Abstract
Tissue factor (TF) triggers upstream coagulation signaling via the activation of protease-activated receptors (PARs) of relevance for inflammation and angiogenesis. TF pathway inhibitor 1 (TFPI-1) is the physiologic inhibitor of TF-initiated coagulation, but its role in regulating TF signaling is poorly understood. Here, we demonstrate that endogenous, endothelial cell-expressed TFPI-1 controls TF-mediated signaling through PARs. In endothelial cells transduced with TF to mimic exacerbated TF expression in vascular cells, TF-VIIa-Xa ternary complex-dependent activation of PAR1 remained intact when TF-mediated Xa generation was blocked with 2.5 to 5 nM recombinant TFPI-1 (rTFPI-1). Concordantly, inhibition of signaling in PAR1-expressing Chinese hamster ovary (CHO) cells required about 30-fold higher rTFPI-1 concentrations than necessary for anticoagulation. Studies with proteoglycan-deficient CHO cells document a crucial role of accessory receptors in supporting the anticoagulant and antisignaling activities of rTFPI-1. Coexpression of PAR2 with TF enhanced rTFPI-mediated inhibition of TF-VIIa-Xa–mediated PAR1 signaling, suggesting an unexpected role of PAR2 in the inhibitory control of TF signaling. These experiments are of potential significance for the limited therapeutic benefit of rTFPI-1 in systemic inflammation and recommend caution in using anticoagulant potency as a measure to predict how efficacious TF-directed inhibitors block cell signaling during initiation of coagulation.
Introduction
Activation of coagulation and cell signaling are interconnected by multiple pathways. Coagulation proteases signal via cleavage of protease-activated receptors (PARs), a family of G protein-coupled receptors with 4 known members (PAR1-PAR4).1-3 The overlap in PAR signaling induced by different coagulation proteases, the partial redundancy between PARs, as well as possible intracellular cross-talk between PARs, make it challenging to distinguish the pathophysiologic significance of these signaling pathways.4 Activation of coagulation by the tissue factor (TF) pathway is common in systemic and local inflammation.5 In vivo, PAR signaling has generally been attributed to activation by thrombin.1 However, our recent study has provided clear evidence for relevant thrombin-independent, TF-mediated PAR2 signaling in angiogenesis in vivo.6 TF-dependent signaling is either by the TF-VIIa protease complex specifically through PAR27,8 or by the nascent product Xa in the ternary TF-VIIa-Xa complex that signals through PAR29,10 as well as PAR1,8-11 the first recognized thrombin receptor.
Tissue factor pathway inhibitor 1 (TFPI-1) is the central endogenous regulator of coagulation activation by TF.12-15 Although purified TFPI-1 at high concentrations can inhibit TF-VIIa,16 efficient inhibition by TFPI-1 is dependent on Xa that is generated during TF-dependent initiation of coagulation. TFPI-1 contains 3 Kunitz-type protease inhibitor domains and a C-terminal polybasic region. The second domain binds and inhibits Xa, and TFPI-1 can thus act as a direct protease inhibitor of Xa. Kunitz domain 1 inhibits VIIa in complex with TF.12,14,15 Endogenous TFPI-1 is directly or indirectly anchored by glycosylphosphatidylinositol (GPI) on cell surfaces.17-23 Lipoprotein receptor-related protein (LRP) and heparan sulfate proteoglycan (HSPG) have been implicated in the binding and internalization of recombinant forms of TFPI-1, depending on their C-terminal polybasic portions.24,25 Endothelial cells, which are normally deficient in LRP, may bind TFPI-1 via HSPG and the very-low-density lipoprotein receptor.26,27 Moreover, intravenously administered [125I]-TFPI-1 is rapidly cleared from the circulation, a process that seems to involve both LRP and HSPG.28 However, there is no direct evidence to support a role for proteoglycans (PGs) in the regulation of TF-dependent PAR signaling by TFPI.
The function of TFPI-1 as the physiologic inhibitor of TF-initiated coagulation has prompted interest in applying recombinant TFPI-1 (rTFPI-1) as a substitution therapy to counterbalance exacerbated coagulant responses in severe inflammation. Although TFPI-1 showed positive effects in several animal sepsis models,29-31 no effect on 28-day mortality in septic patients could be established in a phase 3 clinical study.32,33 Anticoagulant efficacy was documented in this trial by measurements of coagulation markers. However, interference with TF-dependent signaling could not be evaluated because of lack of available surrogate markers. In addition, few data are available on how rTFPI-1 inhibits TF-dependent signaling through PARs in vitro. In this study, we characterized inhibition of cell signaling by endogenous TFPI-1 and rTFPI-1. Because TFPI-1 targets the ternary TF-VIIa-Xa initiation complex, we focused on the characterization of how endogenous or recombinant TFPI-1 inhibited the signaling of this complex via PARs. Although rTFPI-1 is a potent inhibitor of TF-initiated coagulation, signaling of the ternary initiation complex was found to be inhibited with relatively poorer efficiency. We identify cell surface PG and PAR2 expression as additional variables that determine how potently rTFPI-1 inhibits signaling of the TF initiation complex. Because these data indicate that coagulation can be blocked without inhibition of cell signaling, caution is warranted to base clinical usage of TF-targeted inhibitors solely on their anticoagulant potency.
Materials and methods
Materials
Factors VIIa, X, and Xa, polyclonal anti-PAR2 antibody, and anti-TF antibodies were from the same source as described.2,9,34 Chromogenic substrate Spectrozyme FXa (American Diagnostics, Greenwich, CT), anti-PAR2 monoclonal antibody (mAb) SAM11 (Santa Cruz Biotechnology, Santa Cruz, CA), hirudin (Calbiochem, La Jolla, CA), and phospho–extracellular signal–regulated kinase (ERK1/2) and nonphospho-ERK1/2 antibodies (Cell Signaling Technology, Beverly, MA) were obtained commercially. Recombinant human TFPI-1 produced in Escherichia coli was generously provided by Dr A. Creasey (Chiron, Emeryville, CA). Anti-PAR1 antibodies ATAP2 and WEDE-15, and recombinant nematode anticoagulant proteins 5 and c2 (NAP5; NAPc2), were kindly provided by Dr L. F. Brass (University of Pennsylvania, Philadelphia, PA) and Dr G. Vlasuk (Corvas International, San Diego, CA), respectively.
