Abstract
The balance between pro- and anti-inflammatory cytokines modulates inflammation. Intracellular inhibitors of signaling, in turn, contribute to the negative regulation of cytokines. One of these inhibitors is suppressor of cytokine signaling-1 (SOCS-1). Socs1-/- mice die by 3 weeks of age with inflammation and fatty necrosis of the liver. Here, cre/loxP deletion of Socs1 was used to investigate the contribution of specific cells/tissues to inflammatory disease. Mice with SOCS-1 deficiency in myeloid and lymphoid cells, but not lymphoid alone, became ill at 50 to 250 days of age. These mice developed splenomegaly and T-cell/macrophage infiltration of many organs, including liver, lung, pancreas, and muscle. There were also abnormally high levels of the proinflammatory cytokines interferon γ (IFN-γ), tumor necrosis factor (TNF), and interleukin-12 (IL-12), and activated T cells circulating in these mice. Socs1null T cells were found to be hypersensitive to multiple cytokines, including IL-1, IL-2, and IL-12, resulting in IFN-γ production without requiring T-cell receptor (TCR) ligation. Additionally, Socs1null macrophages produced excessive amounts of IL-12 and TNF in response to other cytokines, including IFN-γ. A dysregulated cytokine network between T cells and macrophages is thus associated with this inflammatory disease. These findings indicate that SOCS-1 is critical in both T cells and macrophages for preventing uncontrolled inflammation. (Blood. 2005;106:1668-1675)
Introduction
Inflammation is a critical response to tissue injury, and is required for tissue repair and the clearance of infections. However, uncontrolled inflammation in itself results in further tissue injury. Cytokines play critical roles in modulating inflammation. Proinflammatory cytokines, such as interferons (IFNs), tumor necrosis factor (TNF), interleukin-1 (IL-1), and IL-12, promote inflammation by activating leukocytes, inducing expression of chemoattractants by tissues, and inducing expression of adhesion molecules by endothelial cells.1 In contrast, anti-inflammatory cytokines such as IL-4, IL-10, and transforming growth factor-β suppress the effects and/or expression of proinflammatory cytokines.2 Perturbations in this network of cytokines can therefore result in deleterious consequences.
Suppressor of cytokine signaling-1 (SOCS-1) is an intracellular protein that inhibits Janus kinase (JAK)-signal transducer and activator of transcription (STAT) signaling by cytokines such as interferons and those that signal through the γ-common (γc) receptor chain.3 SOCS-1 is also capable of inhibiting non-JAK-STAT cytokines, such as TNF4,5 and lipopolysaccharide (LPS).6,7 SOCS-1 expression is induced in many cell types in response to cytokine exposure, and thus acts in a classical negative feedback loop to regulate cytokine signaling. In the thymus, SOCS-1 is expressed at very high levels in CD4+CD8+ thymocytes, and this expression appears to be regulated developmentally rather than by cytokines.8,9
The generation of mice deficient in SOCS-1 has demonstrated the critical requirement for negatively regulating cytokine signaling. Socs1-/- mice die within 3 weeks of birth from lymphopenia and an inflammatory disease affecting the liver, pancreas, and heart.10,11 The liver disease is particularly severe with macrophage and T-cell infiltration, and widespread fatty degeneration of hepatocytes. This disease is thought to be dependent on the hypersensitivity of SOCS-1-deficient tissues to IFN-γ. IFN-γ-induced STAT1 activation is prolonged in hepatocytes lacking SOCS-1,12 while the neonatal Socs1-/- disease is ameliorated by administration of neutralizing antibodies to the cytokine, or deletion of the Ifng gene.9,13
T cells and natural killer T (NKT) cells have been implicated as the key cellular mediators of the Socs1-/- disease. This is supported by several pieces of evidence. T cells are required for the Socs1-/- disease, because lymphocyte deficiency caused by Rag1/2 gene inactivation prevents the neonatal lethality.9,14 T cells in Socs1-/- mice appear activated, expressing cell surface markers of activation.9 The increased circulating levels of IFN-γ in these mice also suggest T-cell activation.9 Additionally, T cells from Socs1-/- mice hyperproliferate in response to TCR ligation.9 Finally, Socs1-/- NKT cells are cytotoxic for syngeneic hepatocytes, while NKT-cell depletion reduces the severity of the liver disease in Socs1-/- mice.15
However, more recently we have shown that deletion of Socs1 specifically in T and NKT cells does not recapitulate any of the Socs1-/- inflammatory pathologies.8 These mice remain healthy, but display enhanced differentiation of selected thymocytes toward the CD8+ lineage and a very high percentage of peripheral CD8+ T cells with a memory-like phenotype. These phenotypes appear to be associated with dysregulated signaling by members of the γc family of cytokines.8,16 Therefore, SOCS-1 deficiency in T/NKT cells alone is insufficient to cause inflammation, and disease also depends on abnormalities in other cell types.
Only certain organs are affected by inflammatory infiltration in diseased Socs1-/- mice. These organs are infiltrated with both T cells and macrophages. Therefore, the inflammatory disease in Socs1-/- mice may also be dependent on SOCS-1 deficiency in macrophages and/or target cells, in addition to T cells. To further understand the role SOCS-1 plays in regulating inflammation, we used the cre/loxP system to investigate the consequence of SOCS-1 deficiency in T cells, macrophages, and a target tissue.
Materials and methods
The generation of mice with tissue-specific SOCS-1 deficiency
Tissue-specific SOCS-1-deficient mice were generated using the cre/loxP system by breeding Socs1lox/lox mice, which carry a Socs1 allele flanked by loxP sites,8 with various cre transgenic mice. Mice expressing cre under the endogenous lysozyme M promoter (LysM-cre)17 were used to delete Socs1lox in the myeloid and lymphoid compartment, and cre expressed under the albumin promoter (Alb-cre)18 was used to achieve hepatocyte-specific deletion. Mice in which Socs1lox is deleted specifically in the thymocytes/T-cell compartment (using Lck-cre), or in all cells during embryogenesis (using CMV-cre), are described elsewhere.8 Socs1lox/lox mice were generated on a C57BL/6 genetic background, while all other mice were on their 5th to 10th generation backcross to C57BL/6.
