Abstract
Evidence for the lineage relationship between embryonic and adult hematopoietic stem cells (HSCs) in the mouse is primarily indirect. In order to study this relationship in a direct manner, we expressed the tamoxifen-inducible Cre-ERT recombinase under the control of the stem cell leukemia (Scl) stem-cell enhancer in transgenic mice (HSC-SCL-Cre-ERT). To determine functionality, HSC-SCL-Cre-ERT transgenics were bred with Cre reporter mice. Flow cytometric and transplantation studies revealed tamoxifen-dependent recombination occurring in more than 90% of adult long-term HSCs, whereas the targeted proportion within mature progenitor populations was significantly lower. Moreover, the transgene was able to irreversibly tag embryonic HSCs on days 10 and 11 of gestation. These cells contributed to bone marrow hematopoiesis 5 months later. In order to investigate whether the de novo HSC generation is completed during embryogenesis, HSC-SCL-Cre-ERT–marked fetal liver cells were transplanted into adult recipients. Strikingly, the proportion of marked cells within the transplanted and the in vivo–remaining HSC compartment was not different, implying that no further HSC generation occurred during late fetal and neonatal stages of development. These data demonstrate for the first time the direct lineage relationship between midgestation embryonic and adult HSCs in the mouse. Additionally, the HSC-SCL-Cre-ERT mice will provide a valuable tool to achieve temporally controlled genetic manipulation of HSCs.
Introduction
Hematopoietic stem cells (HSCs) have the capacity to self renew as well as the ability to give rise to all hematopoietic lineages.1 It is generally accepted that HSCs responsible for multilineage hematopoiesis throughout life develop during embryogenesis.2-4 Defining the exact time and site of the first HSC generation within the embryo has been complicated. This is because multiple sites such as the yolk sac, the aorto-gonad-mesonephros (AGM) region, and the fetal liver (FL) harbor hematopoietic progenitors at different developmental stages. However, the de novo generation of HSCs independently at each site cannot be formally excluded. The recent notion that in addition to HSCs, the adult bone marrow (BM) harbors stem cells with more pluripotent properties supports the hypothesis of ongoing de novo generation of HSCs within BM from a pluripotent mesenchymal stem cell during adulthood.5,6 Furthermore, another study has suggested ongoing HSC formation within fetal BM.7 Transplantation studies8-11 support the colonization hypothesis, but demonstrating the direct-lineage relationship between embryonic and adult HSCs without ex vivo manipulation has been difficult.
A system that has been increasingly used for lineage-tracing studies is the Cre/loxP system.12-15 This method relies on the expression of the Cre recombinase in a defined cell type, which mediates irreversible recombination. Any cell that has been tagged in this fashion and all its progeny will be marked for their entire life span.
In this study, we applied this recombination-based system to determine the direct-lineage relationship between embryonic and adult HSCs. We generated transgenic mice, which express the tamoxifen-inducible Cre-ERT recombinase under the control of the stem-cell enhancer16-18 of the stem cell leukemia (Scl) locus (HSC-SCL-Cre-ERT). Gene-targeting studies have demonstrated that Scl is indispensable for the establishment of the hematopoietic system.19,20 Thus, Scl is an ideal genetic marker for the onset of the hematopoietic program in the embryo. The SCL stem-cell enhancer, also known as the Scl-3′-enhancer, is responsible for Scl expression within early hematopoietic progenitors and endothelial cells.16-18
Here we first asked whether the HSC-SCL-Cre-ERT transgenic line is capable of inducing temporally controlled recombination in HSCs. We then used the HSC-SCL-Cre-ERT;R26R-EYFP double-transgenic system to irreversibly mark HSCs during embryonic development to determine their direct-lineage relationship to their adult counterparts in vivo.
Materials and methods
Mice
To construct the plasmid p6E5/Cre-ERT/3′enh (Figure 1A), a 2.1-kb Cre-ERT cDNA fragment was cloned downstream of the 6E5 promoter sequence (2.4-kb Sau3AI/NdeI-fragment)16 and upstream of the Scl-3′ enhancer (5.5-kb BglII/BglII fragment).16 The injection fragment, released by an NheI/SalI digest, was gel purified and used for pronuclear injections of fertilized eggs (C57BL/6xC57BL/6) (Ozgene, Canning Vale, WA, Australia). Mice were genotyped for the presence of Cre-ERT as previously described.21 Transgenic founder lines were bred with R26R22 and R26R-EYFP23 Cre reporter mice.
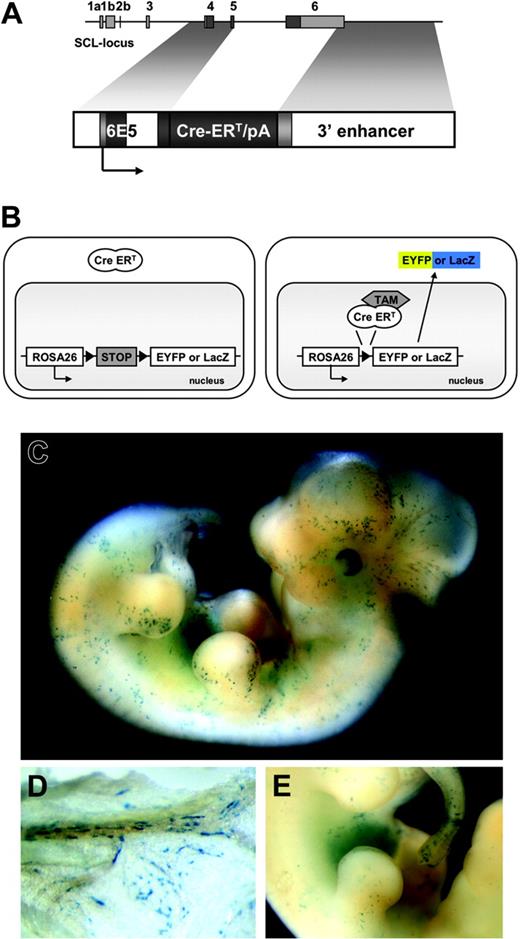
Temporally controlled, tamoxifen-dependent recombination in HSC-SCL-Cre-ERT embryos. (A) The 6E5/Cre-ERT/3′enh construct used to generate HSC-SCL-Cre-ERT transgenic mice. The 6E5 genomic fragment containing the SCL exon 4 promoter combined with a 5.5-kb Scl 3′ enhancer sequence was used to control expression of Cre-ERT. White, light gray, and dark gray boxes represent introns, untranscribed exon, and transcribed exon sequences, respectively. (B) Schematic drawing of the transgenic system used in this study. Upon tamoxifen (TAM) treatment, the Cre-ERT recombinase is translocated and the stop-cassette is removed, inducing expression of Eyfp or lacZ. (C) LacZ staining of E12.5 HSC-SCL-Cre-ERT;R26R embryos revealed positive staining of the fetal liver and scattered LacZ-positive cells throughout the embryo. (D) View on the visceral side of the E12.5 yolk sac, showing spindle-shaped LacZ-positive endothelial cells within blood vessels. (E) Latero-medial view onto the fetal liver. (C-E) LacZ whole-mount staining after maternal tamoxifen injections E9.5 0.5 mg, E10.5 1 mg, and E11.5 2 mg.
Temporally controlled, tamoxifen-dependent recombination in HSC-SCL-Cre-ERT embryos. (A) The 6E5/Cre-ERT/3′enh construct used to generate HSC-SCL-Cre-ERT transgenic mice. The 6E5 genomic fragment containing the SCL exon 4 promoter combined with a 5.5-kb Scl 3′ enhancer sequence was used to control expression of Cre-ERT. White, light gray, and dark gray boxes represent introns, untranscribed exon, and transcribed exon sequences, respectively. (B) Schematic drawing of the transgenic system used in this study. Upon tamoxifen (TAM) treatment, the Cre-ERT recombinase is translocated and the stop-cassette is removed, inducing expression of Eyfp or lacZ. (C) LacZ staining of E12.5 HSC-SCL-Cre-ERT;R26R embryos revealed positive staining of the fetal liver and scattered LacZ-positive cells throughout the embryo. (D) View on the visceral side of the E12.5 yolk sac, showing spindle-shaped LacZ-positive endothelial cells within blood vessels. (E) Latero-medial view onto the fetal liver. (C-E) LacZ whole-mount staining after maternal tamoxifen injections E9.5 0.5 mg, E10.5 1 mg, and E11.5 2 mg.
For the induction of Cre-ERT recombinase, mice received intraperitoneal tamoxifen (1 mg/0.1 mL of corn oil [Sigma, St Louis, MO]) injections. Alternatively, mice were put on a tamoxifen diet (1 g tamoxifen/1 kg diet). This diet was prepared by premixing tamoxifen with sucrose (1:20 ratio) with mortar and pestle. This premix was added to the standard manufacturing process of “rat and mouse cubes” (Glen Forrest Stockfeeders, Glen Forrest, WA, Australia).