Cell culture
Chinese hamster ovary (CHO) K1 cells expressing full-length TF (wildtype CHO/TF) were the same as previously described.35 PG-deficient CHO cells (pgsA-745), which are deficient in xylosyl transferase and express approximately 5% PG as compared with wild-type cells36 (kindly provided by Dr Esko, University of California, San Diego), were transfected with human TF cDNA in pcDNA3.1/Zeo. Stable clones expressing similar levels of TF as wild-type CHO/TF cells were screened by flow cytometry, using fluorescein isothiocyanate (FITC)–labeled anti-TF mAb 10H10. Both TF-expressing CHO cells were routinely grown in Dulbecco modified Eagle medium (DMEM), supplemented with 2 mM glutamine, 1 mM nonessential amino acids (all from Invitrogen, Carlsbad, CA), and 10% newborn calf serum (Omega Scientific, Tarzana, CA). Primary human umbilical vein endothelial cells (HUVECs) were grown in endothelial growth medium (EGM) containing 1 mg/mL hydrocortisone, 10 μg/mL human endothelial growth factor (hEGF), 3 mg/mL bovine brain extract, and 2% fetal bovine serum (Clonetics, Walkersville, MD).
Factor Xa activity analysis and [125I]-Xa internalization assay
Factor X activation was determined at various time intervals after addition of VIIa (10 nM) and X (100 nM) to confluent HUVECs or CHO cells. Aliquots of the incubation medium were quenched with 100 mM EDTA (ethylenediaminetetraacetic acid), and the Xa activity in the supernatant was determined by hydrolysis of the chromogenic substrate Spectrozyme FXa. For [125I]-Xa internalization, wild-type and PG-deficient CHO cells were grown to confluence in 24-well plates, washed with cold phosphate-buffered saline (PBS) 5% bovine serum albumin (BSA), and then incubated in the same buffer with 0, 10, or 100 nM rTFPI-1 for 30 minutes on ice. Cells were then washed with PBS 5% BSA, followed by another incubation with 50 nM [125I]-Xa for 15 minutes at 37°C. Cells were finally detached by trypsin/EDTA treatment and extensively washed, and internalized [125I]-Xa was determined in a γ counter. Results were corrected for nonspecific [125I]-Xa binding by performing identical experiments in wells that did not contain cells.
Adenoviral transduction of endothelial cells
Ad5 serotype vectors expressing either full-length human TF, or Cys245-Ser–mutated TF or PAR2 were described in detail.10,34 HUVECs grown to 50% to 60% confluence were transduced with wild-type or Cys245-Ser–mutated TF (50 virus particles/cell) with or without PAR2 or a PAR2 mutant that carries a Glu mutation at the scissile bond Arg residue (100 virus particles/cell) for 3 hours, and grown for 48 hours, followed by change to serum-free conditions for 5 hours in the presence or absence of tumor necrosis factor-α (TNF-α; 5 ng/mL) prior to the addition of agonists. In all experiments the thrombin inhibitor hirudin (200 nM) was included during agonist stimulation. In some cases cells were incubated with anti-TF, anti-PAR1, or anti-PAR2 antibody, with or without various concentrations of rTFPI-1 for 10 minutes prior to the addition of agonists.
Western blotting and real-time PCR
After agonist incubation for 10 minutes as described, cells were washed with ice-cold PBS and lysed in sodium dodecyl sulfate (SDS) loading buffer for Western blot analysis with phosphorylation-specific (αPTFCD) and pan-specific (αTFCD) antibody to the TF cytoplasmic domain, or with a phospho-specific ERK1/2 and nonphospho-ERK1/2 antibody as previously described.9,10 For TR3 nuclear orphan receptor gene induction, TF ternary initiation reaction was quenched to inhibit Xa activity by the addition of 1 μM NAP5 peptide after 10 minutes of stimulation, followed by incubation for a total of 90 minutes to allow for mRNA induction. TaqMan (Applied Biosystems, Foster City, CA) real-time reverse transcription–polymerase chain reaction (RT-PCR) was performed with reverse-transcribed RNA from stimulated cells. TaqMan probes were custom designed for the genes of interest using primer express program (Applied Biosystems). All samples were normalized with human glyceraldehyde phosphate dehydrogenase (GAPDH) probe and primers obtained from Applied Biosystems.
Results
TFPI-1–mediated inhibition of TF-dependent coagulation and PAR signaling in wild-type and PG-deficient CHO cells
Because of the previously demonstrated utility of CHO cell models to study TF-dependent signaling,9 we first addressed whether regulation of TF initiation complex signaling by rTFPI-1 is dependent on cell surface PG receptors. PG-deficient CHO cells (pgsA-745) that are mutated to express only about 5% PG as compared with wild-type CHO cells36 were stably transfected with TF to achieve comparable levels of TF expression (Figure 1A). TF expression in wild-type and PG-deficient CHO cells yielded similar rates of Xa generation, when cells were incubated with VIIa and X (Figure 1B), indicating that PG per se has no effect on TF-dependent proteolytic activation of macromolecular substrate X. It has previously been shown that cellular uptake of Xa can be mediated via complex formation with rTFPI-1, and that internalization of this complex is inhibited by enzymatic removal of cell surface PG.25 To confirm that the PG-deficient CHO cells are defective in TFPI-1 interaction, we studied rTFPI-1–dependent internalization of radiolabeled Xa. Addition of 10 nM rTFPI-1 to wild-type cells stimulated internalization of Xa about 2.5-fold, but no enhanced uptake was seen in PG-deficient cells. At 100 nM rTFPI-1, Xa uptake was increased 6.5-fold in wild-type cells, but only 2.5-fold in PG-deficient cells (Figure 1C). These results provide genetic evidence for a role of cell surface PG in TFPI-1–mediated internalization of Xa and thus indirectly demonstrate decreased rTFPI-1 binding to PG-deficient CHO cells. These data also indicate that these cells are a suitable model to address the role of PG binding on inhibitory function of rTFPI-1 in TF-initiated coagulation and signaling.