Histologic and hematologic analyses
Tissues were fixed in 10% neutral-buffered formalin and paraffin embedded. Sections were prepared by standard techniques and stained with hematoxylin and eosin. Tissues for immunohistochemistry were fixed in 4% paraformaldehyde in phosphate-buffered saline (PBS) for 2 hours at 4°C, then infused overnight with 30% sucrose in PBS at 4°C, prior to freezing in OCT mounting medium (Sakura Finetechnical, Tokyo, Japan). Sections were stained with monoclonal antibodies (clone name in parentheses) recognizing murine CD4 (H129.19), CD8 (53-6.7), and CD11b/Mac-1 (M1/70) (BD Pharmingen, San Diego, CA). Images were captured by Axiocam attached to an Axioplan 2 microscope (Zeiss, Oberkochen, Germany) using the 20× objective lens. Images were acquired with Axiovision software.
Peripheral blood parameters were determined by automated counting on an ADVIA 120 (Bayer, Tarrytown, NY). Alternatively, differential white cell counts were performed manually on blood smears stained with May-Grünwald-Giemsa.
Flow cytometry
Monoclonal antibodies recognizing the following antigens (clone name in parentheses) were used for flow cytometric analyses: murine CD3 (KT3), CD4 (H129.19), CD8 (53-6.7) CD11b/Mac-1 (M1/70), CD11c (HL3), CD25 (7D4), CD44 (IM7), CD45R/B220 (RA3-6B2), CD62L (MEL-14), CD69 (H1.2F3), NK1.1 (PK136) and Ly6G/Gr-1 (RB6-8C5), and human CD4 (RPA-T4). All antibodies were purchased from BD Pharmingen. Analyses were performed on an LSR cytometer (BD Biosciences, San Diego, CA).
Cytokines and cytokine ELISAs
The cytokines used were recombinant human IL-1 (Genzyme, Cambridge, MA), IL-2 (Roche, Basel, Switzerland), IL-15 (Peprotech, Rocky Hill, NJ), recombinant murine IFN-γ (Genentech, South San Francisco, CA), IL-12 (Roche), TNF (Genentech), and murine IL-7 and LPS (Sigma, St Louis, MO).
Cytokine concentrations were determined using standard sandwich enzyme-linked immunosorbent assays (ELISAs). IFN-γ was measured using the purified monoclonal antibody R4-6A2 (capture) and the biotinylated monoclonal antibody XMG1.2 (detection) (BD Pharmingen). IL-1 (DY401), TNF (DY410), and IL-12p40 (DY499) were measured using DuoSet ELISA Development System antibodies (R&D Systems, Minneapolis, MN).
T-cell and macrophage cultures
T cells were purified from lymph nodes using nylon wool, which achieved more than 95% purity determined by flow cytometry. T cells were plated at 5 × 105 cells/well in 96-well tissue-culture plates.
Resident peritoneal macrophages were obtained by lavage with 5 mL cold PBS/2% fetal calf serum (FCS). The lavage was cultured in Dulbecco modified Eagle medium (DMEM)/10% FCS on tissue-culture plates. After 4 hours, nonadherent cells were washed away with PBS. Adherent cells were then liberated and replated out at 2 × 105 cells/well in 96-well tissue-culture plates.
Bone marrow (BM) transplantation
Eight-week-old C57BL/6 were irradiated with 2 doses of 5.5 Gy (550 rads) given 2 hours apart. Recipients were injected intravenously with 107 BM cells from 100- to 110-day-old donors. Mice were maintained on neomycin sulfate in the drinking water for 2 weeks after receiving the transplant.
Results
LysM-cre deletes Socs1lox in both lymphoid and myeloid lineages
The consequence of SOCS-1 deficiency in myeloid and lymphoid lineages was investigated by breeding Socs1lox/lox mice with Socs1+/- and LysM-cre mice to obtain progeny that were Socs1lox/- heterozygous and LysM-cre positive. The Socs1lox allele carries human CD4 (hCD4) as a reporter of cre-mediated recombination.8 With only one Socs1lox allele in Socs1lox/- mice requiring recombination, any cell that switched on the hCD4 reporter could be considered deficient in both copies of Socs1. The presence of only one copy of Socs1lox also ensured the most efficient deletion.
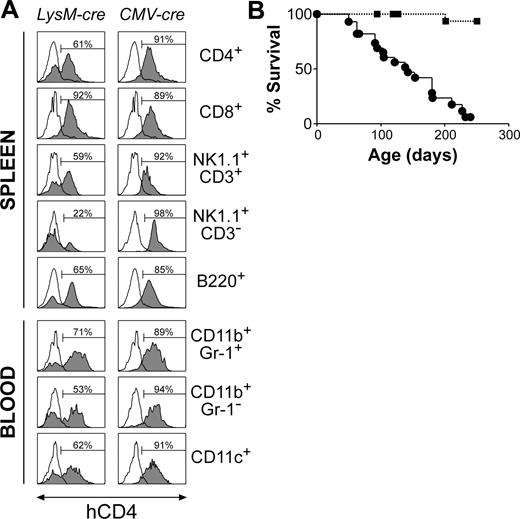
LysM-cre has previously been reported to mediate efficient loxP recombination in myeloid cells.17 However, using the hCD4 reporter in Socs1lox/+LysM-cre mice to track Socs1lox recombination, we found that LysM-cre mediated high levels of recombination in CD4+ T cells, CD8+ T cells, NK1.1+CD3+ NKT cells, and B cells, as well as in monocytes, neutrophils, and CD11c+ dendritic cells (Figure 1A). Low-level deletion was also detected in NK cells. Socs1lox recombination in the T-lymphoid compartment was detected from the late CD4-CD8- double-negative thymocyte stages (not shown). As expected, all cells from positive control Socs1lox/+CMV-cre mice, in which recombination occurs in all cells during embryogenesis,8 expressed hCD4. The Socs1lox/+LysM-cre mouse thus provides a model for examining the effects of SOCS-1 deficiency in lymphoid and myeloid lineages within a SOCS-1-sufficient animal.