As described by others24 tamoxifen treatment during pregnancy interferes with normal delivery. Therefore, to be able to fate-trace cells marked during embryogenesis into adulthood, Cesarean sections were carried out at term, and neonates were cross-fostered to lactating females (Arc:Arc[S], Animal Resources Centre, Canning Vale, WA, Australia).
All animal procedures were performed according to protocols approved by the Animal Ethics Committees of the University of Western Australia and the Princess Margaret Hospital for Children in Perth.
LacZ staining
Whole-mount LacZ staining was performed as previously described.21,25 Specimens were analyzed with an MZ6 stereomicroscope (LacZ whole-mount staining; Leica Microsystems, Gladesville, NSW, Australia) or a DMBL microscope (histological LacZ-analysis; Leica Microsystems). All images were obtained with a DC300 digital camera and IM50 imaging software (Leica Microsystems).
FACS analysis of hematopoietic cells
Cells from BM, thymus, spleen, and peripheral blood were stained as previously described21 with fluorochrome or biotin-conjugated monoclonal antibodies (Pharmingen, San Diego, CA) against c-kit (clone 2B8), Flk-2 (Ly-72), Sca-1 (E13-167.7), CD45.1 (A20), CD45.2 (104), CD3 (145-2C11), CD4 (RM4-5), CD8 (53-6.7), B220 (RA3-6B2), Mac-1 (M1/70), Gr-1 (RB6-8C5), NK1.1 (PK136), and Ter119. Analysis was carried out on a BD FACSCalibur using FlowJo software (Treestar, San Carlos, CA). When enhanced yellow fluorescent protein (EYFP) proportions from different groups or different populations were compared, a 2-tailed Student t test was used to determine the level of significance.
Bone marrow and fetal liver transplantation assays
Repopulation assays were performed using the congenic Ly5.1/Ly5.2 system. B6-SJL recipient mice (Ly5.1/CD45.1; Animal Resources Centre) were lethally irradiated by exposure to 2 administrations of 5.5 Gy (3-hour intervals) from a 137Cs radiation source (Gammacell 3000 Elan, MDS Nordion, Kanata, Canada). BM cells (5 to 10 × 106) or E14.5 FL cells (2 × 106) from tamoxifen-treated HSC-SCL-Cre-ERT;R26R-EYFP and control mice were injected intravenously into the tail vein of recipients. Recipients were maintained on water containing 1.1 g/L neomycin (Sigma) and 106 U/L polymyxin B sulfate (Sigma) for 4 weeks.
The percentage of EYFP-positive day-12 Colony-Forming-Unit-Spleen (CFU-S12) multipotent progenitors was determined by transplanting into lethally irradiated C57BL/6 recipients with 7.5 × 104 BM cells from tamoxifen-treated HSC-SCL-Cre-ERT;R26R-EYFP mice. Spleens of recipient mice were harvested 12 days later. Prior to microdissection, EYFP-positive CFU-S12s were visualized with a Leica MZ6 stereomicroscope fitted with a fluorescence module using a GFP-Plus fluorescence filter (Leica Microsystems). Subsequently, single colonies were fluorescence-activated cell-sorter scanner (FACS) analyzed for Eyfp expression.
Results
Generation of transgenic mice and analysis of embryonic Cre-ERT expression
To create a system for temporally controlled gene targeting in HSCs, we expressed the inducible Cre-ERT recombinase under control of the Scl stem-cell enhancer (3′ enhancer)16-18 and the 6E5 Scl exon 4 promoter16 (Figure 1A).
Three independent transgenic founder lines were obtained. To investigate Cre-ERT–mediated recombination, mice of each founder line were bred with the R26R Cre reporter line.22 This reporter mouse and the reporter mouse used for later experiments (R26R-EYFP23 ) carry a floxed transcriptional STOP cassette inserted into the Rosa26 locus, which prevents expression of the reporter genes lacZ or Eyfp, respectively. When this cassette is removed by Cre-ERT, which is translocated to the nucleus by tamoxifen treatment, lacZ or Eyfp expression is turned on. After recombination occurs, these cells and all their progeny are irreversibly genetically marked (Figure 1B).
Embryos were harvested at E12.5 after maternal tamoxifen treatment (E9.5 0.5 mg, E10.5 1 mg, E11.5 2 mg) and whole-mount stained for LacZ. The FLs of 2 founder lines stained positive for LacZ. One of these 2 lines (founder #42-056), which had the highest proportion of lacZ-expressing cells in adult BM (data not shown), was selected for subsequent experimental use. This transgenic founder line is referred to as HSC-SCL-Cre-ERT.
In addition to strong FL LacZ staining, embryos double-heterozygous for the HSC-SCL-Cre-ERT and the R26R transgenes (HSC-SCL-Cre-ERT;R26R) displayed staining of scattered isolated cells throughout the embryo (Figure 1C,E). LacZ-positive cells included cells with round and spindle-shaped morphology most likely representing hematopoietic and endothelial cells, respectively. LacZ-positive endothelial cells also were present within the yolk sac of HSC-SCL-Cre-ERT;R26R embryos (Figure 1D). No LacZ staining was detected in R26R embryos, which did not carry the HSC-SCL-Cre-ERT transgene (data not shown). The embryonic lacZ-expression pattern of HSC-SCL-Cre-ERT;R26R was similar to the staining pattern in transgenic mice, which directly express lacZ under the control of the Scl 3′ enhancer.16 However, endothelial LacZ staining in HSC-SCL-Cre-ERT;R26R embryos appeared less prominent.
Tamoxifen-dependent recombination within adult hematopoietic progenitor subsets
This study aimed to establish a transgenic mouse model that allows temporally controlled transgenic targeting within early hematopoietic progenitor cells. In order to perform a detailed analysis of recombination induced by the HSC-SCL-Cre-ERT transgene in adult hematopoiesis, HSC-SCL-CreERT transgenics were bred to R26R-EYFP reporter mice. Because the induction of estrogen receptor fusion proteins is known to be dependent on the ligand dose administered,24,26 we initially chose a higher tamoxifen dose for the induction of Cre-ERT than proposed by previous investigators.27 Another group also had chosen to use higher tamoxifen doses and administered up to 9 mg per day for 5 consecutive days to induce Cre recombinase activity without observing any signs of tamoxifen toxicity.24 We treated 12-week-old mice with 2 mg tamoxifen per day for 2 weeks and carried out flow cytometric analysis of BM 2 weeks later (Figure 2). We observed 11.4% ± SD 2.4% (n = 4) of HSC-SCL-Cre-ERT;R26R-EYFP total BM cells to be EYFP positive (data not shown). In BM of untreated HSC-SCL-Cre-ERT;R26R-EYFP we did not detect any Eyfp expression (n = 3, compare to group I, Figure 7C).
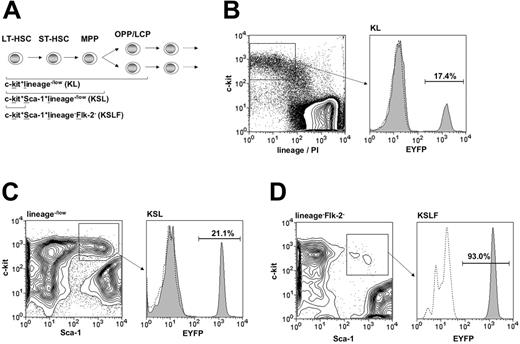
Targeting within hematopoietic progenitor subsets. (A) Diagram of the hierarchical structure of early hematopoietic progenitor populations and the combination of surface markers used for their identification. HSC-SCL-Cre-ERT;R26R-EYFP mice and controls were injected with tamoxifen for 2 weeks (2 mg per day), and bone marrow analysis was carried out 2.5 weeks after the last tamoxifen injection. The percentages of EYFP-positive cells within (B) the c-kit+lineage–/low (KL) (SD ± 3.0%), (C) the c-kit+Sca-1+lineage–/low (KSL) (SD ± 3.9%), and (D) the c-kit+Sca-1+lineage–Flk-2– (KSLF) (SD ± 9.7%) populations were determined by flow cytometry. The highest degree of Cre-mediated recombination was detected within the c-kit+Sca-1+lineage–Flk-2– population and was significantly lower in the c-kit+Sca-1+ lineage–/low and c-kit+lin–/low populations, respectively. Representative plots are shown for each population and the indicated percentages represent means (n = 5). Dashed lines represent negative controls. OPP, oligopotent progenitors; LCP, lineage-committed progenitors; lin, lineage; PI, propidium iodide.