PG-dependence of inhibition of TF-mediated coagulation and signaling. (A) TF expression was determined by fluorescence-activated cell sorting (FACS) analysis of stably expressing human TF in CHO-K1 (wild-type CHO/TF) and pgsA-745 (PG-deficient CHO/TF) cell lines. Isotype-matched immunoglobulin G (IgG) was used as control. MF indicates mean fluorescence values. (B) Xa generation on monolayers of confluent TF-expressing wild-type (□) and PG-deficient CHO cells (○). Cells were incubated at 37°C with 10 nM VIIa and 50 nM X, and Xa generation was determined by chromogenic assay at the indicated times. Results are mean ± SEM (n = 5). (C) rTFPI-1–mediated internalization of Xa. Confluent wild-type (□) and PG-deficient CHO cells (▪) were incubated with rTFPI-1 in PBS 5% BSA for 30 minutes on ice and washed with the same buffer. Internalization of 50 nM [125I]-Xa was carried out for 15 minutes at 37°C, and internalized [125I]-Xa was determined by counting trypsinized and extensively washed cell pellet with background subtraction of cell-free wells subjected to the same procedure. Results are expressed relative to cells receiving no rTFPI-1, mean ± SEM (n = 5). (D) Inhibition of TF-VIIa–dependent Xa generation was determined by adding VIIa (10 nM) and X (50 nM) in the presence of the indicated concentrations of rTFPI-1 for 15 minutes at 37°C and determination of Xa by chromogenic assay; mean ± SEM (n = 6). (E) Representative Western blots of wild-type and PG-deficient CHO cells stimulated with VIIa (10 nM) and X (100 nM) in the presence of the indicated concentrations of rTFPI-1 for 10 minutes at 37°C. Phosphorylated ERK1/2 was detected with phospho-specific ERK1/2 antibody (α-PERK1/2) with loading control with αERK1/2. (F) Densitometric quantitation of rTFPI-1–mediated reduction in ERK1/2 phosphorylation with normalization for loading; mean ± SEM (n = 4). For D and F, ▵ indicates wild-type; □, PG-deficient CHO cells.
PG-dependence of inhibition of TF-mediated coagulation and signaling. (A) TF expression was determined by fluorescence-activated cell sorting (FACS) analysis of stably expressing human TF in CHO-K1 (wild-type CHO/TF) and pgsA-745 (PG-deficient CHO/TF) cell lines. Isotype-matched immunoglobulin G (IgG) was used as control. MF indicates mean fluorescence values. (B) Xa generation on monolayers of confluent TF-expressing wild-type (□) and PG-deficient CHO cells (○). Cells were incubated at 37°C with 10 nM VIIa and 50 nM X, and Xa generation was determined by chromogenic assay at the indicated times. Results are mean ± SEM (n = 5). (C) rTFPI-1–mediated internalization of Xa. Confluent wild-type (□) and PG-deficient CHO cells (▪) were incubated with rTFPI-1 in PBS 5% BSA for 30 minutes on ice and washed with the same buffer. Internalization of 50 nM [125I]-Xa was carried out for 15 minutes at 37°C, and internalized [125I]-Xa was determined by counting trypsinized and extensively washed cell pellet with background subtraction of cell-free wells subjected to the same procedure. Results are expressed relative to cells receiving no rTFPI-1, mean ± SEM (n = 5). (D) Inhibition of TF-VIIa–dependent Xa generation was determined by adding VIIa (10 nM) and X (50 nM) in the presence of the indicated concentrations of rTFPI-1 for 15 minutes at 37°C and determination of Xa by chromogenic assay; mean ± SEM (n = 6). (E) Representative Western blots of wild-type and PG-deficient CHO cells stimulated with VIIa (10 nM) and X (100 nM) in the presence of the indicated concentrations of rTFPI-1 for 10 minutes at 37°C. Phosphorylated ERK1/2 was detected with phospho-specific ERK1/2 antibody (α-PERK1/2) with loading control with αERK1/2. (F) Densitometric quantitation of rTFPI-1–mediated reduction in ERK1/2 phosphorylation with normalization for loading; mean ± SEM (n = 4). For D and F, ▵ indicates wild-type; □, PG-deficient CHO cells.
Factor Xa generation was measured in the presence of increasing concentrations of rTFPI-1 in wild-type and PG-deficient CHO cells. In these experiments, approximately 12 nM Xa was generated in 10 minutes in the absence of rTFPI-1. A 50% reduction in Xa generation was observed at 1 nM rTFPI-1, and at 5 nM rTFPI-1, rates of Xa generation were reduced more than 90% (Figure 1D). In PG-deficient cells, Xa generation was reduced 20% and 38% at 1 nM and 5 nM rTFPI-1, respectively, consistent with a requirement for PG-binding of TFPI-1 to inhibit TF-VIIa complexmediated Xa generation. TFPI-1 is known to act both on the TF-VIIa complex to shut down Xa generation, as well as directly on free Xa.14,15 At 1 nM rTFPI-1, less than 10% of the generated Xa can be directly inhibited by rTFPI-1, assuming a rTFPI-1/Xa binding stoichiometry of 1:1. The presented data thus demonstrate efficiency of rTFPI-1 to inhibit TF-VIIa–mediated Xa generation. Added at a concentration of 50 nM, rTFPI-1 essentially blocked detectable free Xa activity on both cell lines (Figure 1D).
It has previously been shown that the TF-VIIa-Xa ternary complex, but neither TF-VIIa nor free Xa at physiologically achievable concentrations (< 10 nM), induces mitogen-activated protein (MAP) kinase ERK1/2 phosphorylation in CHO cells, which express PAR1.9 The effect of rTFPI-1 on TF initiation complex-mediated PAR1 activation was analyzed by characterizing the dose dependence of inhibition of ERK1/2 phosphorylation by rTFPI-1 in wild-type and PG-deficient CHO cells. Typically, stimulation by VIIa and X for 10 minutes induced MAP kinase phosphorylation about 2- to 3-fold in PG-deficient or wild-type CHO cells (Figure 1E). Surprisingly, at 5 nM TFPI-1 no appreciable decrease in ERK1/2 phosphorylation was observed, but dose-dependent inhibition of signaling was achieved at higher concentrations in wild-type and to a lesser extent in PG-deficient CHO cells (Figure 1E-F). Note that no residual free Xa activity was detectable in the supernatant when rTFPI-1 was present at 50 to 100 nM. Because potential thrombin activity was eliminated by the addition of hirudin, the data suggest that rTFPI-1 is a poor inhibitor of TF-VIIa-Xa ternary complex-mediated PAR1 activation in CHO cells. Moreover, PG deficiency substantially reduced the efficiency of rTFPI-1 to inhibit ternary complex-mediated PAR signaling, further supporting the conclusion that PG-bearing receptors facilitate rTFPI-1–mediated inhibition of the TF initiation complex.
