according to CMV risk status and that CMV-seropositive patients should receive a less extensively T-cell-depleted graft and a CMV-seropositive graft, if possible.42 Our data suggest that if no such CMV-positive donor is available, the T-cell depletion protocol can be adapted to preserve the patient's anti-CMV immunity.
Prepublished online as Blood First Edition Paper, September 20, 2005; DOI 10.1182/blood-2005-07-2746.
Supported by grant 3100-65357.01 from the Swiss National Science Foundation (E.R.) and by the “Dr Henri Dubois-Ferrière-Dinu Lipatti” Foundation (E.R.).
Y.C., S.D., and J.V. contributed equally to this work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Carole Dantin and Jean Ringrose for critical reading of the manuscript.