Targeting within hematopoietic progenitor subsets. (A) Diagram of the hierarchical structure of early hematopoietic progenitor populations and the combination of surface markers used for their identification. HSC-SCL-Cre-ERT;R26R-EYFP mice and controls were injected with tamoxifen for 2 weeks (2 mg per day), and bone marrow analysis was carried out 2.5 weeks after the last tamoxifen injection. The percentages of EYFP-positive cells within (B) the c-kit+lineage–/low (KL) (SD ± 3.0%), (C) the c-kit+Sca-1+lineage–/low (KSL) (SD ± 3.9%), and (D) the c-kit+Sca-1+lineage–Flk-2– (KSLF) (SD ± 9.7%) populations were determined by flow cytometry. The highest degree of Cre-mediated recombination was detected within the c-kit+Sca-1+lineage–Flk-2– population and was significantly lower in the c-kit+Sca-1+ lineage–/low and c-kit+lin–/low populations, respectively. Representative plots are shown for each population and the indicated percentages represent means (n = 5). Dashed lines represent negative controls. OPP, oligopotent progenitors; LCP, lineage-committed progenitors; lin, lineage; PI, propidium iodide.
Fate-tracing embryonic hematopoietic stem cells into adulthood. (A) Outline of the experimental design. Timed matings were set up between HSC-SCL-Cre-ERT males and R26R-EYFP females. Pregnant females were allocated into 3 experimental groups. Group I females did not receive tamoxifen injections, while group II and group III females received tamoxifen injections at E10.5 (1 mg) and E11.5 (2 mg). Group II pregnancies were terminated at E14.5, embryonic fetal liver cells were harvested, analyzed for the expression of Eyfp, and transplanted into lethally irradiated Ly5.1 recipients. Group III neonates were delivered by Caesarean section on day E19.5 and fostered to lactating females. After weaning group I and III, newborn mice were genotyped for the presence of the HSC-SCL-Cre-ERT and R26R-EYFP transgenes. (B) Fate-mapping transplanted (group II) and in vivo–remaining (group III) marked embryonic LT-HSCs: a proportion of group II (left panel) and group III (right panel) embryonic LT-HSCs was genetically marked (EYFP+) by maternal tamoxifen injections. Group II E14.5 fetal liver cells were transplanted into lethally irradiated congenic adult recipients. The proportion of marked fetal liver LT-HSCs was determined by measuring the hematopoietic contribution of these cells within recipients 5 months after transplantation (group II, left panel). In parallel the contribution of in vivo–remaining marked fetal liver LT-HSCs to adult bone marrow hematopoiesis was investigated (group III, right panel). If the proportion of EYFP+ hematopoietic cells within group III adults were found to be significantly lower than within recipient adults (group II), this would mean that the de novo generation of HSCs within the embryo was not completed by the time of transplantation (E14.5). If new LT-HSCs were generated after day 14.5 of gestation, these cells would not be marked (absence of tamoxifen) and would therefore decrease the proportion of group III adult EYFP+ hematopoietic cells compared to group II. (C) Five months after birth or transplantation, respectively, flow cytometric analysis of Eyfp expression in hematopoietic organs of group I, II, and III mice was carried out. Representative plots of total bone marrow from each group are shown. Percentages represent means. FSC, forward light scatter. (D) Comparison of mean percentages of Eyfp-expressing cells within different hematopoietic populations (bone marrow: total cells, neutrophils [Mac-1+Gr-1high], B cells [B220+], erythroid [Ter119+], HSCs [KSL]; thymus: double-positive cells [CD4+CD8+]) of group II (□) and group III mice (▪). No statistical significant difference between the mean percentages of groups II and III was noted. In group II recipients 99.1 ± SD 0.5% of bone marrow cells were donor derived (CD45.2+). Error bars indicate standard deviations. Group I (n = 2); group II (n = 4 donor embryos, n = 2 recipients per donor); group III (n = 4).
Fate-tracing embryonic hematopoietic stem cells into adulthood. (A) Outline of the experimental design. Timed matings were set up between HSC-SCL-Cre-ERT males and R26R-EYFP females. Pregnant females were allocated into 3 experimental groups. Group I females did not receive tamoxifen injections, while group II and group III females received tamoxifen injections at E10.5 (1 mg) and E11.5 (2 mg). Group II pregnancies were terminated at E14.5, embryonic fetal liver cells were harvested, analyzed for the expression of Eyfp, and transplanted into lethally irradiated Ly5.1 recipients. Group III neonates were delivered by Caesarean section on day E19.5 and fostered to lactating females. After weaning group I and III, newborn mice were genotyped for the presence of the HSC-SCL-Cre-ERT and R26R-EYFP transgenes. (B) Fate-mapping transplanted (group II) and in vivo–remaining (group III) marked embryonic LT-HSCs: a proportion of group II (left panel) and group III (right panel) embryonic LT-HSCs was genetically marked (EYFP+) by maternal tamoxifen injections. Group II E14.5 fetal liver cells were transplanted into lethally irradiated congenic adult recipients. The proportion of marked fetal liver LT-HSCs was determined by measuring the hematopoietic contribution of these cells within recipients 5 months after transplantation (group II, left panel). In parallel the contribution of in vivo–remaining marked fetal liver LT-HSCs to adult bone marrow hematopoiesis was investigated (group III, right panel). If the proportion of EYFP+ hematopoietic cells within group III adults were found to be significantly lower than within recipient adults (group II), this would mean that the de novo generation of HSCs within the embryo was not completed by the time of transplantation (E14.5). If new LT-HSCs were generated after day 14.5 of gestation, these cells would not be marked (absence of tamoxifen) and would therefore decrease the proportion of group III adult EYFP+ hematopoietic cells compared to group II. (C) Five months after birth or transplantation, respectively, flow cytometric analysis of Eyfp expression in hematopoietic organs of group I, II, and III mice was carried out. Representative plots of total bone marrow from each group are shown. Percentages represent means. FSC, forward light scatter. (D) Comparison of mean percentages of Eyfp-expressing cells within different hematopoietic populations (bone marrow: total cells, neutrophils [Mac-1+Gr-1high], B cells [B220+], erythroid [Ter119+], HSCs [KSL]; thymus: double-positive cells [CD4+CD8+]) of group II (□) and group III mice (▪). No statistical significant difference between the mean percentages of groups II and III was noted. In group II recipients 99.1 ± SD 0.5% of bone marrow cells were donor derived (CD45.2+). Error bars indicate standard deviations. Group I (n = 2); group II (n = 4 donor embryos, n = 2 recipients per donor); group III (n = 4).
Hematopoietic progenitors are enriched within a population that lacks expression of lineage-specific markers. Additionally, immature hematopoietic cells that include oligopotent progenitors (OPPs) and some already lineage-committed progenitors (LCPs) express c-kit (Figure 2A).28-31 Therefore, we stained adult HSC-SCL-Cre-ERT;R26R-EYFP BM for lineage markers in combination with c-kit. We observed EYFP expression in 17% of c-kit+lineage–/low (KL) cells (Figure 2B). The majority of adult HSC activity within adult BM is contained within the c-kit+Sca-1+lineage–/low (KSL) population of adult BM.32-35 Thus, we performed flow cytometric EYFP analysis of this population (Figure 2C). Compared to KL cells we found a significantly higher EYFP percentage (21%) within the KSL population (P < .05). We also analyzed Eyfp expression within a population, which is enriched for LT-HSCs. Increasing expression of the marker Flk-2 is correlated with HSC maturation from a population with extensive self-renewal potential to a multipotent progenitor (MPP) population with only limited self-renewal potential (Figure 2A).36,37 We exploited this phenomenon to be able to determine the extent of HSC-SCL-Cre-ERT– mediated recombination within LT-HSCs. We performed this by adding an Flk-2–specific antibody to the panel of lineage-specific antibodies. By excluding Flk-2–positive cells together with lineage-positive cells, we were able to determine the proportion of Eyfp-expressing cells within c-kit+Sca-1+lineage–Flk-2– (KSLF) cells. Strikingly, more than 90% of KSLF cells were positive for EYFP (Figure 2D). These data imply that the activity of the HSC-SCL-Cre-ERT transgene was correlated with the level of immaturity of hematopoietic progenitors. Taken together, the flow cytometric analysis demonstrated genetic targeting in the great majority of HSCs of HSC-SCL-Cre-ERT;R26R-EYFP mice.
If HSC-SCL-Cre-ERT transgenic mice preferentially target progenitor cells, the proportion of Eyfp-expressing cells should increase when progenitor cells proliferate and differentiate over time. Therefore, we compared the percentage of BM-Eyfp– expressing cells one day after the last tamoxifen injection with the percentage of Eyfp-expressing cells 2 weeks later. During this time period we observed a 3-fold increase (P < .05) of the total EYFP percentage of adult BM, suggesting that a subset of Cre-recombined cells were proliferating progenitor cells (Table 1). To further determine whether HSC-SCL-Cre-ERT–targeted progenitor cells undergo proliferation, we studied changes within the KSL EYFP percentage over time. We found a 1.6-fold increment (P < .05) in EYFP-positive cells within the KSL population 2 weeks after a 5-day tamoxifen protocol (Table 1). However, the increase of the EYFP percentage within KSLF cells within the same time period was not significant (P = .26). The increment of EYFP-positive cells within KSL was about half that observed for total BM during the same time period. This finding is consistent with the notion that the turnover rate of more mature progenitors is higher than that of immature progenitors within the KSL population.