Regulation of TF signaling by endogenous and recombinant TFPI-1 in endothelial cells
TF is induced on cytokine or endotoxin stimulation of endothelial cells or monocytes. However, endogenous, GPI-anchored TFPI-1 controls TF procoagulant activity in these cell types.18-23 TNF-α–stimulated HUVECs show increased ERK1/2 phosphorylation when triggered with a stabilized ternary TF-VIIa-Xa complex using the nematode inhibitor NAPc2.9 We had also used mRNA expression levels of the TR3 orphan receptor gene, which has been demonstrated in endothelial cells of atherosclerotic lesions in vivo,37 as an additional, potentially relevant readout for TF-dependent signaling. The efficiency of ERK1/2 phosphorylation or TR3 induction on stimulation with NAPc2-stabilized ternary complex did not change appreciably when endogenous TFPI-1 was blocked with antibody (Figure 2A-B), indicating that NAPc2, by binding to the Xa exosite or the VIIa catalytic site, prevents endogenous TFPI-1 from inhibiting signaling by Xa. In addition, these data provide collateral evidence that the anti-TFPI-1 antibody does not nonspecifically influence the TF-dependent cell signaling pathways. Treatment with cleavage-blocking antibodies to PAR1 or PAR2 showed that TR3 induction is predominantly mediated by PAR1 signaling, whereas ternary complex-mediated ERK1/2 phosphorylation was dependent on PAR2. Thus, there appears to be selectivity in TF-triggered cellular responses downstream of PAR1 and PAR2 signaling.
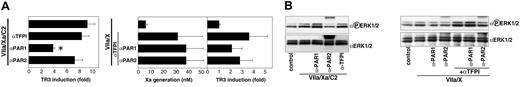
Inhibition of TF-dependent signaling by endogenous TFPI-1 in TNF-α–stimulated HUVECs. (A) TR3 induction by NAPc2 stabilized ternary complex (VIIa/Xa/C2) or by addition of VIIa (10 nM) and X (100 nM) in the presence of the indicated antibodies. The amount of Xa generated was determined after 10 minutes, prior to quenching of all free Xa with the specific inhibitor NAP5. Mean and SD (n = 3); asterisk indicates statistically different from control (P < .05, t test). (B) ERK1/2 phosphorylation in HUVECs stimulated for 10 minutes as in panel A was detected by Western blotting with phosphorylation-specific αP-ERK1/2. Equal amount of protein loading was confirmed by Western blotting with a nonphospho-specific α-ERK1/2 antibody.
Inhibition of TF-dependent signaling by endogenous TFPI-1 in TNF-α–stimulated HUVECs. (A) TR3 induction by NAPc2 stabilized ternary complex (VIIa/Xa/C2) or by addition of VIIa (10 nM) and X (100 nM) in the presence of the indicated antibodies. The amount of Xa generated was determined after 10 minutes, prior to quenching of all free Xa with the specific inhibitor NAP5. Mean and SD (n = 3); asterisk indicates statistically different from control (P < .05, t test). (B) ERK1/2 phosphorylation in HUVECs stimulated for 10 minutes as in panel A was detected by Western blotting with phosphorylation-specific αP-ERK1/2. Equal amount of protein loading was confirmed by Western blotting with a nonphospho-specific α-ERK1/2 antibody.
Stimulation of TNF-α–treated HUVECs with VIIa and X resulted in little Xa generation, marginal ERK1/2 phosphorylation, and no detectable induction of TR3 expression (Figure 2A-B). However, antibody blockade of endogenous TFPI-1 increased Xa generation about 5-fold and promoted TF-dependent cell signaling. These data indicate that signaling of the TF-VIIa-Xa complex that forms transiently during initiation of coagulation is effectively suppressed by endogenous TFPI-1. When TFPI-1 was blocked to induce signaling, anti-PAR1 antibody was more efficient in suppressing TR3 induction, whereas ERK1/2 phosphorylation was appreciably reduced only with anti-PAR2 antibody (Figure 2A-B). These data are consistent with the PAR selectivity observed on stimulation with NAPc2-stabilized ternary complex. To study the inhibition of TF signaling in HUVECs by rTFPI-1, we developed an adenoviral transduction protocol that minimally overexpressed TF to overcome the inhibitory threshold of endogenous TFPI-1. Because endogenous TFPI-1 efficiently suppressed cell signaling of the TF initiation reaction, we reasoned that exceeding the inhibitory capacity of endogenous TFPI-1 will result in TF-dependent cell signaling and thus mimic the excessive upregulation of TF during escalation of systemic inflammation, such as in severe sepsis.
Effects of rTFPI-1 on TF-dependent PAR1 signaling in primary human endothelial cells
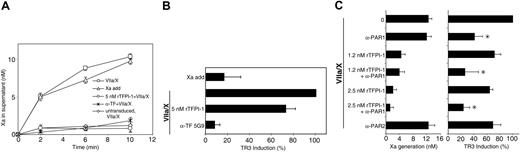
We first established conditions where TF was overexpressed in HUVECs that were also stimulated with TNF-α (5 ng/mL) for 5 hours to maintain other changes induced by inflammatory cytokines, such as up-regulated PAR2 expression38 or reduced cell surface PG.39 Figure 3A shows progress curves for Xa generation on addition of VIIa and, as a control, Xa levels on cells to which purified Xa was added throughout the time course to mimic the endogenous Xa generation by cells that received VIIa and X. Typically, 10 nM Xa was generated in 10 minutes. This is one third of the generated Xa activity obtained in α-TFPI-1–treated, TNF-α–stimulated HUVECs (Figure 2A), demonstrating that no excessive overexpression of functional TF was introduced. Activation of coagulation was blocked by adding either 5 nM rTFPI-1 or the monoclonal anti-TF antibody 5G9 that occupies the macromolecular substrate exosite and thus prevents TF-VIIa-Xa ternary complex formation and signaling. Both rTFPI-1 and 5G9 reduced Xa generation more than 80%, comparable to the very low levels of Xa generation on TNF-α–stimulated and nontransduced HUVECs (Figure 3A). TR3 induction was about 4-fold in cells stimulated with VIIa and X as compared with nonstimulated control cells. Xa added to mimic the kinetics of endogenous Xa generation on cells treated with VIIa and X did not elicit signaling (Figure 3B), demonstrating conditions that measure ternary complex signaling. Disruption of the TF-VIIa-Xa ternary complex by the exosite-directed anti-TF antibody 5G9 reduced TR3 induction more than 90%, but inhibition of Xa generation by 5 nM rTFPI-1 to a similar extent reduced TR3 induction only by about 30% (Figure 3B). These results suggest that TF-VIIa-Xa ternary complex signaling can proceed in the presence of rTFPI-1 concentrations that shut off coagulation, which is consistent with the CHO cell data.
