Abstract
The single nucleotide polymorphism (SNP) Ser128Arg in the E-selectin gene is overrepresented in certain patient groups with atherosclerosis or restenosis. We hypothesized and tested whether it may affect cytokine-induced levels of soluble (s) E-selectin, or be associated with proinflammatory or procoagulant properties in a well-standardized inflammation model. Healthy male volunteers (n = 157) received a lipopolysaccharide (LPS) infusion and were genotyped for the S128R SNP, and outcome parameters were measured by enzyme immunoassays and real-time polymerase chain reaction (RT-PCR, Taqman). The S128R SNP had no pronounced effects on basal or inducible sE-selectin levels, or levels of tumor necrosis factor or interleukin-6. However, carriers of the S128R SNP had 20% higher monocyte counts at 24 hours after LPS infusion. Importantly, the S128R allele enhanced thrombin generation by 50% to 80%, as measured by prothrombin fragment F1+2 (P < .01), and hence fibrin formation (D-dimer) 2-fold (P = .01 to P = .002). However, tissue factor (TF) mRNA levels were not affected. The S128R E-selectin genotype is associated with procoagulant effects in a human model of endotoxin-induced, TF-triggered coagulation. This could contribute to its linkage with various thrombotic cardiovascular disorders.
Introduction
The selectins mediate the rolling of leukocytes on endothelial cells (ECs).1 E-selectin is exclusively expressed on ECs, mostly after their activation.2 E-selectin is expressed3 and proteolytically cleaved from the surface of ECs upon activation by different stimuli,4 including endotoxin. In vivo, such a shedding leads to an increase in plasma levels of soluble (s) E-selectin.5 Further, sE-selectin levels correlate with its surface expression on ECs in vitro.6
Thus, sE-selectin serves as an excellent marker for endothelial activation in numerous cardiovascular and inflammatory diseases.7 Interestingly, the common Ser128Arg polymorphism has been reported to regulate plasma levels of sE-selectin.8,9 This polymorphism is functional in that it alters ligand affinity:10 E-selectin has an aminoterminal C-type lectin domain that is thought to possess the carbohydrate binding site that binds the sialylated Lewisx antigen (sLex or CD15s) (Neu5Acalpha2-3Galbeta1-4(Fucalpha1-3)GlcNAc). Substitution of E-selectin endothelial growth factor (EGF) domain residue Ser128 with an arginine results in E-selectin proteins that have lost the requirement for alpha1-3–linked fucose and are thus able to bind to sialyllactosamine.10 Likely as a consequence, this SNP enhances tethering of myeloid cells11 and extends the range of lymphocytes recruited by E-selectin.12 Further, S128R-transduced ECs support significantly more rolling and adhesion of neutrophils and mononuclear cells compared with ECs transduced with wild-type E-selectin. These S128R-transduced ECs also exhibit significantly greater levels of phosphorylation of extracellular signal–regulated kinase 1 and 2 and p38 mitogen-activated protein kinase. This suggests that an altered endothelial signaling pathway is associated with this polymorphism.13 Clinically, the polymorphism has been associated with atherosclerosis,14-16 myocardial infarction,13 and restenosis after angioplasty,9,17 but the underlying mechanism is unclear.
The low-grade endotoxemia model in humans is well standardized and permits to elucidate potential roles and interactions of key players during systemic inflammation and coagulation.18,19 It has been proposed that the S123R genotype may modulate cytokine-induced E-selectin expression in diabetic patients.8 Accordingly, we hypothesized that and tested whether the polymorphism may increase sE-selectin levels induced by cytokines after EC activation by endotoxin. Further, we hypothesized that the S128R SNP may be associated with procoagulant properties, because an animal model suggests that E-selectin may regulate thrombus formation and its fibrin content.20,21
Materials and methods
The study protocols were approved by the ethics committee of the Medical University Vienna, and all participants gave written informed consent before entering the study. We obtained data and DNA samples from 157 healthy male volunteers who had participated in several clinical trials in which they had received endotoxin (LPS) only, including recently published studies22-26 and several unpublished trials.
All participants were nonsmokers, 19 to 40 years of age, with a body mass index between the 15th and 85th percentiles. Determination of health status included a medical history, physical examination, laboratory values, and virologic and standard drug screening as previously described.27 Exclusion criteria were regular or recent intake of medications, including nonprescription medications, and clinically relevant abnormal findings in the medical history or laboratory values.