In order to be able to reliably administer tamoxifen for a longer time period, we manufactured a rodent diet that contained tamoxifen (1 g tamoxifen/kg feed). Consumption of this diet did not lead to any obvious changes in appearance, behavior, or feeding of the mice over the period of study. We achieved a significantly higher degree of targeting within all populations investigated (P < .01) using this tamoxifen diet (Table 1). After 21 days of consumption of the tamoxifen rodent diet nearly all KSLF cells were EYFP positive (Table 1).
In summary, the extent of HSC-SCL-Cre-ERT–mediated recombination in hematopoietic progenitors appears to be dependent both on the total tamoxifen dose and on the length of the time between cessation of tamoxifen treatment and analysis.
Demonstrating temporally controlled transgenic targeting of hematopoietic stem cells in HSC-SCL-Cre-ERT transgenic mice using transplantation assays
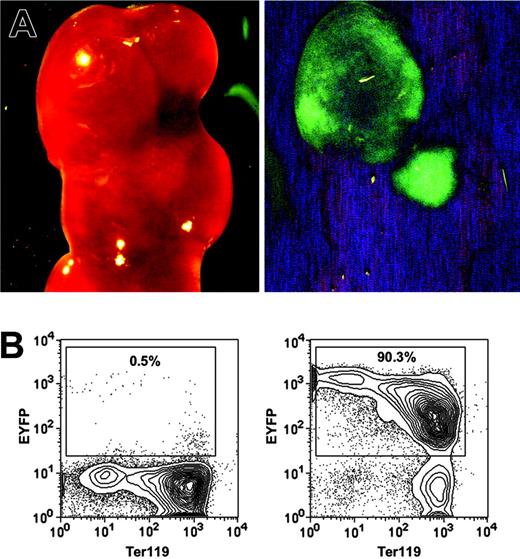
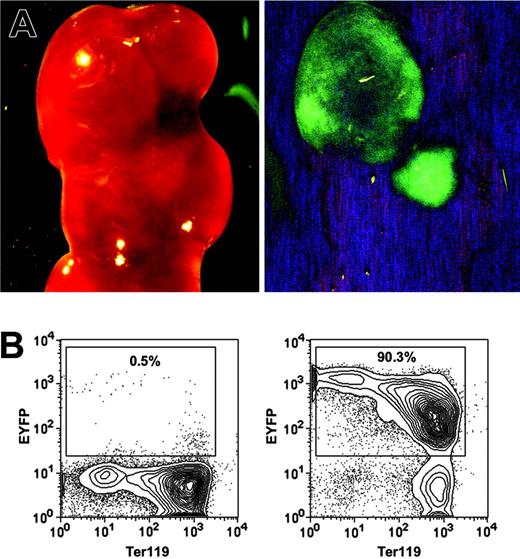
The most rigorous way to identify HSCs is to perform transplantation assays and demonstrate LT-multilineage engraftment. To test whether the HSC-SCL-Cre-ERT transgenics allow targeting of HSCs, we exploited the fact that all progeny of a marked progenitor of HSC-SCL-Cre-ERT;R26R-EYFP mice are positive for EYFP. Therefore, if tagged progenitors give rise to mature hematopoietic cells, those cells should express Eyfp. We first examined to what extent the HSC-SCL-Cre-ERT transgene was capable of targeting CFU-S12 multipotent progenitors.38 Lethally irradiated recipients were transplanted with BM of tamoxifen-treated HSC-SCL-Cre-ERT;R26R-EYFP mice (n = 4), and spleens were harvested 12 days later. We found an average of 7.6 colonies per recipient spleen. The presence of EYFP-positive colonies in spleens was confirmed with a stereo fluorescence microscope (Figure 3A). After microdissection, cells of single colonies were stained for Ter119 and analyzed by flow cytometry (Figure 3B). We found that the Eyfp expression of erythroid (Ter119+) cells was relatively low compared to other hematopoietic cells (Figures 3B, 6D). In order to prevent the oversight of EYFP-positive primarily erythroid colonies by fluorescent microscopy, we used flow cytometry to analyze Eyfp expression in all nonselected single spleen colonies (Figure 3B). The analysis revealed that 29% of CFU-S12 colonies were positive for EYFP (Table 2).
HSC-SCL-Cre-ERT–mediated recombination in CFU-S12 of adult bone marrow. Lethally irradiated recipients were transplanted with 7.5 × 104 bone marrow cells of tamoxifen-treated HSC-SCL-Cre-ERT;R26R-EYFP mice (n = 4, 2 mg tamoxifen per day for 14 days, transplanted 2 weeks after last injection). The recipients were killed 12 days later, and the spleens were harvested for analysis. (A) EYFP-positive CFU-S12 colonies were visualized with a fluorescent dissection microscope (left panel, bright field; right panel, eGFP filter). (B) Single CFU-S12 colonies were microdissected, stained for Ter119, and analyzed by flow cytometry. Representative plots of an EYFP-negative (left panel) and an EYFP-positive colony (right panel) are shown. Percentages refer to the proportion of EYFP-positive cells within single microdissected CFU-S12 colonies.
HSC-SCL-Cre-ERT–mediated recombination in CFU-S12 of adult bone marrow. Lethally irradiated recipients were transplanted with 7.5 × 104 bone marrow cells of tamoxifen-treated HSC-SCL-Cre-ERT;R26R-EYFP mice (n = 4, 2 mg tamoxifen per day for 14 days, transplanted 2 weeks after last injection). The recipients were killed 12 days later, and the spleens were harvested for analysis. (A) EYFP-positive CFU-S12 colonies were visualized with a fluorescent dissection microscope (left panel, bright field; right panel, eGFP filter). (B) Single CFU-S12 colonies were microdissected, stained for Ter119, and analyzed by flow cytometry. Representative plots of an EYFP-negative (left panel) and an EYFP-positive colony (right panel) are shown. Percentages refer to the proportion of EYFP-positive cells within single microdissected CFU-S12 colonies.
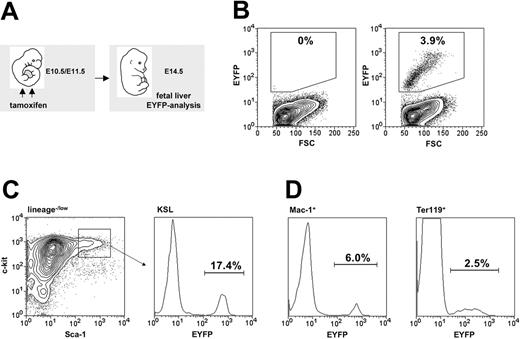
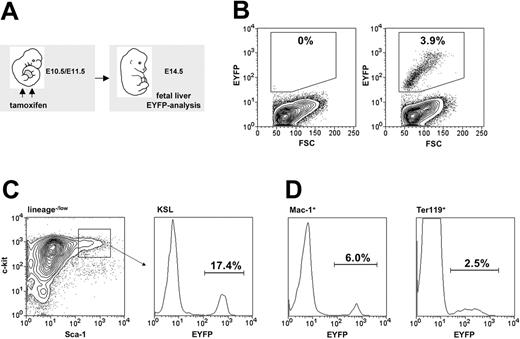
Tamoxifen-induced recombination within embryonic hematopoiesis. (A) Maternal tamoxifen injections were carried out on day E10.5 (1 mg) and E11.5 (2 mg). Embryonic fetal livers were harvested for flow cytometric analysis 3 days later (E14.5). (B) The proportion of Eyfp-expressing cells within total fetal liver cells from R26R-EYFP (left panel) and HSC-SCL-Cre-ERT;R26R-EYFP (right panel, SD ± 1.0%) littermates was determined. (C) The percentage of Eyfp-expressing cells also was determined within the c-kit+Sca-1+lineage–/low population (SD ± 2.2%). (D) Additionally, Eyfp expression within lineage-committed fetal liver cells was determined: Mac-1+ (SD ± 1.3%) and Ter119+ (SD ± 0.8%) populations. Representative plots are shown, and indicated percentages represent means (n = 7).