Lymphoid- and myeloid-specific SOCS-1 deficiency causes multiorgan inflammation and premature lethality
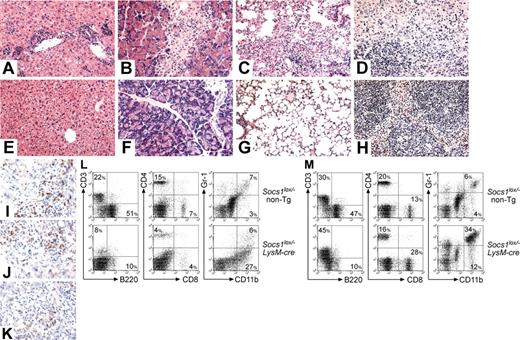
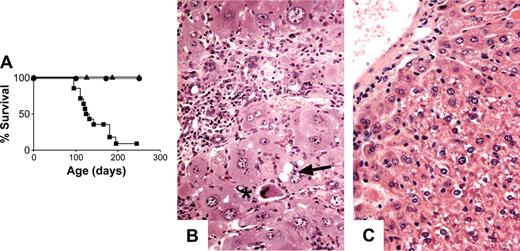
Unlike Socs1-/- or Socs1lox/-CMV-cre mice,8 Socs1lox/-LysM-cre mice survived past weaning, and by 6 weeks of age remained healthy and did not exhibit any tissue pathology. To investigate if long-term survival may be affected in these mice, groups of 28 Socs1lox/-LysM-cre mice and 21 Socs1lox/- nontransgenic littermate controls were monitored to determine their life spans. Socs1lox/-LysM-cre mice began to become ill from 50 days of age, and by 250 days, almost all animals were moribund (Figure 1B). Smaller numbers of Socs1+/+LysM-cre and Socs1lox/+LysM-cre mice were also monitored, but the survivals of these mice were indistinguishable from Socs1lox/- nontransgenic mice (data not shown). When Socs1lox/-LysM-cre mice became ill, randomly chosen Socs1lox/- nontransgenic controls were also analyzed in parallel. Organs were harvested for histologic and/or flow cytometric analyses. Serum was also collected, which was subsequently analyzed at the end of the survival study. Table 1 and Figure 2 detail the pathology of Socs1lox/-LysM-cre mice.
Premature mortality in mice with Socs1 gene inactivation in lymphoid and myeloid lineages. (A) Cre expressed under the lysozyme promoter (LysM-cre) deletes Socs1lox in both lymphoid and myeloid lineages. Socs1lox recombination switches on an hCD4 reporter within the targeted locus, and thus recombination was tracked by analyzing hCD4 expression by flow cytometry. Shown are leukocyte populations (□, Socs1lox/+ nontransgenic; ▦, Socs1lox/+cre) in the spleen and blood. Cells were stimulated with 100 U/mL IFN-γ for 4 hours to increase hCD4 reporter activity. As a positive control, CMV-cre was used to delete Socs1lox during embryogenesis, and therefore in all cells. The percentage of hCD4+ cells is indicated in each histogram. (B) Survival curves of Socs1lox/-LysM-cre (•; n = 28) and littermate control Socs1lox/- nontransgenic (▪; n = 21) mice.
Premature mortality in mice with Socs1 gene inactivation in lymphoid and myeloid lineages. (A) Cre expressed under the lysozyme promoter (LysM-cre) deletes Socs1lox in both lymphoid and myeloid lineages. Socs1lox recombination switches on an hCD4 reporter within the targeted locus, and thus recombination was tracked by analyzing hCD4 expression by flow cytometry. Shown are leukocyte populations (□, Socs1lox/+ nontransgenic; ▦, Socs1lox/+cre) in the spleen and blood. Cells were stimulated with 100 U/mL IFN-γ for 4 hours to increase hCD4 reporter activity. As a positive control, CMV-cre was used to delete Socs1lox during embryogenesis, and therefore in all cells. The percentage of hCD4+ cells is indicated in each histogram. (B) Survival curves of Socs1lox/-LysM-cre (•; n = 28) and littermate control Socs1lox/- nontransgenic (▪; n = 21) mice.
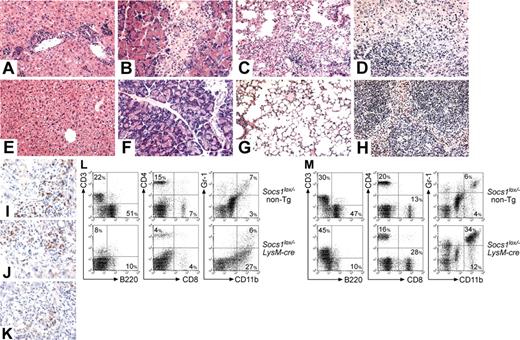
Splenomegaly was the most prominent feature in sick Socs1lox/-LysM-cre mice. Socs1lox/-LysM-cre spleen weights were on average 9 times those of controls (Table 1). Lymphadenopathy was also apparent. There was massive overgrowth of reticuloendothelial cells (with a macrophage appearance) in both the spleen (Figure 2D) and lymph node (not shown). Flow cytometric analysis confirmed the presence of a large splenic Gr-1-CD11b+ macrophage population (Figure 2L). This was paralleled by relative decreases in lymphocyte populations. Socs1lox/-LysM-cre spleens and lymph nodes also lacked germinal centers.
The cortex of the thymus of most Socs1lox/-LysM-cre mice was atrophied, and there was a high frequency of BMs in which granulocytic cells were more numerous than mononuclear cells. Although total blood leukocyte numbers appeared normal, sick Socs1lox/-LysM-cre mice displayed a relative loss of B220+ B cells and substantial increases in Gr-1+CD11b+ neutrophils and Gr-CD11b+ monocytes (Figure 2M).
The livers of all Socs1lox/-LysM-cre mice were infiltrated by CD4+ and CD8+ T cells, and macrophages, with infiltrating cells localizing to portal tracts or in isolated foci (Figure 2A, I-K). Few B cells, neutrophils, or eosinophils were present (not shown). Fatty degeneration of hepatocytes, typical of Socs1-/- mice, was not observed.
Socs1lox/-LysM-cre mice succumb to a multiorgan inflammatory disease. Representative hematoxylin and eosin-stained sections of the (A,E) liver, (B,F) pancreas, (C,G) lung, and (D,H) spleen from (A-D) moribund Socs1lox/-LysM-cre mice and (E-H) healthy Socs1lox/- nontransgenic littermates. Frozen sections of liver from moribund Socs1lox/-LysM-cre mice were stained with antibodies recognizing (I) CD4, (J) CD8, and (K) CD11b. (L) Splenocytes and (M) blood leukocytes from moribund Socs1lox/-LysM-cre and age-matched Socs1lox/- nontransgenic mice were analyzed for expression of the indicated markers by flow cytometry. Quadrant percentages are shown.