The experimental procedures of our LPS infusion studies have been described in different studies.28 Briefly, volunteers were admitted to the study ward at 8:00 am after an overnight fast. Throughout the entire study period, participants were confined to bed rest and kept fasting for 8.5 hours after LPS infusion. Participants received an intravenous bolus containing 2 ng/kg LPS (National Reference Endotoxin, Escherichia coli; U.S. Pharmacopeia, Rockville, MD).
Blood samples were collected by venipuncture into vacutainer tubes containing EDTA (ethylenediaminetetraacetic acid) anticoagulant (Becton Dickinson, Vienna, Austria) before LPS infusion and thereafter at the times indicated in Figures 1 and 2. Plasma samples were processed immediately by centrifugation at 2000g at 4°C for 15 minutes and stored at -80°C before batch analysis. The following commercially available enzyme immunoassays were used: plasma levels of prothrombin fragment (F1+2; Enzygnost TAT micro; Behring, Marburg, Germany) were used as a marker of in vivo thrombin generation, D-dimer (Boehringer Mannheim, Mannheim, Germany) as an indicator of fibrin formation (STA-analyzer; Diagnostica Stago, Parsippany, NJ), and plasmin-antiplasmin complexes (PAPs; Enzygnost PAP micro; Behring) as a marker of endogenous fibrinolytic capacity. Tumor necrosis factor (TNF), interleukin-6 (IL-6), and soluble E-selectin levels were measured with enzyme immunoassays from R&D Systems (Oxon, United Kingdom). Interassay variability of all assays ranges from 3% to 9%. Differential blood counts were obtained with a cell counter (XE-2100; Sysmex, Milton Keynes, United Kingdom). Plasma levels of C-reactive protein were determined by a nephelometric assay using reagents from Olympus, on an automated analyzer (Olympus System Reagent C-reactive protein, OSR 6147; Olympus, Melville, NY).
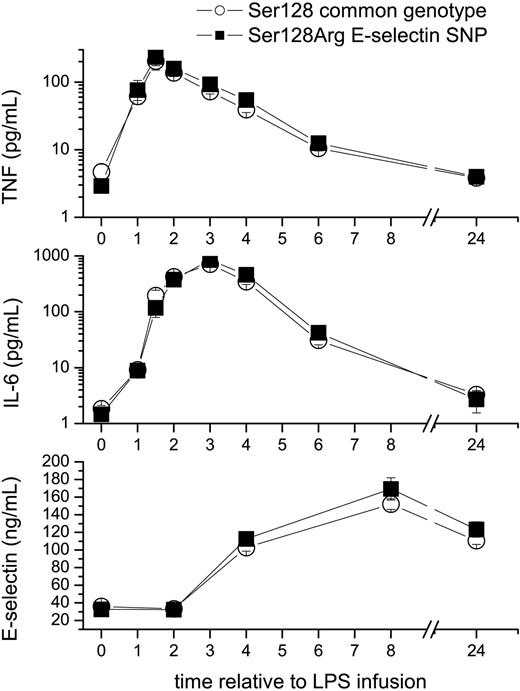
Effects of the S128R E-selectin polymorphism on LPS-induced plasma levels of cytokines and soluble (s) E-selectin. Healthy male volunteers (n = 157) received an intravenous infusion of 2 ng/kg endotoxin (LPS). The polymorphism (▪) slightly enhanced plasma levels of tumor necrosis factor alpha (TNF, top) at 3 hours, and interleukin-6 (IL-6) levels at 6 hours (middle) by 30%, but had no major effect on basal or cytokine-induced plasma levels of soluble (s) E-selectin (bottom). □ indicates Ser128 common genotype. Data are mean plus or minus standard error of the mean (SEM). To enhance the visibility values in the low range, cytokines are plotted on a logarithmic scale.