rTFPI-1 is a poor inhibitor of TF-dependent PAR1 signaling in endothelial cells. (A-B) HUVECs were transduced with TF (50 particles/cell) for 3 hours, followed by growth for 2 days. Confluent cells were rendered quiescent in serum-free medium for 5 hours in the presence of TNF-α prior to stimulation with VIIa (10 nM) and X (100 nM) without (□) or with 5 nM rTFPI-1 (○)or50 μg/mL 5G9 anti-TF antibody (*). Alternatively, purified Xa was added at 1, 3, 5, 7, and 9 minutes (5, 2, 1, 1, and 1 nM, respectively; □) to mimic the kinetics of endogenous Xa generation. (A) Xa activity was determined at the indicated times by chromogenic assay. (B) Xa activity was quenched with 1 μM NAP5 at 10 minutes, and mRNA levels of TR3 were determined at 90 minutes by real-time PCR; mean ± SD (n = 6). (C) HUVECs were transduced with virus encoding TF (50 particles/cell) for 3 hours, then grown for 2 days and incubated in serum-free medium for 5 hours. After treatment with anti–PAR-1 or rTFPI-1 (or both) for 10 minutes, cells were stimulated with VIIa (10 nM) and X (100 nM). (Left) Xa activity in the supernatant at 10 minutes. (Right) Xa was quenched with 1 μM NAP5 at 10 minutes and TR3 mRNA levels at 90 minutes were determined by real-time PCR. Mean ± SD (n = 4); asterisk indicates the reduction in TR3 induction on anti-PAR1 addition was statistically significant (P < .01, t test) for each of the rTFPI-1 concentrations.
rTFPI-1 is a poor inhibitor of TF-dependent PAR1 signaling in endothelial cells. (A-B) HUVECs were transduced with TF (50 particles/cell) for 3 hours, followed by growth for 2 days. Confluent cells were rendered quiescent in serum-free medium for 5 hours in the presence of TNF-α prior to stimulation with VIIa (10 nM) and X (100 nM) without (□) or with 5 nM rTFPI-1 (○)or50 μg/mL 5G9 anti-TF antibody (*). Alternatively, purified Xa was added at 1, 3, 5, 7, and 9 minutes (5, 2, 1, 1, and 1 nM, respectively; □) to mimic the kinetics of endogenous Xa generation. (A) Xa activity was determined at the indicated times by chromogenic assay. (B) Xa activity was quenched with 1 μM NAP5 at 10 minutes, and mRNA levels of TR3 were determined at 90 minutes by real-time PCR; mean ± SD (n = 6). (C) HUVECs were transduced with virus encoding TF (50 particles/cell) for 3 hours, then grown for 2 days and incubated in serum-free medium for 5 hours. After treatment with anti–PAR-1 or rTFPI-1 (or both) for 10 minutes, cells were stimulated with VIIa (10 nM) and X (100 nM). (Left) Xa activity in the supernatant at 10 minutes. (Right) Xa was quenched with 1 μM NAP5 at 10 minutes and TR3 mRNA levels at 90 minutes were determined by real-time PCR. Mean ± SD (n = 4); asterisk indicates the reduction in TR3 induction on anti-PAR1 addition was statistically significant (P < .01, t test) for each of the rTFPI-1 concentrations.
In TNF-α–stimulated HUVECs, TR3 induction was predominantly mediated by PAR1 signaling, and we hypothesized that PAR1 signaling was poorly inhibited by rTFPI. To simplify the experimental protocol, we omitted stimulation with TNF-α of TF-transduced HUVECs. Incubation with VIIa (10 nM) and X (100 nM) for 10 minutes yielded about 12 nM Xa under these conditions (Figure 3C). TF-VIIa-Xa ternary initiation complex-induced TR3 gene induction was sensitive by about 70% to anti-PAR1 and by about 30% to anti-PAR2 antibodies (Figure 3C). Very little inhibition of the TR3 response was observed by rTFPI-1 at 1.2 and 2.5 nM, and the simultaneous blockade of PAR1 cleavage showed that PAR1-dependent signaling remained insensitive to these concentrations of rTFPI-1 that efficiently blocked TF-initiated Xa generation. However, 1.2 and 2.5 nM rTFPI-1 reduced TR3 induction to an extent that was consistent with inhibition of the PAR2 component in the signaling response, raising the possibility that PAR2 signaling was relatively more sensitive to inhibition by rTFPI-1.
Inhibition of TF signaling by rTFPI-1 in cells overexpressing PAR2
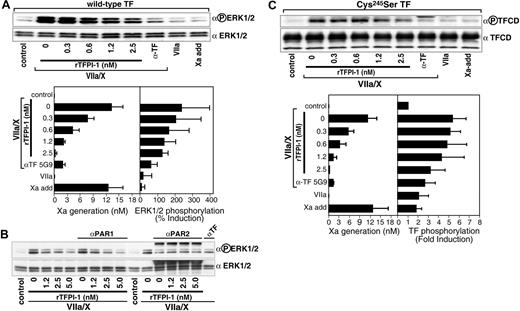
Primary HUVECs express variable but low levels of PAR2. To analyze PAR2 signaling in these cells, we used our previously established adenoviral cotransduction system that expresses TF and PAR2 at close to stoichiometric levels in HUVECs.10 In line with previously published data,9,10 neither 10 nM VIIa nor mimicking levels of free Xa (a cumulative final concentration of 12 nM) induced significant ERK1/2 phosphorylation, demonstrating that under the experimental conditions this response was specific for the transient TF-VIIa-Xa complex (Figure 4A). There was no apparent effect of PAR2 overexpression on the potency of rTFPI-1 to block TF-VIIa–mediated Xa generation (compare Figures 3C and 4A), and rTFPI-1 dose dependently reduced ERK1/2 phosphorylation. However, relatively higher concentrations of rTFPI-1 were required to achieve a similar degree of inhibition of ERK1/2 phosphorylation, as compared to the inhibition of Xa generation measured in the supernatant (Figure 4A). Consistent with the notion that this represents signaling of the TF initiation complex, anti-TF 5G9 that disrupts ternary complex formation appeared to be more efficient than rTFPI-1 at similar residual generation of Xa during the experiment.
Inhibition of TF-dependent PAR2 signaling by rTFPI-1 in HUVECs transduced with TF and PAR2. HUVECs were cotransduced with virus encoding TF (50 particles/cell) and PAR2 (100 particles/cell) for 3 hours, then grown for 2 days, rendered quiescent in serum-free medium for 5 hours, and pretreated with anti-PAR1 (WEDE-15, 20 μg/mL; ATAP-2, 10 μg/mL), polyclonal rabbit anti-PAR2 (200 μg/mL), the indicated concentrations of rTFPI-1, or 50 μg/mL anti-TF exosite antibody 5G9 prior to stimulation with VIIa (10 nM) and X (100 nM) or with Xa added at 1, 3, 5, 7, and 9 minutes (5, 2, 1, 1, and 1 nM, respectively) for 10 minutes. (A) Representative Western blot and densitometric quantitation of inhibition of VIIa/X-induced ERK1/2 phosphorylation by rTFPI-1 or 5G9 antibody. The left panel shows Xa activity in the supernatant at 10 minutes and the right panel the fold induction of ERK1/2 phosphorylation; mean ± SD (n = 3). (B) Preincubation with anti-PAR1 or anti-PAR2 demonstrates that TF initiation phase-mediated ERK1/2 phosphorylation is PAR2 dependent. (C) TF cytoplasmic domain (TFCD) phosphorylation was determined in cells transduced under identical conditions as in panel A with a palmitoylation-deficient mutant of TF (Cys245-Ser TF) and treated as described. Representative Western blot for dose-dependent inhibition of TF cytoplasmic domain phosphorylation by rTFPI-1. In the bottom graph, the left panel indicates Xa activity by chromogenic assay at 10 minutes; the right panel, densitometric quantification of TF phosphorylation given as fold increase over control; mean ± SD (n = 3).