Tamoxifen-induced recombination within embryonic hematopoiesis. (A) Maternal tamoxifen injections were carried out on day E10.5 (1 mg) and E11.5 (2 mg). Embryonic fetal livers were harvested for flow cytometric analysis 3 days later (E14.5). (B) The proportion of Eyfp-expressing cells within total fetal liver cells from R26R-EYFP (left panel) and HSC-SCL-Cre-ERT;R26R-EYFP (right panel, SD ± 1.0%) littermates was determined. (C) The percentage of Eyfp-expressing cells also was determined within the c-kit+Sca-1+lineage–/low population (SD ± 2.2%). (D) Additionally, Eyfp expression within lineage-committed fetal liver cells was determined: Mac-1+ (SD ± 1.3%) and Ter119+ (SD ± 0.8%) populations. Representative plots are shown, and indicated percentages represent means (n = 7).
Based on these results, the targeting efficiency of the HSC-SCL-Cre-ERT line in CFU-S12 progenitors was comparable to the targeting efficiency found in the KSL population (Figure 2C). This was in agreement with the fact that CFU-S12 activity is known to primarily reside within the KSL of adult BM.39
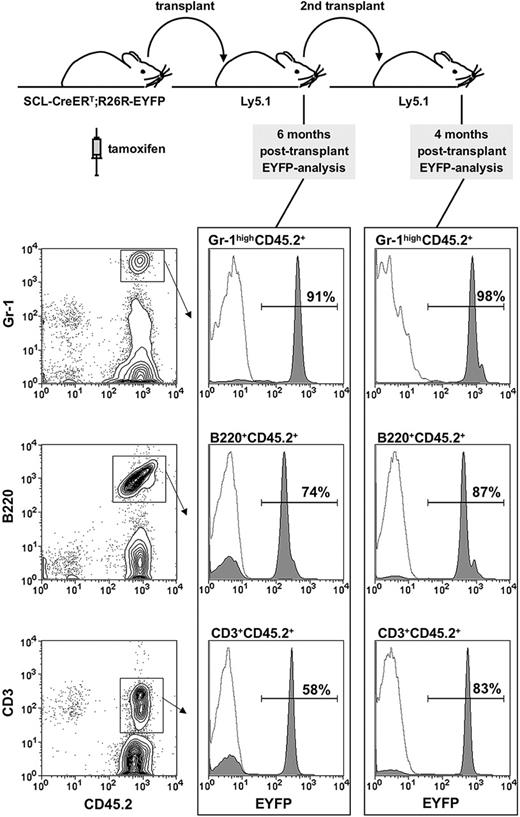
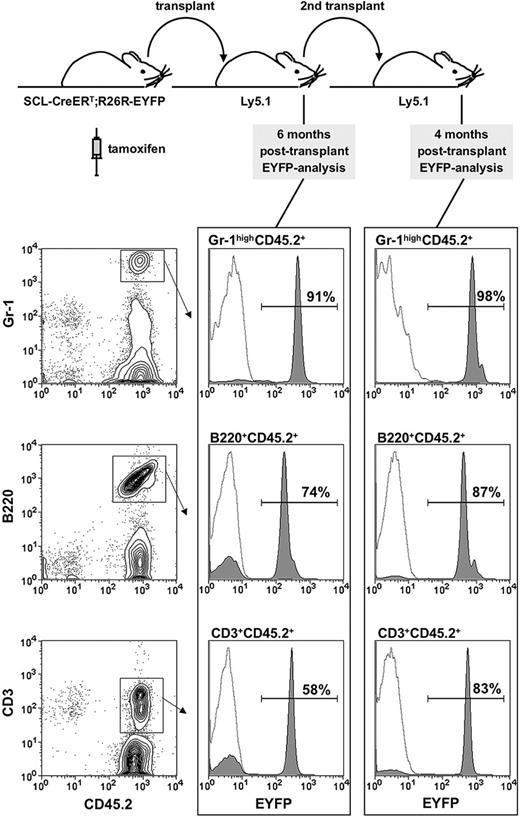
The ability of CFU-S12 multipotent progenitors to give rise to mature hematopoietic cells ceases after 12 days. Therefore, ST- and LT-reconstitution assays were performed to determine the targeting efficiency in HSCs. Irradiated recipients (Ly5.1/CD45.1) were reconstituted with 5 to 10 × 106 BM cells from tamoxifen-treated HSC-SCL-Cre-ERT;R26R-EYFP mice. We chose to analyze Eyfp expression in granulocytes because these cells have a short life span and must therefore be regenerated de novo from immature progenitors in recipient mice. We investigated the proportion of donor-derived peripheral blood granulocytes (Gr-1highCD45.2+), which was generated by a targeted, EYFP-positive HSC 6 weeks (ST) and 4 to 6 months (LT) after transplantation. Six weeks after transplantation we observed that 81% ± SD 7.1% of peripheral blood Gr-1highCD45.2+ cells were EYFP positive (data not shown, n = 4 donors, 2 mg tamoxifen per day for 14 days, transplanted 2 weeks after the last injection). We found that 91% of peripheral blood granulocytes were EYFP-positive after 6 months (Figure 4). The self-renewing capacity of LT-HSCs can be assayed by their ability to reconstitute secondary recipients. Thus, we carried out secondary transplants into congenic hosts with BM from mice, which had been reconstituted with tamoxifen-treated HSC-SCL-Cre-ERT;R26R-EYFP BM 6 months earlier (Figure 4). Strikingly, 98% of peripheral blood granulocytes from these secondary recipients were EYFP-positive 4 months after the secondary transplant (Figure 4). These results were confirmed with a different tamoxifen protocol: we observed that 4 months after primary transplantation of BM from HSC-SCL-Cre-ERT;R26R-EYFP mice (tamoxifen diet for 21 days, n = 3), 89% ± SD 5.4% of BM Gr-1highMac-1+ cells were EYFP-positive. Importantly, congenic mice receiving BM from HSC-SCL-Cre-ERT;R26R-EYFP mice, not receiving any tamoxifen treatment, did not show any Eyfp expression (data not shown). Additionally, we analyzed Eyfp expression within donor-derived lymphoid cells (Figure 4). It is important to note that total bone marrow, which contains significant numbers of unmarked mature lymphoid cells, was transplanted. These lymphocytes have a long life span and might even proliferate in recipient animals. Therefore, we expected a lower proportion of EYFP-marked lymphocytes compared to granulocytes. We observed that 74% of B cells and 58% of T cells were marked in primary recipients after 6 months, which increased to 87% and 83%, respectively, in secondary recipients (Figure 4). These data demonstrate that the majority of the lymphoid compartment in irradiated recipients was replenished by transplanted marked HSCs.
Analysis of Eyfp expression in primary and secondary recipients. (A) Overview of the experimental strategy: bone marrow of tamoxifen treated (5 mg day 1, 2 mg days 2-5) HSC-SCL-Cre-ERT;R26R-EYFP mice (n = 2) was transplanted (day 8) into lethally irradiated congenic recipients (Ly5.1/CD45.1). After 6 months primary recipient bone marrow was transplanted into secondary recipients (Ly5.1/CD45.1). Peripheral blood was analyzed 6 months after the primary and 4 months after secondary transplants. The proportion of EYFP-positive cells was determined within donor-derived peripheral blood granulocytes (Gr-1highCD45.2+), B cells (B220+CD45.2+), and T cells (CD3+CD45.2+). Representative plots are shown, and the indicated percentages represent means of 2 to 4 recipient mice. Dashed lines represent negative controls.
Analysis of Eyfp expression in primary and secondary recipients. (A) Overview of the experimental strategy: bone marrow of tamoxifen treated (5 mg day 1, 2 mg days 2-5) HSC-SCL-Cre-ERT;R26R-EYFP mice (n = 2) was transplanted (day 8) into lethally irradiated congenic recipients (Ly5.1/CD45.1). After 6 months primary recipient bone marrow was transplanted into secondary recipients (Ly5.1/CD45.1). Peripheral blood was analyzed 6 months after the primary and 4 months after secondary transplants. The proportion of EYFP-positive cells was determined within donor-derived peripheral blood granulocytes (Gr-1highCD45.2+), B cells (B220+CD45.2+), and T cells (CD3+CD45.2+). Representative plots are shown, and the indicated percentages represent means of 2 to 4 recipient mice. Dashed lines represent negative controls.
In conclusion, the transplantation data confirmed the flow cytometric data (Figure 2) and demonstrated that the HSC-SCL-Cre-ERT transgene targeted the great majority of adult BM HSCs.
Activity of the HSC-SCL-Cre-ERT transgene within adult organs
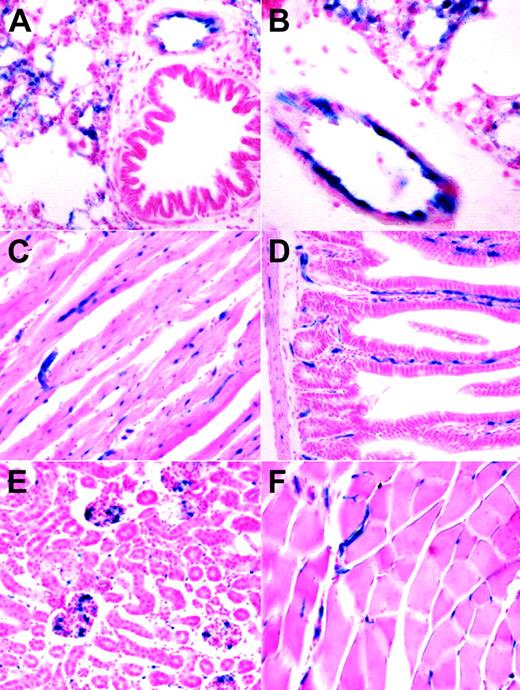
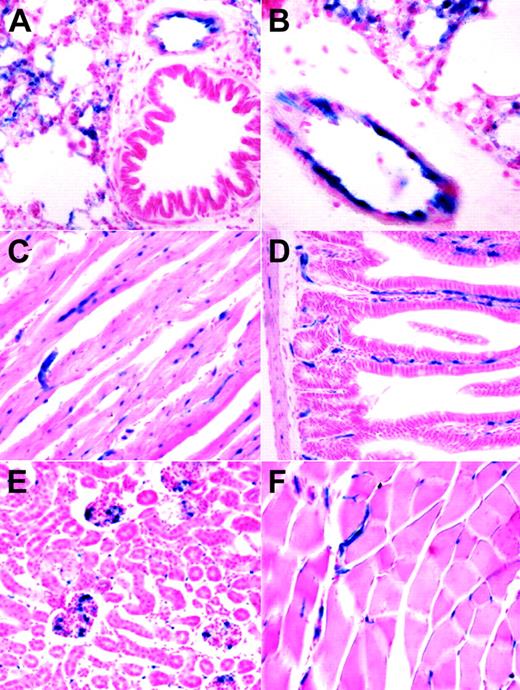
The embryonic endothelial activity of the HSC-SCL-Cre-ERT line was less pronounced than anticipated from earlier transgenic studies with the Scl-3′ stem-cell enhancer.16,18 However, the endothelial activity of the Scl-3′ enhancer in adult animals has not been previously reported. We investigated the ability of the HSC-SCL-Cre-ERT transgenic line to induce recombination in endothelial cells of adult animals. Organs from tamoxifen-treated HSC-SCL-Cre-ERT;R26R mice were harvested and subjected to LacZ analysis. In lung, lacZ-positive endothelium was present on the inner lining of bigger blood vessels (Figure 5A-B). However, not the entire lining of lung vessel walls appeared LacZ positive. Within adult myocardium capillary-like structures stained positive for LacZ (Figure 5C). In liver we could not detect any LacZ staining; neither sinusoidal nor portal endothelial cells were positive for LacZ (data not shown). However, capillaries within intestinal villi and the intestinal wall showed endothelial LacZ staining (Figure 5D). In kidney, scattered glomerular and intertubular LacZ-positive cells were present (Figure 5E). In striated muscle capillary LacZ staining was present (Figure 5F).
Analysis of lacZ expression within adult organs of tamoxifen-treated HSC-SCL-Cre-ERT;R26R mice. Mice were injected with tamoxifen (2 mg every 48 hours for 2 weeks), and adult organs were harvested for lacZ analysis one day after the last injection. (A, B) In adult lung, lacZ expression was present in endothelial cells of larger vessels. LacZ activity also was present in smaller lung capillaries. (C) Within adult myocardium, capillaries stained positive for LacZ. (D) Capillaries of intestinal villi and the intestinal wall were LacZ positive. (E) In kidney, scattered LacZ staining was observed within glomeruli and intertubular space. (F) In skeletal muscle, LacZ-positive vessels were present between muscle fibers. Original magnification (panels A, C-F), × 20; (panel B), × 40.
Analysis of lacZ expression within adult organs of tamoxifen-treated HSC-SCL-Cre-ERT;R26R mice. Mice were injected with tamoxifen (2 mg every 48 hours for 2 weeks), and adult organs were harvested for lacZ analysis one day after the last injection. (A, B) In adult lung, lacZ expression was present in endothelial cells of larger vessels. LacZ activity also was present in smaller lung capillaries. (C) Within adult myocardium, capillaries stained positive for LacZ. (D) Capillaries of intestinal villi and the intestinal wall were LacZ positive. (E) In kidney, scattered LacZ staining was observed within glomeruli and intertubular space. (F) In skeletal muscle, LacZ-positive vessels were present between muscle fibers. Original magnification (panels A, C-F), × 20; (panel B), × 40.
Taken together, the HSC-SCL-Cre-ERT line has relevant endothelial activity within adult organ endothelial cells. However, compared to another Cre-ERT transgenic line we generated (end-SCL-Cre-ERT),21 endothelial targeting by HSC-SCL-Cre-ERT transgenic mice was less prominent.
Fate-tracing embryonic HSCs into adulthood
In order to be able to trace embryonic HSCs, we investigated whether the HSC-SCL-Cre-ERT transgene was capable of marking embryonic HSCs (Figure 6A). We chose to inject tamoxifen at E10.5 and E11.5 because within this timeframe the first adult LT-HSCs are generated in the AGM and begin to colonize the FL.40 Another reason for commencing injections on E10.5 was our observation that earlier tamoxifen applications lead to reduced survival frequencies to term, which would have complicated subsequent tracing studies. Tamoxifen injections at E10.5 and E11.5 resulted in EYFP-marked cells in the FL on day E14.5 (Figure 6B). The proportion of EYFP-positive cells within the FL KSL population (Figure 6C) was higher than in erythroid (Ter119+) and myeloid (Mac-1+) FL cells (Figure 6D). Thus, the HSC-SCL-Cre-ERT transgene was capable of inducing recombination in the embryonic HSC compartment. Therefore, recombination observed in the mature myeloid and erythroid cells represented a combination of direct targeting of these cells and, more likely, the generation of progeny of transgene-targeted progenitor cells.
To confirm that the transgene targets embryonic HSCs, transplant experiments with HSC-SCL-Cre-ERT;R26R-EYFP FL cells were performed (Figure 7A, group II). Five months after transplantation the percentage of EYFP-positive cells within recipient BM populations was determined. Unexpectedly, we observed that only 8% to 10% of donor-derived (CD45.2+) hematopoietic cells were EYFP positive (Figure 7C, middle panel; Figure 7D, open bars). These data demonstrated that the high level of recombination achieved by the HSC-SCL-Cre-ERT transgene in adult HSCs could not be reached in embryonic HSCs. The reason for this observation could be (1) that tamoxifen levels achieved within the embryo proper were lower than the levels achieved in the adult BM cavity, (2) that the AGM region as the primary site of HSC emergence already has down-regulated transgene expression by the time of tamoxifen administration at E10.5/11.5, or (3) that the Cre-ERT expression level is lower in embryonic HSCs than in adult HSCs. We favor the first option because a previous study has demonstrated that the Scl–stem cell enhancer is highly active in the AGM region at E1116 and should thus mediate adequate Cre-ERT transgene expression at this time point in our system. Nevertheless, these transplantation data confirmed that the HSC-SCL-Cre-ERT transgenic line could be used to efficiently mark approximately 10% of embryonic HSCs between E10.5 and E11.5 in vivo.
We exploited this ability to study the direct-lineage relationship between embryonic and adult HSCs in the murine system. Additionally, we examined whether all adult HSCs originated from an early embryonic counterpart or if a subset of HSCs was newly generated from an additional source during the late fetal or neonatal period.
We genetically marked FL HSCs of E10.5/E11.5 HSC-SCL-Cre-ERT;R26R-EYFP embryos by maternal tamoxifen injections (Figure 7A, group III) and allowed these mice to reach adulthood. At 5 months of age their hematopoietic system was analyzed for the expression of Eyfp. In these mice, 9.6% of all BM cells were EYFP positive (Figure 7C, group III). A similar proportion of EYFP-positive cells was observed throughout all lineages and populations included in the analysis (Figure 7D, closed bars), indicating that these cells had arisen from a cell with adult multilineage hematopoietic potential. Thus, around 10% of the adult HSCs were derived from embryonic HSCs, marked on days 10.5/11.5 of gestation. There are 2 possibilities that explain the origin of the remaining 90% of unmarked HSCs. One possibility is that these cells also were of embryonic origin but were not successfully marked by the transgene. Alternatively, it is possible that these cells were generated after the genetic marking took place. In age-matched HSC-SCL-Cre-ERT;R26R-EYFP mice, which did not receive any tamoxifen treatment, no Eyfp expression could be detected (Figure 7C, group I). These lineage-tracing data demonstrate for the first time in a direct manner that a significant proportion of HSC cells generated in the E10.5/E11.5 embryo give rise to the adult hematopoietic system in the mouse.
To address the question of whether the de novo generation of the majority of LT-HSCs was completed in the midgestation embryo, we compared the EYFP percentage of mice that had been transplanted with previously HSC-SCL-Cre-ERT–marked E14.5 FL cells (group II) with the EYFP percentage of HSC-SCL-Cre-ERT; R26R-EYFP mice whose HSCs were marked at the same time in utero (group III) (Figure 7A). As described, the percentage of Eyfp-expressing cells within recipients 5 months after transplantation reflected the proportion of marked FL LT-HSCs at the time of transplantation (E14.5) (Figure 7B, left panel). We chose E14.5 as the time point for transplantation because at this stage the FL is the exclusive location of LT-HSCs within the embryo. By this time the progenitor generation within the AGM region has ceased, and LT repopulating activity cannot yet be detected in BM and spleen.41-43 In contrast to FL cells that remain in situ (group III), marked transplanted E14.5 FL cells (group II) bypassed their normal fetal and neonatal microenvironment and were transferred directly into an adult recipient. Because we chose to transplant into congenic recipients (Ly5.1/CD45.1), we were able to discriminate between potential recipient-derived (CD45.1+) and FL graft–derived hematopoietic cells (CD45.2+). If in HSC-SCL-Cre-ERT;R26R-EYFP embryos (group III), significant numbers of HSCs were newly generated from non-FL residing cells (for example, in the adult bone medullary cavity) after E14.5, these HSCs would be EYFP negative (absence of tamoxifen) and would therefore decrease the proportion of EYFP-positive cells detected later during adulthood (Figure 7B, right panel). In contrast, the EYFP proportion within the adult-engrafted FL HSC compartment could not be altered by ongoing late fetal or neonatal de novo HSC generation because these developmental stages were bypassed by transplantation. Additionally, any newly generated hematopoietic cell from a source other than the transplanted FL cells was excluded by using a congenic transplantation system. Therefore, within the transplanted FL HSC compartment the EYFP percentage should stay constant after transplantation (Figure 7B, left panel).
We found that percentages of EYFP-positive cells within total BM group II and group III did not differ significantly (Figure 7C-D). The same observation was made throughout all hematopoietic populations included in the analysis (Figure 7D). These data strongly suggest that the generation of HSCs is completed by midgestation because the proportion of tagged cells was not altered by transplanting marked HSCs at E14.5. The fact that the proportion of unmarked cells (90%) in the transplanted FL-HSC compartment (group II) was similar to the unmarked cell proportion in the in vivo–remaining HSC compartment (group III) imply that the unmarked HSCs in the in vivo fate-traced HSC compartment (group III) were generated before E14.5 (the time point of the FL transplantation of group II).
Discussion
Resolving the self-renewal and multilineage differentiation program of stem cells is one of the central interests of current research. We have established a Cre transgenic mouse line, which allows temporally controlled gene targeting in stem cells of the hematopoietic system. To our knowledge, this is the first mouse model that allows the genetic manipulation of this unique cell type in a temporally controlled and highly specific manner. The Cre/loxP technology allows multiple different experimental approaches: this system facilitates the generation of conditional knock-out and overexpression models.44 Importantly, this system also can be used to trace the fate of genetically marked hematopoietic progenitors in vivo throughout life without the need of transplantation.
We have demonstrated by using a Rosa26-based reporter system that in HSC-SCL-Cre-ERT mice recombination can reliably be induced in adult HSCs by the application of tamoxifen. The reporter gene Eyfp was expressed in cells that expressed HSC markers. Moreover, the transplantation of BM from tamoxifen-treated HSC-SCL-Cre-ERT mice resulted in Eyfp expression by the vast majority of peripheral blood leukocytes after LT reconstitution.
As null mutations of genes implicated in HSC function often cause lethal phenotypes due to compromised embryonic hematopoiesis,19,20,45,46 the temporally controlled targeting provided by HSC-SCL-Cre-ERT transgenics will be valuable to induce HSC-specific null mutations in the adult. Furthermore, long-term expression of Cre has been associated with toxic effects such as illegitimate chromosomal recombinations and suppression of growth.47-50 These toxic effects are circumvented by an inducible transgenic system.
Multiple Cre transgenic lines mediating recombination in different hematopoietic lineages and at different developmental stages have been described.51-55 To our knowledge, the only line providing temporally controlled gene targeting in hematopoiesis is the interferon-inducible Mx1-Cre strain.56 The Mx1-Cre strain has been widely used to induce null mutations in adult HSCs.57-61 Compared to Mx1-Cre, the described transgenic system provides a number of advantages. The HSC-SCL-Cre-ERT line is characterized by high-level specificity within hematopoiesis for HSCs, while the Mx1-Cre line deletes efficiently in the entire hematopoietic system and other organs such as liver and heart.56 Furthermore, the HSC-SCL-Cre-ERT line provides a tighter control of Cre induction than Mx1-Cre. Due to endogenous interferon α or β production, Mx1-Cre–mediated recombination can be observed without the exogenous application of inducer.56,62 The induction of Mx1-Cre with the synthetic double-stranded RNA PI-PC also leads to unspecific hematologic effects, such as rapid drops in leukocyte and platelet counts.63 In contrast, tamoxifen is regarded as a relatively safe drug, and the long-term application of tamoxifen to mice did not alter the histopathology of major organs and tissues.24,64 Nevertheless, in order to control for unspecific effects, tamoxifen was administered to all experimental groups under investigation.
Tenen and coworkers have used regulatory sequences of the human CD34 locus to target expression of the transactivator gene tta of the tetracycline-responsive expression system65 to hematopoietic progenitor cells (CD34-tTA).66,67 Those mice, when intercrossed with mice transgenic for Cre under the control of the tetracycline-responsive element Tet-O-cre, could provide temporally controlled recombination in HSCs. However, using that system to achieve the conditional null mutation of a gene requires complex breeding strategies because 4 genetically modified alleles are required to be combined.
The Scl-3′ enhancer previously has been used in a retrovirus to target the expression of a transgene to hematopoietic progenitors.68 In contrast to those studies we have found a relatively low targeting efficiency in CFU-S: while we observed recombination in 30% of CFU-S12, Murphy et al68 showed 70% of CFU-S12 were targeted. This discrepancy might be due to transgene integration effects or to differences in the efficiency of Cre-ERT translocation to the nucleus.
In agreement with previous reports regarding the activity of the Scl-3′ enhancer, we observed some activity of the HSC-SCL-Cre-ERT line in endothelial cells. Given the close relationship between the hematopoietic and endothelial lineages, especially during ontogeny, activity in both lineages may be an inseparable feature of enhancers targeting early hematopoietic progenitors.54,67,69
Other groups already have demonstrated that embryonic HSCs were capable of long-term reconstitution in adults,10,11,43,70 suggesting that these cells colonize the bone medullary cavity via the FL and contribute to adult multilineage hematopoiesis. However, these data do not demonstrate this relationship directly. Additionally, it cannot be excluded that de novo–generated HSCs displace their embryonic counterparts during development. Therefore, we genetically marked HSCs on days E10.5/E11.5 of development, because during this time frame the majority of adult LT-HSCs are thought to arise in the AGM region.40 The HSC generation in the AGM has ceased by E13.5.43 By this time the tamoxifen-dependent recombination events in HSCs also should have ceased. Remarkably, we observed that EYFP-tagged embryonic HSCs or their progeny gave rise to a significant proportion of adult hematopoiesis. In parallel to the in vivo fate-tracing approach, we studied the contribution of transplanted EYFP-tagged embryonic HSCs to reconstituting hematopoiesis in adult recipients. Strikingly, the proportions of marked HSCs contributing to multilineage adult hematopoiesis in both approaches were the same. Therefore, we concluded that the de novo generation of HSCs within the embryo must have ceased by E14.5 and that adult BM hematopoiesis arises solely by the colonization of HSCs generated in the midgestation embryo.
Two groups have previously studied the origin of adult BM HSCs. Their data already implied that BM HSCs are derived from the FL.71,72 However, those studies were performed in different species (sheep and rat) and involved transplantation, retroviral marking, and manipulation of the fetus in utero. Additionally, those studies did not allow any quantitative assessment of fetal contribution to adult hematopoiesis.
Others have applied a similar approach to ours using the gpIIb-promoter element driving the expression of Cre recombinase to study the fate of fetal progenitor cells. In that study gpIIb-Cre– mediated recombination was achieved in 60% to 90% of E14.5 FL cells.73 Even though gpIIb (CD41) expression recently has been described as one of the hallmarks of emerging primitive and definitive hematopoietic progenitors,74,75 they were unable to find significant contribution of genetically marked cells to adult BM hematopoiesis. Accordingly, they hypothesized the existence of a separate wave of hematopoiesis, which arises in parallel or at later stages of development. In contrast, our data do not suggest the emergence of a second wave of hematopoiesis during late fetal development because the proportion of embryonically marked cells in adults was identical to the proportion found in adult recipients of HSC-SCL-Cre-ERT–marked E14.5 FL cells. Alternatively, the low adult contribution of gpIIb-cre–marked fetal progenitors could be explained by the absence of critical regulatory elements in the gpIIb-Cre construct, which are responsible for gpIIb expression in the cells that give rise to adult hematopoiesis.
In conclusion, we have derived an HSC-SCL-Cre-ERT transgenic line that can successfully be used to specifically manipulate HSCs in vivo. Using this system we demonstrated the existence of a direct-lineage relationship between embryonic and adult HSCs. Additionally, our results imply that the de novo generation of stem cells of the hematopoietic system is completed in the midgestation embryo.
Prepublished online as Blood First Edition Paper, December 14, 2004; DOI 10.1182/blood-2004-08-3037.
Supported by grants from the Deutsche Forschungsgemeinschaft (GO 953/1-1, J.R.G.), the Wellcome Trust (A.R.G.), the Leukaemia Research Fund (B.G.), and the National Health and Medical Research Council, Australia (#139108, C.G.B.).
An Inside Blood analysis of this article appears in the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Kelly Taggart for excellent technical assistance. We are grateful to Prof Pierre Chambon and Dr Daniel Metzger for providing the plasmid pCre-ERT. The ROSA26R and R26R-EYFP mice were generously provided by Dr Edouard Stanley and Dr Frank Costantini, respectively.


![Figure 7. Fate-tracing embryonic hematopoietic stem cells into adulthood. (A) Outline of the experimental design. Timed matings were set up between HSC-SCL-Cre-ERT males and R26R-EYFP females. Pregnant females were allocated into 3 experimental groups. Group I females did not receive tamoxifen injections, while group II and group III females received tamoxifen injections at E10.5 (1 mg) and E11.5 (2 mg). Group II pregnancies were terminated at E14.5, embryonic fetal liver cells were harvested, analyzed for the expression of Eyfp, and transplanted into lethally irradiated Ly5.1 recipients. Group III neonates were delivered by Caesarean section on day E19.5 and fostered to lactating females. After weaning group I and III, newborn mice were genotyped for the presence of the HSC-SCL-Cre-ERT and R26R-EYFP transgenes. (B) Fate-mapping transplanted (group II) and in vivo–remaining (group III) marked embryonic LT-HSCs: a proportion of group II (left panel) and group III (right panel) embryonic LT-HSCs was genetically marked (EYFP+) by maternal tamoxifen injections. Group II E14.5 fetal liver cells were transplanted into lethally irradiated congenic adult recipients. The proportion of marked fetal liver LT-HSCs was determined by measuring the hematopoietic contribution of these cells within recipients 5 months after transplantation (group II, left panel). In parallel the contribution of in vivo–remaining marked fetal liver LT-HSCs to adult bone marrow hematopoiesis was investigated (group III, right panel). If the proportion of EYFP+ hematopoietic cells within group III adults were found to be significantly lower than within recipient adults (group II), this would mean that the de novo generation of HSCs within the embryo was not completed by the time of transplantation (E14.5). If new LT-HSCs were generated after day 14.5 of gestation, these cells would not be marked (absence of tamoxifen) and would therefore decrease the proportion of group III adult EYFP+ hematopoietic cells compared to group II. (C) Five months after birth or transplantation, respectively, flow cytometric analysis of Eyfp expression in hematopoietic organs of group I, II, and III mice was carried out. Representative plots of total bone marrow from each group are shown. Percentages represent means. FSC, forward light scatter. (D) Comparison of mean percentages of Eyfp-expressing cells within different hematopoietic populations (bone marrow: total cells, neutrophils [Mac-1+Gr-1high], B cells [B220+], erythroid [Ter119+], HSCs [KSL]; thymus: double-positive cells [CD4+CD8+]) of group II (□) and group III mice (▪). No statistical significant difference between the mean percentages of groups II and III was noted. In group II recipients 99.1 ± SD 0.5% of bone marrow cells were donor derived (CD45.2+). Error bars indicate standard deviations. Group I (n = 2); group II (n = 4 donor embryos, n = 2 recipients per donor); group III (n = 4).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/105/7/10.1182_blood-2004-08-3037/6/m_zh80070576250007.jpeg?Expires=1768023162&Signature=YiiqsK2nVZoI~5qZn-pMrgx66qDRtF5z8-AxTZbwGqlN1zR8Hcw7m2J23wZxBDepqsQNTvFzT3afsLgFoiVph4yVsm2w-KuHZbjR4vOPXZegv3Usz35a3RyI8C~j7~lwi7uTsFbzuhqlwq4iFVI5Km2Ka-s3-4A2JwWBrBLGtj~3iLkfwBFN1FhaBpOiYc5DudPvQZ27LJ15AHs-DEnKU4~WY7I2ZyF8djjSpC0dXwF8cRlLHkDeSk7Vl~AkKnasEYUmJ0uAclQaqJ4w8jAG6tTRKQIh2bBaFSoZawlo88wJTOIcVOmp0N0KanQS47ZOuqQUsKv3oSI0oaE2FwbVmg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)


![Figure 7. Fate-tracing embryonic hematopoietic stem cells into adulthood. (A) Outline of the experimental design. Timed matings were set up between HSC-SCL-Cre-ERT males and R26R-EYFP females. Pregnant females were allocated into 3 experimental groups. Group I females did not receive tamoxifen injections, while group II and group III females received tamoxifen injections at E10.5 (1 mg) and E11.5 (2 mg). Group II pregnancies were terminated at E14.5, embryonic fetal liver cells were harvested, analyzed for the expression of Eyfp, and transplanted into lethally irradiated Ly5.1 recipients. Group III neonates were delivered by Caesarean section on day E19.5 and fostered to lactating females. After weaning group I and III, newborn mice were genotyped for the presence of the HSC-SCL-Cre-ERT and R26R-EYFP transgenes. (B) Fate-mapping transplanted (group II) and in vivo–remaining (group III) marked embryonic LT-HSCs: a proportion of group II (left panel) and group III (right panel) embryonic LT-HSCs was genetically marked (EYFP+) by maternal tamoxifen injections. Group II E14.5 fetal liver cells were transplanted into lethally irradiated congenic adult recipients. The proportion of marked fetal liver LT-HSCs was determined by measuring the hematopoietic contribution of these cells within recipients 5 months after transplantation (group II, left panel). In parallel the contribution of in vivo–remaining marked fetal liver LT-HSCs to adult bone marrow hematopoiesis was investigated (group III, right panel). If the proportion of EYFP+ hematopoietic cells within group III adults were found to be significantly lower than within recipient adults (group II), this would mean that the de novo generation of HSCs within the embryo was not completed by the time of transplantation (E14.5). If new LT-HSCs were generated after day 14.5 of gestation, these cells would not be marked (absence of tamoxifen) and would therefore decrease the proportion of group III adult EYFP+ hematopoietic cells compared to group II. (C) Five months after birth or transplantation, respectively, flow cytometric analysis of Eyfp expression in hematopoietic organs of group I, II, and III mice was carried out. Representative plots of total bone marrow from each group are shown. Percentages represent means. FSC, forward light scatter. (D) Comparison of mean percentages of Eyfp-expressing cells within different hematopoietic populations (bone marrow: total cells, neutrophils [Mac-1+Gr-1high], B cells [B220+], erythroid [Ter119+], HSCs [KSL]; thymus: double-positive cells [CD4+CD8+]) of group II (□) and group III mice (▪). No statistical significant difference between the mean percentages of groups II and III was noted. In group II recipients 99.1 ± SD 0.5% of bone marrow cells were donor derived (CD45.2+). Error bars indicate standard deviations. Group I (n = 2); group II (n = 4 donor embryos, n = 2 recipients per donor); group III (n = 4).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/105/7/10.1182_blood-2004-08-3037/6/m_zh80070576250007.jpeg?Expires=1768023163&Signature=0Ty2dBGF338M4O8odRFLEJwn6LYZ3beBGrNSavCnGoFToxqmnqOT32lSjFQLfFGVMg3C6DXQspXiE~fz7iF~otSy8ZMvk7S6HW~H5gjHIS38AnatNoqLVp-3bih-OXsEbA7E721XTIqzRKgVUQvhfT4h1WdJB4mxCvCqyNOumiUtckponv5lHfnCUHqyU-NBwBdymLHcGKNSCg8riOgZyLuM8SenbO7uhikLLhQT8ud-hmO12Sm~ncilrJogo1kjPz73S~hzgXFRe-Ca24oI91iMMs-4PYfEgoOLQ058ISetMB8KHyYcv29A-Bz8rJQqGzrkf0ZvO3UI13sJJJEZ3w__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)