Effects of the S128R E-selectin polymorphism on LPS-induced plasma levels of cytokines and soluble (s) E-selectin. Healthy male volunteers (n = 157) received an intravenous infusion of 2 ng/kg endotoxin (LPS). The polymorphism (▪) slightly enhanced plasma levels of tumor necrosis factor alpha (TNF, top) at 3 hours, and interleukin-6 (IL-6) levels at 6 hours (middle) by 30%, but had no major effect on basal or cytokine-induced plasma levels of soluble (s) E-selectin (bottom). □ indicates Ser128 common genotype. Data are mean plus or minus standard error of the mean (SEM). To enhance the visibility values in the low range, cytokines are plotted on a logarithmic scale.
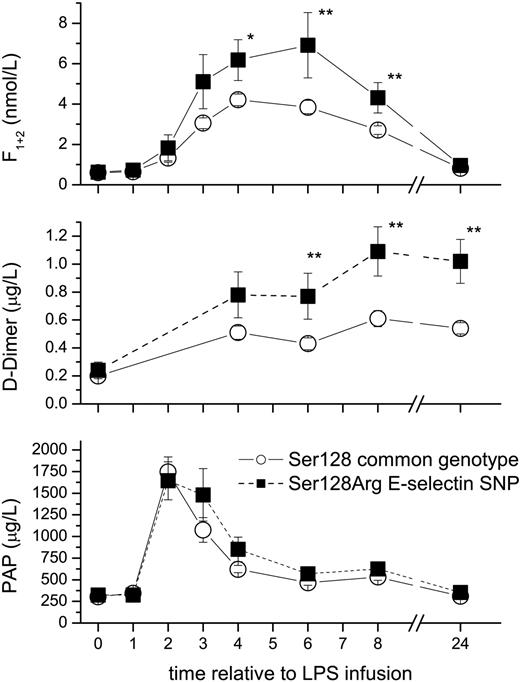
The S128R E-selectin genotype enhances LPS-induced activation of coagulation. Healthy male volunteers (n = 157) received an intravenous infusion of 2 ng/kg LPS. The polymorphism (▪) enhanced thrombin generation by 50% to 80% as measured by prothrombin fragment F1+2 levels (top), and increased fibrin generation 2-fold as measured by D-dimer levels (middle), but had no significant effect on fibrinolysis as measured by plasmin antiplasmin (PAP) complexes (bottom). □ indicates Ser128 common genotype. Data are mean plus or minus SEM; *P < .05, **P < .01 between genotypes.
The S128R E-selectin genotype enhances LPS-induced activation of coagulation. Healthy male volunteers (n = 157) received an intravenous infusion of 2 ng/kg LPS. The polymorphism (▪) enhanced thrombin generation by 50% to 80% as measured by prothrombin fragment F1+2 levels (top), and increased fibrin generation 2-fold as measured by D-dimer levels (middle), but had no significant effect on fibrinolysis as measured by plasmin antiplasmin (PAP) complexes (bottom). □ indicates Ser128 common genotype. Data are mean plus or minus SEM; *P < .05, **P < .01 between genotypes.
RT-PCR analysis of tissue factor messenger RNA levels and PCR analysis of the Ser128Arg polymorphism
At 0, 3, 5, and 24 hours after LPS infusion, blood was analyzed for TF-mRNA, which was determined by real-time–polymerase chain reaction (RT-PCR) reagents and equipment as previously described in detail.22,23,25 Presence of the S128R E-selectin polymorphism was determined exactly as described in different studies.9,29
Statistics
A sample size calculation was performed, which indicated that we had an 80% power (∝ = 0.05) to detect a 10% difference in basal or peak sE-selectin levels.30 This was deemed sufficient in view of the reported effect size (20%-40%) of the polymorphism on sE-selectin levels in different patient groups.8,9 Data are expressed as mean and 95% confidence interval (CI). Because of skewed data, repeated measures analysis of variance (ANOVA) was performed on log transformed data, and differences between groups were confirmed by U tests. The Spearman rank correlation was used to measure correlation between parameters. A 2-tailed P value of less than .05 was considered significant. All statistical calculations were performed using Statistica (Vers. 6.0; Stat Soft, Tulsa, OK).
Results
Distribution of genotypes
Of all 157 individuals tested, 114 (72.6%) exhibited the E-selectin S128 genotype, 40 (25.5%) were heterozygous S128R, and 3 (1.9%) were homozygous for the 128R allele. The 3 homozygous individuals were not analyzed separately because of insufficient power but were pooled with the heterozygous subjects for statistical comparisons. The genotype distribution was within the Hardy Weinberg equilibrium and was as expected from previous studies in the Austrian population.9,29 Basal values of all parameters are presented in Table 1 and were not different between genotypes.