Inhibition of TF-dependent PAR2 signaling by rTFPI-1 in HUVECs transduced with TF and PAR2. HUVECs were cotransduced with virus encoding TF (50 particles/cell) and PAR2 (100 particles/cell) for 3 hours, then grown for 2 days, rendered quiescent in serum-free medium for 5 hours, and pretreated with anti-PAR1 (WEDE-15, 20 μg/mL; ATAP-2, 10 μg/mL), polyclonal rabbit anti-PAR2 (200 μg/mL), the indicated concentrations of rTFPI-1, or 50 μg/mL anti-TF exosite antibody 5G9 prior to stimulation with VIIa (10 nM) and X (100 nM) or with Xa added at 1, 3, 5, 7, and 9 minutes (5, 2, 1, 1, and 1 nM, respectively) for 10 minutes. (A) Representative Western blot and densitometric quantitation of inhibition of VIIa/X-induced ERK1/2 phosphorylation by rTFPI-1 or 5G9 antibody. The left panel shows Xa activity in the supernatant at 10 minutes and the right panel the fold induction of ERK1/2 phosphorylation; mean ± SD (n = 3). (B) Preincubation with anti-PAR1 or anti-PAR2 demonstrates that TF initiation phase-mediated ERK1/2 phosphorylation is PAR2 dependent. (C) TF cytoplasmic domain (TFCD) phosphorylation was determined in cells transduced under identical conditions as in panel A with a palmitoylation-deficient mutant of TF (Cys245-Ser TF) and treated as described. Representative Western blot for dose-dependent inhibition of TF cytoplasmic domain phosphorylation by rTFPI-1. In the bottom graph, the left panel indicates Xa activity by chromogenic assay at 10 minutes; the right panel, densitometric quantification of TF phosphorylation given as fold increase over control; mean ± SD (n = 3).
Figure 4B shows that 5 nM rTFPI-1 further reduced ERK1/2 phosphorylation, thus substantiating the conclusion that a fraction of the ERK1/2 response was still intact at conditions when coagulation was effectively inhibited by lower concentrations of rTFPI-1. In TNF-α–stimulated endothelial cells, TF-dependent ERK1/2 phosphorylation was mediated by PAR2 signaling (Figure 2B). Antibodies to PAR1 did not influence ERK1/2 phosphorylation, but inhibition of PAR2 cleavage completely blocked TF initiation complex-mediated ERK1/2 activation in transduced HUVECs. These data demonstrate that the transduction model properly recapitulates the ERK1/2 signaling response of cytokine-stimulated HUVECs. These data also suggest that PAR2 signaling responses are more efficiently inhibited at 2.5 and 5 nM rTFPI, as compared to the inhibition of PAR1-dependent TR3 induction (Figure 3). This notion is further supported by the analysis of another PAR2-specific signaling response, the phosphorylation of the TF cytoplasmic domain. Because thioester modification of TF negatively regulates TF cytoplasmic domain phosphorylation,10,34 experiments were performed with Cys245-Ser–mutated TF to facilitate detection of PAR2-dependent TF phosphorylation. TF cytoplasmic domain phosphorylation was progressively inhibited by 1.2 and 2.5 nM rTFPI-1 (Figure 4C). These data provide clear evidence that rTFPI-1 regulates ternary TF-VIIa-Xa–mediated PAR2 activation.
PAR2 expression facilitates inhibition of PAR1 signaling by rTFPI-1
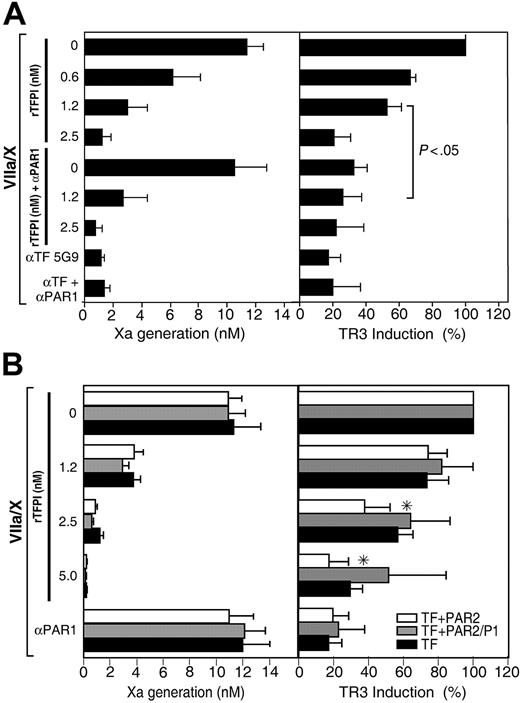
In PAR2-tranduced HUVECs, a PAR1-blocking antibody reduced VIIa/X-mediated induction of TR3 by about 70% to 80%, suggesting that even at high PAR2 expression levels the majority of the TF-dependent TR3 response is triggered by PAR1 signaling (Figure 5). Residual TR3 induction at 1.2 nM rTFPI-1 in PAR2-transduced cells was sensitive to anti-PAR1 antibody, confirming that PAR1 signaling escaped inhibition at lower concentration of rTFPI-1 (Figure 5A). Interestingly, 2.5 nM rTFPI-1 inhibited TR3 induction, which should be compared with minimal inhibition by 2.5 or 5 nM rTFPI-1 in cells that were not cotransduced with PAR2 (Figure 3). Thus, expression of PAR2 appears to enhance rTFPI-1 inhibition of TF-mediated PAR1 signaling.