Socs1lox/-LysM-cre mice succumb to a multiorgan inflammatory disease. Representative hematoxylin and eosin-stained sections of the (A,E) liver, (B,F) pancreas, (C,G) lung, and (D,H) spleen from (A-D) moribund Socs1lox/-LysM-cre mice and (E-H) healthy Socs1lox/- nontransgenic littermates. Frozen sections of liver from moribund Socs1lox/-LysM-cre mice were stained with antibodies recognizing (I) CD4, (J) CD8, and (K) CD11b. (L) Splenocytes and (M) blood leukocytes from moribund Socs1lox/-LysM-cre and age-matched Socs1lox/- nontransgenic mice were analyzed for expression of the indicated markers by flow cytometry. Quadrant percentages are shown.
Lymphocyte and macrophage foci were present in half of the Socs1lox/-LysM-cre pancreas sections (Figure 2B).
All lungs displayed thickening of alveolar walls and cuffing of vessels by lymphocytes and macrophages, as well as isolated foci of the same cells (Figure 2C).
Small foci of lymphocytes, macrophages, and eosinophils were found in skeletal muscle of half the Socs1lox/-LysM-cre mice, but there was no damage to muscle cells (not shown).
Half the Socs1lox/-LysM-cre mice also displayed infiltration of the gut (not shown).
There were minor patches of epithelial thickening in the skin (not shown).
Lesions were not apparent in heart, cornea, knee joints, salivary glands, bladder, kidney, brains, or reproductive organs of Socs1lox/-LysM-cre mice (not shown).
T cells are aberrantly activated in Socs1lox/-LysM-cre mice
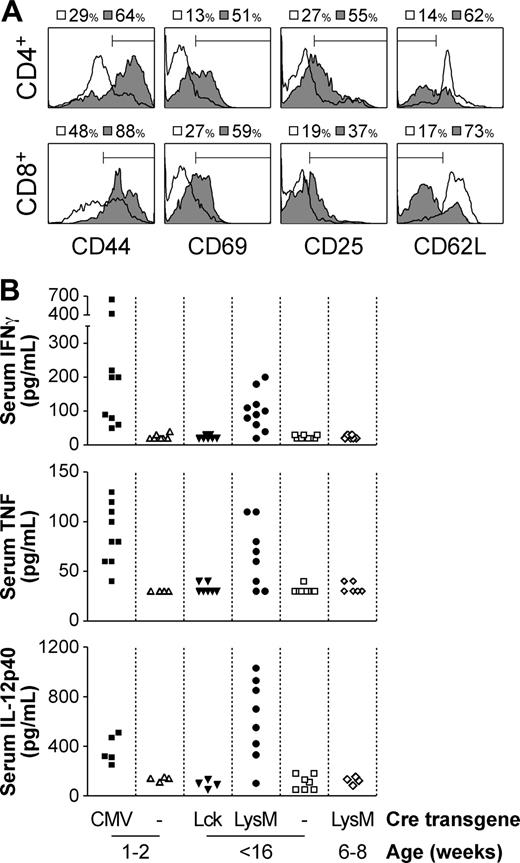
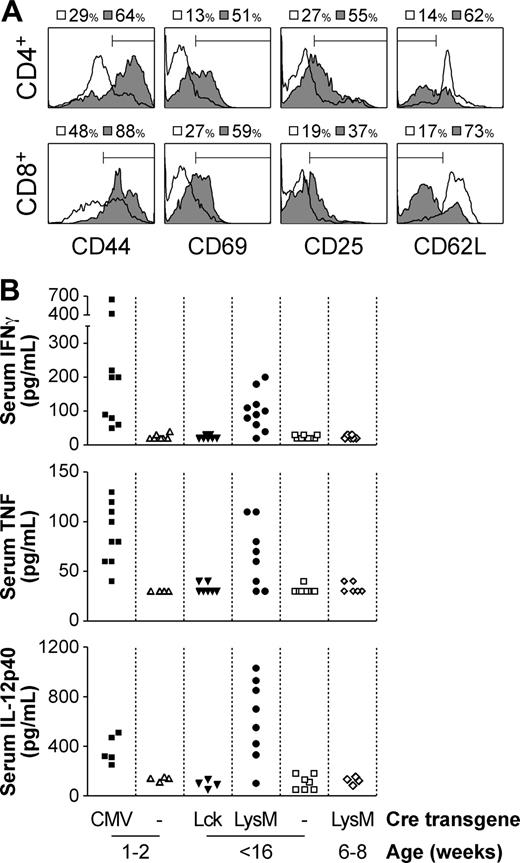
We have previously shown that in Socs1lox/-Lck-cre mice, in which SOCS-1 deficiency is restricted only to the T-cell compartment, abnormal activation of T cells does not occur, in that while CD44 expression is increased there is no increase in CD69 or CD25.8 However in Socs1lox/-LysM-cre mice, almost all blood, lymph node, and splenic CD4+ and CD8+ T cells appeared activated, up-regulating CD44, CD69, and CD25 expression, and down-regulating CD62L (Figure 3A and not shown). Also suggestive of T-cell activation was the increased levels of IFN-γ in the serum of moribund mice (Figure 3B). Class I major histocompatibility complex (MHC) expression, which is highly susceptible to induction by IFN-γ, was likewise elevated in many organs, including liver, spleen, and pancreas (not shown).
Consistent with systemic inflammation and elevated IFN-γ levels, there was a reduction in B cells and an increase in granulocytes in the blood of Socs1lox/-LysM-cre mice (Figure 2M). This has previously been shown to occur in neonatal Socs1-/- mice and is dependent on IFN-γ.13
In addition to IFN-γ, serum TNF and IL-12p40 levels were also elevated in moribund Socs1lox/-LysM-cre mice (Figure 3B). IL-1 was also measured, but was not detected (not shown). Elevated levels of IFN-γ, TNF, and IL-12p40 were also detected in the serum of Socs1lox/-CMV-cre mice, in which the Socs1-/- disease is recapitulated. However, serum cytokine levels were not increased in Socs1lox/-Lck-cre mice.
Activated T cells were evident only in moribund Socs1lox/-LysM-cre mice. In young healthy Socs1lox/-LysM-cre mice, T cells did not express markers of activation (not shown). Similarly, serum cytokine levels were not elevated in young mice (Figure 3B).
SOCS-1-deficient T cells are hypersensitive to cytokine-induced IFN-γ production
T cells are potentially the source of the increased circulating IFN-γ in Socs1lox/-LysM-cre mice. We therefore investigated the effect of SOCS-1 deficiency on IFN-γ production by T cells. Young Socs1lox/-Lck-cre mice were used as the source of SOCS-1-deficient T cells, because unlike in Socs1-/- or Socs1lox/-LysM-cre mice, there is no abnormal activation in vivo.8 Therefore, intrinsic T-cell responses can be examined in vitro, independent of effects of SOCS-1 deficiency in other cell types, such as antigen-presenting cells.