Effects on soluble (s)E-selectin levels and differential blood counts
The S128R single nucleotide polymorphism (SNP) had no pronounced effects on basal or inducible sE-selectin levels (Figure 1). As the S128R SNP may affect tethering of leukocytes,11-13 we were interested in studying the kinetics of leukocyte counts. Although nadir levels of monocyte counts were identical between groups (0.05 109/L [CI: 0.04-0.06]), monocyte counts were 20% higher in heterozygous S128R subjects (0.63 109/L [CI: 0.54-0.71]) as compared with the S128 genotype (0.50 109/L [CI: 0.45-0.54]; P = .003) 24 hours after LPS infusion. In contrast, nadir lymphocyte counts were slightly lower in S128R SNP carriers (0.52 109/L [CI:0.47-0.56]) than in the S128 genotype (0.61 109/L [CI: 0.56-0.67]).
Effects on proinflammatory cytokines TNF and IL-6
The S128R SNP was not associated with pronounced differences in cytokine levels between groups. SNP carriers had slightly higher TNF levels 3 hours after LPS challenge (92 pg/mL [CI: 76-108]) than carriers of the common variant (72 pg/mL [CI: 61-84]). Interleukin-6 levels were also slightly higher in the S128R SNP carriers (42 pg/mL [CI: 20-64]) compared with the S128 genotype (31 pg/mL; [CI: 20-42]) at 6 hours.
Effects on coagulation and fibrinolysis
LPS infusion increases thrombin generation with peak levels seen at 4 to 6 hours (Figure 2). The S128R SNP enhanced peak thrombin generation by 50% as measured by F1+2 levels at 4 hours (P = .045; Figure 2), and by 60% to 80% during the later phase of coagulation (6 hours and 8 hours; P < .01). Consistently and consequently, fibrin generation as measured by D-dimer levels was up to 2-fold higher in SNP carriers than in the subjects homozygous for S128 at 6, 8, and 24 hours (P = .01 to P = .002). In contrast, there was no difference in the induction of tissue factor expression as quantified by TF-mRNA levels (25-fold [CI: 5-44] in S128R SNP carriers 5 hours after LPS versus 19-fold [CI: 12-25] in S128 homozygous subjects) or activation of fibrinolysis as quantified by PAP levels (Figure 2) between genotypes.
Correlations
As TNF levels determine sE-selectin, in this model we looked for a correlation between those parameters: there was a consistent and highly significant correlation (P < .001) between TNF levels measured from 1 hour to 6 hours and peak E-selectin levels measured at 8 hours and 24 hours (r = 0.25-0.69).
Adverse effects
As reported in different previous studies, the 2 ng/kg dose of endotoxin was tolerated well by all the volunteers without any serious or severe adverse events. As expected, transient flulike symptoms (average temperature 37.5°C) were encountered and treated with paracetamol on demand, which does not affect inflammation.5,31
Discussion
Our study provides 2 findings of interest. First, the S128R polymorphism in the E-selectin gene is not a major regulator of sE-selectin levels in healthy men (Figure 1). Second, the major finding is a hitherto undiscovered procoagulant effect associated with the S128R SNP (Figure 2).