Enhanced inhibition of ternary complex PAR1 signaling by rTFPI-1 in HUVECs coexpressing PAR2. HUVECs were cotransduced with virus encoding TF (50 particles/cell) and PAR2 or cleavage-insensitive PAR2/P1 mutant (100 particles/cell) for 3 hours, then grown for 2 days, rendered quiescent in serum-free medium for 5 hours, and pretreated with anti-PAR1 (WEDE-15, 20 μg/mL; ATAP-2, 10 μg/mL), the indicated concentrations of rTFPI-1, or 50 μg/mL anti-TF 5G9 prior to stimulation with VIIa (10 nM) and X (100 nM) for 10 minutes. Thrombin signaling was excluded by the routine addition of 200 nM hirudin prior to stimulation with proteases. (Left) Xa activity in the supernatant at 10 minutes. (Right) Xa was quenched with 1 μM NAP5 at 10 minutes, and TR3 mRNA levels at 90 minutes were determined by real-time PCR. (A) Mean ± SD (n = 3); significantly different based on t test. (B) Mean ± SD (n = 4). *PAR2-transduced statistically different (t test) from non–PAR2-transduced (2.5 nM rTFPI-1, P < .005; 5 nM rTFPI-1, P < .05) or PAR2/P1-transduced (2.5 nM rTFPI-1, P < .05). □ indicates TF + PAR2; ▦, TF + PAR2/P1; ▪, TF only.
Enhanced inhibition of ternary complex PAR1 signaling by rTFPI-1 in HUVECs coexpressing PAR2. HUVECs were cotransduced with virus encoding TF (50 particles/cell) and PAR2 or cleavage-insensitive PAR2/P1 mutant (100 particles/cell) for 3 hours, then grown for 2 days, rendered quiescent in serum-free medium for 5 hours, and pretreated with anti-PAR1 (WEDE-15, 20 μg/mL; ATAP-2, 10 μg/mL), the indicated concentrations of rTFPI-1, or 50 μg/mL anti-TF 5G9 prior to stimulation with VIIa (10 nM) and X (100 nM) for 10 minutes. Thrombin signaling was excluded by the routine addition of 200 nM hirudin prior to stimulation with proteases. (Left) Xa activity in the supernatant at 10 minutes. (Right) Xa was quenched with 1 μM NAP5 at 10 minutes, and TR3 mRNA levels at 90 minutes were determined by real-time PCR. (A) Mean ± SD (n = 3); significantly different based on t test. (B) Mean ± SD (n = 4). *PAR2-transduced statistically different (t test) from non–PAR2-transduced (2.5 nM rTFPI-1, P < .005; 5 nM rTFPI-1, P < .05) or PAR2/P1-transduced (2.5 nM rTFPI-1, P < .05). □ indicates TF + PAR2; ▦, TF + PAR2/P1; ▪, TF only.
It was of concern to us that the signaling responses in PAR2-expressing and -nonexpressing cells were not evaluated strictly in parallel. We therefore carried out another set of experiments in which cells were stimulated in the presence of the identical dilutions of rTFPI-1. In addition, we addressed whether PAR2 cleavage and activation were required to enhance rTFPI-1's inhibition of TF-dependent signaling. To this end, we cotransduced HUVECs with uncleavable PAR2/P1 mutant in which the scissile bond Arg residue was replaced by Glu. Figure 5B shows the dose dependence of rTFPI-1–mediated inhibition of TR3 induction in HUVECs expressing TF alone, TF and PAR2, or TF and uncleavable PAR2/P1. Induction of the TR3 transcript was significantly more inhibited at 2.5 nM rTFPI-1 in PAR2-expressing HUVECs compared to cells that were not PAR2 transduced or that expressed an uncleavable PAR2/P1. These data suggest that PAR2 activation, rather than a bystander effect of PAR2 expression, is necessary for the better inhibition by rTFPI-1. However, 5 nM rTFPI-1 appeared to be more potent to inhibit PAR1-mediated signaling as compared to TNF-α–stimulated HUVECs (Figure 3B). In TNF-α–stimulated endothelial cells, PAR2 up-regulation may remain modest relative to levels of adenoviral-transduced TF. In addition, inflammatory perturbation may change additional factors, such as PG coreceptors, required for efficient inhibition by rTFPI-1.
Discussion
This study provides clear experimental evidence that inhibition of TF-VIIa–dependent Xa generation, an important initial step that triggers the downstream coagulation cascade, is a poor predictor for the efficiency by which rTFPI-1 inhibits signaling of the TF initiation complex. TF initiation phase signaling is primarily mediated by the activation of PAR1 in CHO cells. In this model, we show that rTFPI-1 incompletely inhibits cell signaling at concentrations that abolish detectable Xa generation. Relatively poor inhibition of PAR1-signaling responses by rTFPI-1 was also seen in HUVECs. In contrast, an inhibitory antibody that prevents formation of the TF-VIIa-X initiation complex and the ternary TF-VIIa-Xa–signaling complex9 inhibited signaling when reducing Xa generation with similar efficiency as rTFPI-1. This further supports the concept that rTFPI-1 is a relatively poor inhibitor of TF initiation phase signaling. The reduced potency of rTFPI-1 can be rationalized by kinetic aspects of its inhibitory mechanism, which depends on the formation of a ternary TF-VIIa-Xa complex or the presence of nascent product Xa in very close proximity.14
Efficacy of inhibition of TF ternary complex signaling may thus require the proper localization of rTFPI-1 to the cell surface. rTFPI-1 and a wide variety of other extracellular ligands, such as growth factors, lipoproteins, and microbes, are known to associate with cells via binding to highly sulfated cell surface PGs.40 However, in many cases the functional significance of these interactions has not been established. TFPI-1-Xa complex internalization was significantly reduced in PG-deficient cells, corroborating indirect evidence that PG-receptors are required, in addition to LRP family members, for the cellular uptake of TFPI-1.24,25 In addition, this study provides new genetic evidence that PG binding facilitates the inhibitory activities of rTFPI-1 on TF-dependent coagulation. Even more strikingly, the rTFPI-1 inhibition of PAR signaling by the ternary initiation complex was highly dependent on PG, indicating that positioning of TFPI-1 by binding to PG receptors is crucial for rapid inhibition of the nascent product Xa and thus control of signaling that accompanies initiation of coagulation.
The crucial role for proper positioning of TFPI-1 for efficient inhibition of TF initiation phase signaling is further underscored by the finding that endogenous TFPI-1, which is known to be GPI anchored,17-23 suppressed signaling in HUVECs in which TF was up-regulated by inflammatory cytokine stimulation (Figure 2). Although localization of TFPI-1 to lipid rafts is not required for inhibition of TF procoagulant activity,41 the efficiency of GPI-anchored TFPI-1 to block signaling may in part originate from its ability to translocate the TF initiation complex into caveolae.20 However, inflammatory cytokine stimulation of HUVECs also induces the expression of PAR2.38 Thus, more efficient inhibition of TF by endogenous TFPI-1 may in part result from the balanced up-regulation of PAR2 that enhances the inhibition of TF signaling by rTFPI-1.