Aberrant T-cell activation and increased serum inflammatory cytokine levels in moribund Socs1lox/-LysM-cre mice. (A) Peripheral blood CD4+ and CD8+ T cells from moribund Socs1lox/-LysM-cre (▦) and age-matched Socs1lox/- nontransgenic (□) mice were analyzed for expression of CD44, CD69, CD25, and CD62L by flow cytometry. (B) The serum of neonatal moribund Socs1lox/-CMV-cre and age-matched Socs1lox/- nontransgenic mice, adult moribund Socs1lox/-LysM-cre and age-matched Socs1lox/-Lck-cre and Socs1lox/- nontransgenic mice, and young healthy Socs1lox/-LysM-cre mice were measured for IFN-γ, TNF, and IL-12p40 levels by ELISA.
Aberrant T-cell activation and increased serum inflammatory cytokine levels in moribund Socs1lox/-LysM-cre mice. (A) Peripheral blood CD4+ and CD8+ T cells from moribund Socs1lox/-LysM-cre (▦) and age-matched Socs1lox/- nontransgenic (□) mice were analyzed for expression of CD44, CD69, CD25, and CD62L by flow cytometry. (B) The serum of neonatal moribund Socs1lox/-CMV-cre and age-matched Socs1lox/- nontransgenic mice, adult moribund Socs1lox/-LysM-cre and age-matched Socs1lox/-Lck-cre and Socs1lox/- nontransgenic mice, and young healthy Socs1lox/-LysM-cre mice were measured for IFN-γ, TNF, and IL-12p40 levels by ELISA.
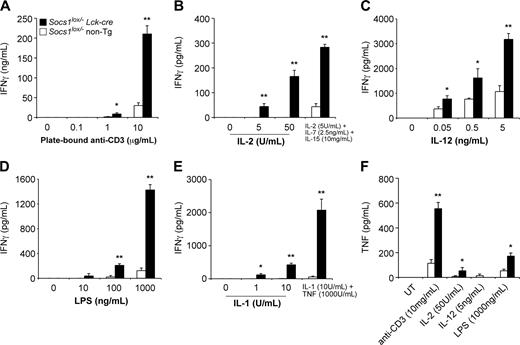
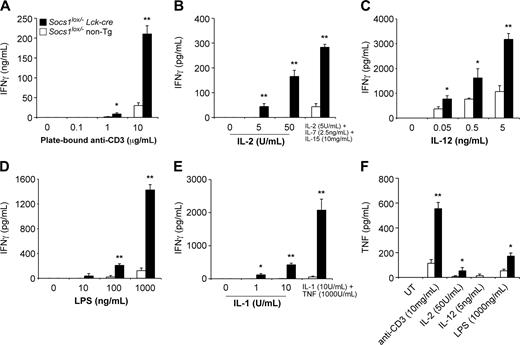
As shown in Figure 4A, SOCS-1-deficient T cells produced substantially greater levels of IFN-γ in response to stimulation by plate-bound anti-CD3 antibody compared with control T cells. SOCS-1-deficient but not wild-type T cells were capable of producing large quantities of IFN-γ in response to IL-2, LPS, or IL-1, independent of a TCR signal (Figure 4B, D, and E). The addition of IL-7 and IL-15 enhanced the effect of IL-2, while TNF greatly enhanced the effect of IL-1. IFN-γ was not produced in response to IL-7, IL-15, or TNF alone (not shown). SOCS-1-deficient T cells were also hypersensitive to IL-12-induced IFN-γ production (Figure 4C). By intracellular cytokine staining and flow cytometric analysis, we found that both CD4+ and CD8+ T cells were hypersensitive to stimuli and produced increased levels of IFN-γ (Figure S1, available on the Blood website; see the Supplemental Figure link at the top of the online article).
SOCS-1-deficient T cells also produced more TNF in response to anti-CD3, IL-2, or LPS, compared with control cells (Figure 4F). SOCS-1-deficient but not control T cells also produced IL-1 in response to anti-CD3 treatment (not shown).
The same experiments were also performed on T cells from 4-week-old Socs1lox/-LysM-cre mice. As with Socs1lox/-Lck-cre T cells, Socs1lox/-LysM-cre T cells were capable of producing IFN-γ and TNF, in response to other cytokines, independent of TCR ligation (not shown).
SOCS-1-deficient macrophages are hypersensitive to LPS, IFN-γ, and TNF
Both T cells and macrophages were found in the inflammatory lesions of Socs1lox/-LysM-cre mice. SOCS-1 deficiency in T cells alone is insufficient to cause aberrant T-cell activation and IFN-γ production.8 Therefore, the elevated IFN-γ levels in Socs1lox/-LysM-cre mice may depend on an interaction between SOCS-1-deficient T cells and SOCS-1-deficient macrophages. To examine the effect of SOCS-1 deficiency on macrophages, 4-week-old Socs1lox/-LysM-cre mice were used as the source of SOCS-1-deficient macrophages. This is well before the onset of disease. Peritoneal macrophages were obtained by lavage and then used for experiments in vitro.
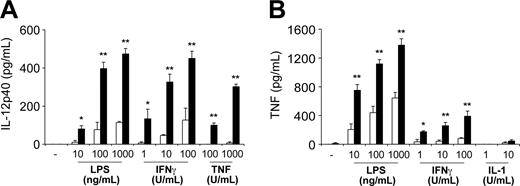
SOCS-1-deficient macrophages were found to be hypersensitive to several cytokines. They produced larger quantities of IL-12p40 in response LPS, IFN-γ, and TNF compared with control macrophages (Figure 5A). Similarly, SOCS-1-deficient macrophages produced more TNF in response to LPS and IFN-γ, but not IL-1 (Figure 5B). These macrophages also produced more IL-1 in response to LPS and TNF, but not IFN-γ (not shown). Therefore, the hypersensitivity of SOCS-1-deficient macrophages to IFN-γ, TNF, and/or LPS may contribute to disease in Socs1lox/-LysM-cre mice.
BM from Socs1lox/-LysM-cre donors induces multiorgan inflammatory disease when transplanted into wild-type recipients
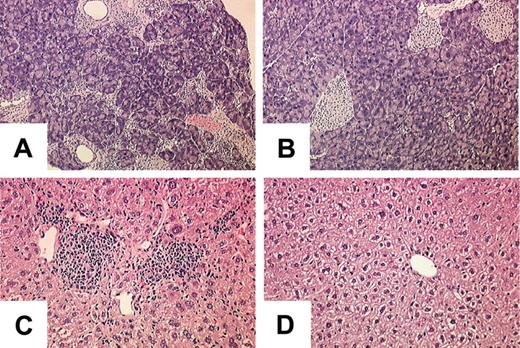
We have shown that LysM-cre deletes the Socs1lox allele in the lymphoid and myeloid lineages, and this was sufficient to cause spontaneous inflammatory disease. We therefore postulated that SOCS-1-deficient hematopoietic cells should also be able to induce inflammatory disease in wild-type animals. To investigate this, wild-type C57BL/6 mice were lethally irradiated, then received transplants of BM from Socs1lox/-LysM-cre or Socs1lox/- nontransgenic donors to reconstitute both the lymphoid and myeloid compartments. Twelve recipients received Socs1lox/-LysM-cre BM and 8 received Socs1lox/- nontransgenic BM. Four of the animals that received Socs1lox/-LysM-cre BM died within 2 weeks of transplantation because of poor engraftment, and thus were excluded from the experiment. Three months after receiving the BM transplant, the organs of the remaining recipients were analyzed for inflammatory pathologies. As shown in Figure 6, all animals that received Socs1lox/-LysM-cre BM developed a multiorgan inflammatory disease similar to the Socs1lox/-LysM-cre donor mice, whereas those animals that received Socs1lox/- nontransgenic remained free of pathology. This result confirms that SOCS-1 deficiency in hematopoietic cells alone is sufficient to cause aberrant inflammatory disease.
SOCS-1 deficiency in hepatocytes exacerbates the hepatitis in Socs1lox/-LysM-cre mice
SOCS-1 has previously been shown to regulate the duration of STAT1 activation in hepatocytes in response to IFN-γ, and the loss of SOCS-1 in hepatocytes is thought to be important in the neonatal Socs1-/- hepatitis.12,13 We therefore wanted to examine whether SOCS-1 expression in hepatocytes modulates the chronic liver disease in Socs1lox/-LysM-cre mice. To address this, cre expressed under the albumin promoter (Alb-cre) was used to delete Socs1lox in hepatocytes. By analyzing the expression of the hCD4 reporter in Socs1lox mice, Alb-cre appeared to mediate loxP recombination in almost all hepatocytes, but not in other tissues (not shown).
SOCS-1 deficiency in T cells causes hyperproduction of IFN-γ in response to multiple stimuli. Nylon wool-purified lymph node T cells from 4-week-old Socs1lox/-Lck-cre mice and Socs1lox/- nontransgenic mice as controls were cultured for 48 hours in the presence of (A) plate-bound anti-CD3, (B) IL-2 or IL-2/7/15 in combination, (C) IL-12, (D) LPS, or (E) IL-1 or IL-1 + TNF in combination. Culture supernatants were then assayed for IFN-γ by ELISA. (F) T cells were simulated for 48 hours as indicated; then culture supernatants were assayed for TNF by ELISA. UT indicates untreated. The data represent the mean ± SEM of 4 to 6 experiments. Statistical significance (Mann-Whitney test): *P < .05 or **P < .001 compared with Socs1lox/- nontransgenic.
SOCS-1 deficiency in T cells causes hyperproduction of IFN-γ in response to multiple stimuli. Nylon wool-purified lymph node T cells from 4-week-old Socs1lox/-Lck-cre mice and Socs1lox/- nontransgenic mice as controls were cultured for 48 hours in the presence of (A) plate-bound anti-CD3, (B) IL-2 or IL-2/7/15 in combination, (C) IL-12, (D) LPS, or (E) IL-1 or IL-1 + TNF in combination. Culture supernatants were then assayed for IFN-γ by ELISA. (F) T cells were simulated for 48 hours as indicated; then culture supernatants were assayed for TNF by ELISA. UT indicates untreated. The data represent the mean ± SEM of 4 to 6 experiments. Statistical significance (Mann-Whitney test): *P < .05 or **P < .001 compared with Socs1lox/- nontransgenic.
SOCS-1 deficiency in macrophages causes hyperproduction of IL-12p40 and TNF in response to multiple stimuli. Resident peritoneal macrophages from healthy 4-week-old Socs1lox/-LysM-cre mice (▪) and Socs1lox/- nontransgenic mice (□) as controls were cultured for 48 hours in the presence of LPS, IFN-γ, TNF, or IL-1. Culture supernatants were then assayed for (A) IL-12p40 or (B) TNF by ELISA. The data represent the mean ± SEM of 3 experiments. Statistical significance (Mann-Whitney test): *P < .05 or **P < .001 compared with Socs1lox/- nontransgenic.
SOCS-1 deficiency in macrophages causes hyperproduction of IL-12p40 and TNF in response to multiple stimuli. Resident peritoneal macrophages from healthy 4-week-old Socs1lox/-LysM-cre mice (▪) and Socs1lox/- nontransgenic mice (□) as controls were cultured for 48 hours in the presence of LPS, IFN-γ, TNF, or IL-1. Culture supernatants were then assayed for (A) IL-12p40 or (B) TNF by ELISA. The data represent the mean ± SEM of 3 experiments. Statistical significance (Mann-Whitney test): *P < .05 or **P < .001 compared with Socs1lox/- nontransgenic.
The Socs1lox/-LysM-cre inflammatory disease can be transferred to wild-type recipients by BM transplantation. Lethally irradiated C57BL/6 recipients received transplants of BM from (A,C) Socs1lox/-LysM-cre or (B,D) Socs1lox/- nontransgenic donors. Organs were harvested for analysis 3 months after transplantation. Shown are the (A-B) pancreas and (C-D) liver.
The Socs1lox/-LysM-cre inflammatory disease can be transferred to wild-type recipients by BM transplantation. Lethally irradiated C57BL/6 recipients received transplants of BM from (A,C) Socs1lox/-LysM-cre or (B,D) Socs1lox/- nontransgenic donors. Organs were harvested for analysis 3 months after transplantation. Shown are the (A-B) pancreas and (C-D) liver.
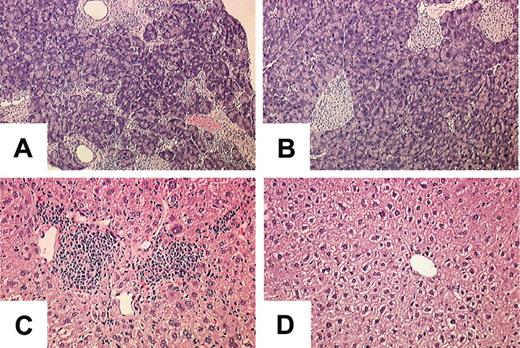
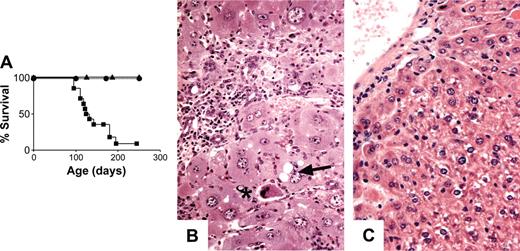
Survival over a 250-day period appeared not to be affected by SOCS-1 deficiency in hepatocytes alone (Figure 7A), and histologically, the livers of Socs1lox/-Alb-cre mice appeared normal. Similarly, simultaneous deletion of Socs1lox in hepatocytes and T cells (Socs1lox/-Alb-cre/Lck-cre) did not affect the survival of mice (Figure 7A). Lethality was not accelerated in Socs1lox/-LysM-cre mice that were also Alb-cre transgenic compared with Socs1lox/-LysM-cre mice alone (Figures 1B and 7A). Socs1lox/-Alb-cre/LysM-cre mice developed the same pathologies as Socs1lox/-LysM-cre mice, but developed a more severe hepatitis (Table 1). Liver infiltration was more extensive and hepatocyte damage was much more prominent in Socs1lox/-Alb-cre/LysM-cre mice than in Socs1lox/-LysM-cre mice (Figure 7B). In the livers of Socs1lox/-Alb-cre/LysM-cre mice, there was obvious swelling of hepatocytes and focal areas of necrosis immediately adjacent to infiltrates. Loss of nuclei and vacuole formation by hepatocytes were also evident. This suggests that SOCS-1-deficient hepatocytes are more susceptible to damage.
Discussion
Perturbations in cytokine networks are known to have deleterious effects. It is therefore critical that mechanisms exist to regulate the effects of cytokines. The targeted deletion of Socs1 in mice has clearly demonstrated that SOCS-1 is a critical regulator of inflammation, because deficiency of this protein results in spontaneous inflammation.10,11
To define which SOCS-1-deficient cell type causes aberrant inflammation, the cre/loxP system was used to investigate the effects of SOCS-1 deficiency in selective cell populations. We found that Socs1lox/-LysM-cre mice, in which SOCS-1 is deficient from both lymphoid and myeloid cells, develop severe splenomegaly and chronic inflammation of many organs. When lethally irradiated wild-type recipients received transplants of BM from Socs1lox/-LysM-cre donors to reconstitute both the lymphoid and myeloid compartments, spontaneous multiorgan inflammation also developed. Previously, it was shown that inflammatory lesions also develop in wild-type mice that received transplants of BM from sick Socs1-/- donors.19 While these previous transplant experiments demonstrated that BM from sick Socs1-/- mice is autoaggressive, these studies did not clarify whether Socs1-/- BM is autoaggressive because of an intrinsic defect in SOCS-1-deficient BM cells or as a result of interactions with other SOCS-1-deficient tissues in Socs1-/- donors. Therefore, the findings reported here indicate that the autoaggressive nature of Socs1-/- hematopoietic cells is due to an intrinsic defect caused by SOCS1 deficiency rather than interactions with other SOCS1-deficient tissues.
Targeting SOCS-1 deficiency to hepatocytes exacerbates the hepatocyte damage in Socs1lox/-LysM-cre mice. (A) Survival curves of Socs1lox/-Alb-cre (▴; n = 6), Socs1lox/-Alb-cre/LysM-cre (▪; n = 14), and Socs1lox/-Alb-cre/Lck-cre (•; n = 6) mice. (B) Liver pathology in Socs1lox/-Alb-cre/LysM-cre mice compared with (C) Socs1lox/-Alb-cre mice. Note the swelling of hepatocytes that is not seen in Socs1lox/-LysM-cre mice (Figure 2A). Collapsed hepatocytes (*) and vacuole formation within hepatocytes (arrow) are also readily identifiable.
Targeting SOCS-1 deficiency to hepatocytes exacerbates the hepatocyte damage in Socs1lox/-LysM-cre mice. (A) Survival curves of Socs1lox/-Alb-cre (▴; n = 6), Socs1lox/-Alb-cre/LysM-cre (▪; n = 14), and Socs1lox/-Alb-cre/Lck-cre (•; n = 6) mice. (B) Liver pathology in Socs1lox/-Alb-cre/LysM-cre mice compared with (C) Socs1lox/-Alb-cre mice. Note the swelling of hepatocytes that is not seen in Socs1lox/-LysM-cre mice (Figure 2A). Collapsed hepatocytes (*) and vacuole formation within hepatocytes (arrow) are also readily identifiable.
Neither Socs1lox/-LysM-cre mice nor Socs1-/- mice that received a BM transplant developed the characteristic pathologies of Socs1-/- mice. Absent in particular was the fatty necrosis of hepatocytes that occurs in the neonatal Socs1-/- disease. This is despite SOCS-1-deficient hematopoietic cells being present in Socs1lox/-LysM-cre mice even during the neonatal period. Therefore, hematopoietic SOCS-1 deficiency alone is insufficient to cause the neonatal Socs1-/- disease. In fact, neonatal Socs1lox/-LysM-cre mice did not exhibit any pathology. Inflammation began to become apparent only well after weaning age. Moreover, T-cell activation and increased serum cytokine levels paralleled this later development of inflammatory lesions.
SOCS-1 regulates cytokine signaling in parenchymal cells. SOCS-1 deficiency prolongs IFN-γ signaling in hepatocytes12 and increases the sensitivity of pancreatic β cells and fibroblasts to TNF-induced cell death.4,5 We therefore examined whether aberrantly activated hematopoietic cells combined with defective cytokine signaling in target tissues are required for the rapid neonatal Socs1-/- disease. In this study, we addressed the contribution of SOCS-1 deficiency in hepatocytes, one of the primary targets in the neonatal disease. In Socs1lox/-Alb-cre/LysM-cre double-transgenic mice, there was a more severe liver disease and greater destruction of hepatocytes. This indicates that SOCS-1-deficient cells are more susceptible to inflammatory damage. However, Socs1lox/-Alb-cre/LysM-cre mice succumbed at the same kinetics as Socs1lox/-LysM-cre mice. There was no evidence of liver pathology in neonatal Socs1lox/-Alb-cre/LysM-cre mice. Thus, the precise identities of all the cellular components that participate to bring about the Socs1-/- phenotype remain unclear.
Clearly, SOCS-1 deficiency in hematopoietic cells causes lethality, albeit later in life compared with germ-line deficiency. Why are SOCS-1-deficient hematopoietic cells aggressive? Recent evidence suggests that SOCS-1 may be important in regulating autoimmunity. In Socs1-/- mice that have enforced expression of SOCS-1 in the lymphoid compartment, a lupuslike disease develops.20 This was associated with dendritic cells and B-cell abnormalities and anti-dsDNA autoantibody production. In Socs1lox/-LysM-cre mice, however, we found no evidence of autoimmunity. These mice do not develop glomerulonephritis, a characteristic of systemic autoimmunity. We also did not detect antinuclear autoantibodies in these mice (data not shown). An important difference is that in this previously reported study, enforced SOCS-1 expression was achieved by a constitutive promoter,20 and thus expression was not regulated in the appropriate manner (ie, cytokine inducible). Moreover, it is known that B cells and TCR signaling are not required for inflammation caused by SOCS-1 deficiency, because inflammation still occurs in Socs1-/-Rag-/- OT-1 transgenic mice, which lack B cells and have T cells that recognize only the exogenous antigen ovalbumin.14
T/NKT cells are essential for inflammation caused by SOCS-1 deficiency, because removal of these cells prevents or reduces disease.9,14,15 In Socs1lox/-LysM-cre mice, serum levels of IFN-γ, TNF, and IL-12 were elevated. T cells were the likely source of the increased IFN-γ, because T cells constituted the majority of infiltrating cells in the inflammatory lesions and almost all T cells had an activated phenotype. It has been shown that T-cell activation and elevated serum IFN-γ levels in Socs1-/- mice do not require antigen and TCR signaling.14 SOCS-1 is an intracellular inhibitor of cytokine signaling. We therefore examined whether abnormal responses of SOCS-1-deficient T cells to cytokines may be responsible for the elevated serum IFN-γ levels in Socs1lox/-LysM-cre mice. It has previously been suggested that the elevated IFN-γ levels in Socs1-/- mice are also due to abnormal responses of SOCS-1-deficient T cells.9 However, because these studies relied on germ-line SOCS-1-deficient mice, they could not determine whether the increase in IFN-γ production was due to intrinsic defects in T cells or due to other SOCS-1-deficient cell types interacting with the T cells. By examining SOCS-1-deficient T cells from Socs1lox/-Lck-cre mice, in which disease and aberrant T-cell activation in vivo do not occur, we showed that SOCS-1-deficient T cells were indeed capable of producing excessive quantities of IFN-γ in response IL-1, IL-2, and LPS, independent of TCR ligation. These cells were also hypersensitive to IL-12-induced IFN-γ production. Additionally, IL-7, IL-15, and TNF were found to enhance the effects of IL-1 or IL-2 on SOCS-1-deficient T cells, which again was not observed in wild-type T cells independent of TCR ligation. Excessive IFN-γ production is thus an intrinsic defect of SOCS-1-deficient T cells.
We have previously shown that other TCR-dependent responses in T cells can also be bypassed by SOCS-1 deficiency and cytokine hypersensitivity. γc cytokines alone can induce proliferation and the up-regulation of CD44 expression in SOCS-1-deficient CD8+ T cells.8,16 T-cell abnormalities, similar to those seen in the various SOCS-1-deficient mouse models, have been observed in mice overexpressing or administrated with many of the cytokines examined here. For example, aberrant T-cell activation, proliferation, and/or cytokine production, as well as inflammatory disease occur in mice with excess IL-2, IL-7, IL-12, IL-15, or TNF.21-25 SOCS-1 deficiency therefore has the same effect as excessive quantities of cytokines and is consistent with the loss of a negative regulator of cytokine signaling.
Hypersensitivity of SOCS-1-deficient T cells to multiple cytokines alone, however, is insufficient to induce aberrant IFN-γ production in vivo, because Socs1lox/-Lck-cre mice do not display elevated IFN-γ levels or inflammatory lesions. Macrophages were also present in the lesions of Socs1lox/-LysM-cre mice. Additionally, there were increased levels of TNF and IL-12 in the serum of these mice. Monocytes/macrophages are a major source of IL-1226 and TNF.27 Therefore, we postulated that the inflammatory disease in Socs1lox/-LysM-cre mice might involve an interaction between abnormal T cells and abnormal macrophages. Indeed, SOCS-1-deficient macrophages were found to be hypersensitive to LPS, IFN-γ, and TNF, resulting in hyperproduction of IL-12 and TNF. Potentially, SOCS-1-deficient macrophages are hyperresponsive to normal levels of cytokines produced by T cells, such as IFN-γ, leading to minor increases in TNF and IL-12 production. Conversely, SOCS-1-deficient T cells may be hyperresponsive to normal levels of cytokines produced by macrophages, such as IL-12 and TNF, leading to a minor increase in IFN-γ production. In isolation, these 2 situations may not be pathogenic. However, if both are present concurrently, a dysregulated cytokine network might result whereby the more SOCS-1-deficient T cells produce cytokines that stimulate macrophages, the more SOCS-1-deficient macrophages produce cytokines that stimulate T cells.
This study has clearly shown that SOCS-1 deficiency in T cells and macrophages is the primary lesion underlying inflammation cause by SOCS-1 deficiency, although other cell types must also be participating to bring about the rapid disease in germ-line Socs1-/- mice. This finding raises the possibility that loss of SOCS-1 function in hematopoietic cells may be a potential contributing mechanism in human inflammatory diseases. Approaches to increase SOCS-1 expression or function may therefore be of therapeutic benefit in such settings.
Prepublished online as Blood First Edition Paper, May 17, 2005; DOI 10.1182/blood-2004-08-3049.
Supported by the National Health and Medical Research Council of Australia, and the Juvenile Diabetes Research Foundation International.
The online version of the article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We wish to thank Jason Corbin for the automated blood analysis; Steven Mihajlovic, Helen Thomas, and Eveline Angstetra for assistance with histology; Joy Melny for the autoantibody analyses; and Dannielle Cooper and Melanie Mager for expert animal husbandry.