Low-grade endotoxemia induces marked endothelial cell activation as demonstrated by an increase in plasma levels of sE-selectin (Figure 1), von Willebrand factor, or thrombomodulin.5,27 The lack of an effect on inducible E-selectin release can be explained by the finding that sE-selectin release is strongly inhibited by a TNF antagonist.32 Thus, the primary regulator of sE-selectin in this model is TNF, which is also indicated by the correlation between those parameters, but peak levels of TNF (at 2 hours) were only slightly (10%) higher in carriers of the S128R allele. However, the lack of difference in basal sE-selectin levels between genotypes complements previous studies: we observed 20% higher sE-selectin levels in patients with peripheral arterial disease carrying the S128R SNP as compared with S128 homozygotes.9 sE-selectin levels were also 40% higher in diabetic S128R SNP carriers.8 However no difference between genotypes (< 2%) was seen in relatives of these diabetic patients or in healthy controls8 as well as in another study in patients undergoing coronary angioplasty.17
Recently, it has been demonstrated that E-selectin is an important regulator of thrombus formation and fibrin content in a mouse venous thrombosis model.20,21 The thrombus weight in E-selectin wild-type mice was 3-fold higher than in the E-selectin knockout mice. Our major finding is in line with this observation and suggests that particularly the S128R allele of E-selectin regulates coagulation in humans: it markedly increased thrombin and fibrin formation in response to LPS (Figure 2). As sE-selectin levels were similar between groups (Figure 1), the observed effect is unlikely due to differences in the amount of released sE-selectin. The same may apply for endothelial expressed E-selectin, although this can only be assessed by invasive biopsies in future studies. The minor increase in cytokine release is unlikely of clinical relevance and/or responsible for the enhanced activation of coagulation, not only because experimental inhibition of TNF or IL-6 activity has little effect on coagulation in this model.23,32 Conversely, effective anticoagulation does not affect cytokine release in this setting.27,33,34 However, monocytes are highly responsive to LPS due to their high CD14 expression and TLR expression and are capable of secreting cytokines and initiating coagulation. The increased monocyte counts 24 hours after LPS infusion could point toward a potential change in the leucokinetics of monocytes. Monocytes can interact with E-selectin, and the S128R SNP–associated alterations in E-selectin enhance the interaction with myeloid cells.10,11 One may speculate that this observed in vitro interaction could be the operative mechanism of action in vivo, how the S128R SNP enhances coagulation. Apart from modulation in cytokine or TF transcription, changes in TF encryption or release of TF-containing microparticles could result in monocytes. No differences in TF mRNA levels were seen at least in circulating monocytes, which could have provided an explanation for heightened procoagulant responses in S128R SNP carriers. However, extrapolation to the large pool of marginated monocytes (> 90%) is impossible during times of profound monocytopenia, so that we cannot entirely rule out an effect on TF transcription. Further, S128R-transduced ECs also exhibit more pronounced phosphorylation of extracellular signal–regulated kinase 1 and 2 and p38 mitogen-activated protein (MAP) kinase. This suggests that an altered endothelial signaling pathway is associated with this polymorphism.13 However, it is uncertain whether the effects of the S128R SNP on coagulation were mediated by p38 MAP kinase, because various effects on cytokines or fibrinolysis would be expected from previous studies employing a p38 MAP kinase inhibitor.35 Interestingly, the sLex blocking selectin inhibitor bimosiamose does not decrease coagulation in our LPS model (Florian B. Mayr and B.J., unpublished data, 2005). The S128R mutation results in E-selectin that has lost the requirement for a 1- to 3-linked fucose.10 Thus, the S128R SNP effects on coagulation could be mediated by sLex-independent E-selectin ligands. However, further trials are necessary to clarify the precise mechanism how the S128R polymorphism in the E-selectin gene enhances coagulation.
Nonetheless, the current study is the first to demonstrate that the S128R allele is associated with procoagulant effects. This is of potential clinical relevance: it may not only contribute to the association of the S128R SNP to atherosclerosis and restenosis9,13-16 but may have far-reaching consequences for many thrombotic vascular disorders. This should be the subject of further scrutinized studies.
There are obvious limitations to our study: first, the observed effect could also be caused by less-characterized polymorphisms, which could be in linkage disequilibrium with the examined E-selectin polymorphism. Second, the number of homozygous S128R SNP carriers was too small to allow any conclusions. As previously discussed,22 our inflammation model, although standardized and highly reproducible, is by no means a sepsis model. Thus, extrapolation to the potential role of this SNP in septic patients is not possible. Also, women were not studied.
In conclusion, the S128R genotype of the E-selectin gene is associated with a pronounced procoagulant effect, which could contribute to its linkage with thrombotic cardiovascular disorders.
Prepublished online as Blood First Edition Paper, November 16, 2004; DOI 10.1182/blood-2004-09-3752.
Supported in part by grants from the Austrian Science Fund (FWF no. P15363) and the Austrian National Bank (no. 9584) and die Virologie-Fonds zur Förderung der Molekularen Grundlagenforschung (O.F.W.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.