The presented data show that TF-induced PAR2 signaling is more efficiently inhibited by rTFPI-1 in comparison to PAR1-signaling responses. In addition, PAR2 expression shifted the dose response for inhibition of TF-dependent PAR1 signaling by rTFPI-1. With a cleavage-resistant mutant of PAR2 we show that the activation of PAR2, most likely by the TF ternary complex, is responsible for this improved inhibition by rTFPI-1. Our recent studies provided in vitro and in vivo evidence for a close reciprocal signaling cross-talk between TF and PAR2.6,10 This close connection makes it desirable that TF-dependent signaling is tightly controlled in PAR2-expressing cells. The data presented here expand on this concept by suggesting that PAR2 is a key component that contributes to the regulatory control of TF initiation phase signaling through PARs.
However, PAR2 and TF are not necessarily coregulated in physiologically relevant cell types. For example, in cultured HUVECs, proinflammatory cytokines up-regulate PAR2 slowly,38 reaching peak mRNA levels after 24 to 72 hours, whereas TNF-α–induced TF expression peaks at 4 hours.42 TF is rapidly induced in monocytes, but TF and PAR2 are typically coexpressed only on macrophage differentiation.43 Alternatively, there are synergistic pathways to potentiate TF expression in endothelial cells,44 which may lead to an imbalance of TF and PAR2 expression in systemic inflammation and sepsis. Thus, PAR1-mediated signaling of TF may require regulation during relative PAR2 deficiency, and rTFPI-1 may be inefficient to regulate such signaling events.
Excessive TF-dependent activation of coagulation is a common finding in severe sepsis, and increased TF expression or decreased anticoagulant inhibitory potential may both contribute to the exacerbation of the coagulopathy. Moreover, HUVECs exposed to inflammatory mediators respond by a loss of cell-associated PG,39 which may contribute to relative inefficiency of rTFPI-1 to inhibit TF initiation phase signaling. Unlike the successful clinical testing of activated protein C in sepsis,45 rTFPI-1 failed to reduce overall 28-day mortality in a phase 3 sepsis trial.32,33 The therapeutically applied dose of rTFPI-1 (∼5 nM) achieved measurable anticoagulant benefit in the patients. However, the data presented here using a similar concentration of rTFPI-1 strongly suggest that antisignaling potency may not have been achieved. Indeed, Taylor and colleagues46 demonstrated that in a subgroup of primates exposed to a lethal dose of E coli, rTFPI-1 conferred anticoagulant protection without providing anti-inflammatory and survival benefit that is typically achieved with higher doses of rTFPI-1 or other TF-directed inhibitors. The presented data caution that anticoagulant potency of TF-targeted inhibitors may not properly reflect on antisignaling and anti-inflammatory potency, an important consideration for potential future anticoagulant trials in systemic inflammatory diseases.
Prepublished online as Blood First Edition Paper, November 18, 2004; DOI 10.1182/blood-2004-09-3422.
Supported by the National Heart Lung Blood Institute, Bethesda, MD (W.R.); Medical Faculty, Lund University, Lund, Sweden; Swedish Research Council and the Zoegás Foundation, Sweden (M.B.); and the American Heart Association, Western Affiliate, Burlingame, CA (J.A.). J.A. and M.B. contributed equally to this study.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Jennifer Royce, Pablito Tejada, David Revak, and Cindi Biazak for excellent technical assistance and Barbara Parker for preparation of figures. We thank A. Creasey for rTFPI-1, G. Vlasuk for recombinant NAPs, and L. Brass for anti-PAR1 antibodies.

![Figure 1. PG-dependence of inhibition of TF-mediated coagulation and signaling. (A) TF expression was determined by fluorescence-activated cell sorting (FACS) analysis of stably expressing human TF in CHO-K1 (wild-type CHO/TF) and pgsA-745 (PG-deficient CHO/TF) cell lines. Isotype-matched immunoglobulin G (IgG) was used as control. MF indicates mean fluorescence values. (B) Xa generation on monolayers of confluent TF-expressing wild-type (□) and PG-deficient CHO cells (○). Cells were incubated at 37°C with 10 nM VIIa and 50 nM X, and Xa generation was determined by chromogenic assay at the indicated times. Results are mean ± SEM (n = 5). (C) rTFPI-1–mediated internalization of Xa. Confluent wild-type (□) and PG-deficient CHO cells (▪) were incubated with rTFPI-1 in PBS 5% BSA for 30 minutes on ice and washed with the same buffer. Internalization of 50 nM [125I]-Xa was carried out for 15 minutes at 37°C, and internalized [125I]-Xa was determined by counting trypsinized and extensively washed cell pellet with background subtraction of cell-free wells subjected to the same procedure. Results are expressed relative to cells receiving no rTFPI-1, mean ± SEM (n = 5). (D) Inhibition of TF-VIIa–dependent Xa generation was determined by adding VIIa (10 nM) and X (50 nM) in the presence of the indicated concentrations of rTFPI-1 for 15 minutes at 37°C and determination of Xa by chromogenic assay; mean ± SEM (n = 6). (E) Representative Western blots of wild-type and PG-deficient CHO cells stimulated with VIIa (10 nM) and X (100 nM) in the presence of the indicated concentrations of rTFPI-1 for 10 minutes at 37°C. Phosphorylated ERK1/2 was detected with phospho-specific ERK1/2 antibody (α-PERK1/2) with loading control with αERK1/2. (F) Densitometric quantitation of rTFPI-1–mediated reduction in ERK1/2 phosphorylation with normalization for loading; mean ± SEM (n = 4). For D and F, ▵ indicates wild-type; □, PG-deficient CHO cells.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/105/6/10.1182_blood-2004-09-3422/6/m_zh80060575810001.jpeg?Expires=1769086151&Signature=3AD8LYcc7sZGBhwVWMzOmOCkoseI8aMQf8fTdWMr8bMnl4v3J92HCv1jHjXKXXNW6g7ob9P9jCYETC36qEwisgMAbAW0LH7E19xwiJ6YhlgnqoBn9Vq~waOxHUu1aAw-U39MnpHctkPVavc9vqdgobp9VC7sD1VTcRxvoAMwT5UuaZdvy4oZF6VmqfnV8ZayVVkcYlj1LkX4Ki~jqY5U1UfLPcUT-4kgKfXDHIlqPwjzTlnYxQPQ-sCXy7IVO0SF38XDXPUoJKIDLjlhPAOUzkmRL1PbVyWwijjjFTdLQXCzb0IN6e~wNnIWBaKNtEo6Ipd5VFR8luwmoCxIk2p2pw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal