Abstract
The combination of cyclophosphamide, vincristine, and prednisone (CVP) is one of several standard treatment options for advanced follicular lymphoma. This, like similar chemotherapeutic regimens, induces response rates of 60% to 80%, with a median response duration of under 2 years. Rituximab, a chimeric monoclonal antibody against CD20, is active in follicular lymphoma, both as monotherapy and in combination with chemotherapy. Previously untreated patients with stages III to IV follicular lymphoma were randomly assigned to receive either 8 cycles of CVP plus rituximab (R-CVP; n = 162) or CVP (n = 159). Overall and complete response rates were 81% and 41% in the R-CVP arm versus 57% and 10% in the CVP arm, respectively (P < .0001). At a median follow-up of 30 months, patients treated with R-CVP had a very significantly prolonged time to progression (median 32 months versus 15 months for CVP; P < .0001). Median time to treatment failure was 27 months in patients receiving R-CVP and 7 months in the CVP arm (P < .0001). Rituximab did not add significantly to the toxicity of CVP. The addition of rituximab to the CVP regimen significantly improves the clinical outcome in patients with previously untreated advanced follicular lymphoma, without increased toxicity.
Introduction
Follicular lymphoma is the most common form of indolent lymphoma, accounting for about 70% of indolent lymphomas and 20% to 25% of all cases of non-Hodgkin lymphoma (NHL).1 Most patients have advanced disease—stage III or IV—at diagnosis and cannot be cured with currently available therapy. Median survival is 6 to 10 years, and the majority of patients eventually die of their disease after multiple remissions and subsequent relapses.2-4
Initial treatment with chemotherapy is associated with a high rate of clinical response, followed invariably by relapse. Subsequent remissions can be obtained with further treatment but at a progressively lower rate and with remissions of shorter duration.5 There is, however, no evidence that early initiation of therapy improves survival.6,7 For this reason, patients who are asymptomatic at diagnosis are often referred to a “watch and wait” strategy until clinical signs or symptoms warrant intervention. Patients with adverse prognostic indicators, rapidly progressive disease, bone marrow failure, or life-threatening organ involvement require earlier intervention.
There is as yet no universally accepted first-line treatment for stage III/IV follicular lymphoma. Chlorambucil as a single agent, and the combination of cyclophosphamide, vincristine, and prednisone (CVP), have become standard treatments in many centers.8-11 The addition of anthracyclines to CVP adds significantly to the toxicity of the regimen and has not been shown conclusively to prolong disease-free or overall survival, though complete remission rates may be increased.12,13
Adding interferon (IFN)–α to chemotherapeutic regimens can increase the rate and duration of response, and one study suggests both progression-free and overall survival benefits, but many patients are unable to tolerate the accompanying side effects.14
Nucleoside analogs, such as fludarabine, are also promising agents in this setting, but have yet to be proved superior to existing treatments. Hagenbeek et al15 compared fludarabine monotherapy with CVP in previously untreated patients with follicular NHL. Fludarabine yielded an increase in response rates, but there was no difference in time to progression (TTP) (21 months vs 15 months; P = nonsignificant [NS]) or overall survival. Thus, there is no proven, clinically relevant benefit from more toxic first-line chemotherapy regimens compared with alkylator-based therapy.15-20
The chimeric mouse/human monoclonal antibody, rituximab, has been shown to be effective in the treatment of follicular lymphoma. Directed against CD20+ cells, it has been demonstrated both in vitro and in vivo to cause lysis of CD20+ lymphoma cells via complement-mediated cytotoxicity, antibody-dependent cellular cytotoxicity, and, directly, by causing apoptosis.21 In a phase 2 trial,22 166 patients with relapsed/refractory indolent NHL received 4 weekly infusions with rituximab doses of 375 mg/m2 of body surface area. In this heavily pretreated group, a response rate of 48% was obtained, with a median time to progression of 9 months—similar results to those obtained with other cytotoxic salvage therapies. In contrast to standard chemotherapy, side effects were modest (chills, fevers, and headache) and mainly confined to the first infusion of rituximab. No significant opportunistic infections were seen. The same rituximab regimen was given to previously untreated patients with follicular lymphoma in an early phase 2 study.23 A response rate of 73% was observed in these good-prognosis, low-tumor-burden patients. This is no worse—though not significantly better—than that obtained with chemotherapy.
Data from in vitro studies suggest that rituximab can sensitize lymphoma cell lines to chemotherapy.24 In addition, a synergistic effect between rituximab and various cytotoxic agents has been demonstrated.25 In a phase 2 study of rituximab in combination with CHOP (cyclophosphamide, doxorubicin, vincristine, and prednisone) chemotherapy in 40 patients with previously untreated and relapsed low-grade or follicular lymphoma, the overall response rate (ORR) was 95%, with a 55% complete response rate.26
In view of these results, we decided to evaluate the addition of rituximab to a widely used standard chemotherapy regimen (CVP) in a prospective randomized trial against CVP alone, in previously untreated patients with stage III/IV follicular lymphoma.
Patients, materials, and methods
Patients
Eligible patients were 18 years or older with untreated CD20+ follicular lymphoma (National Cancer Institute [NCI] Working Formulation Groups B, C, D; WHO follicular lymphoma Grades 1-3) confirmed by lymph node biopsy. All patients had to have stage III or IV disease, a performance status of 0 to 2 according to Eastern Clinical Oncology Group (ECOG) criteria, a life expectancy of more than 3 months, and a need for therapy in the opinion of the participating clinician. Patients were ineligible if there was evidence of histologic transformation to high-grade or diffuse large B-cell lymphoma, central nervous system involvement, or a history of severe cardiac disease or previous malignancy other than in situ carcinoma of the cervix and basal cell carcinoma of the skin. Patients were also excluded if they had impaired renal or hepatic function not caused by lymphoma, or if they had known infection with HIV, or with hepatitis B or C.
Trial design
Patients were randomly assigned (1:1) at trial entry to treatment with CVP plus rituximab (R-CVP) or CVP alone. No crossover between the 2 arms was planned. Randomization was performed centrally, stratifying by center and International Prognostic Index (IPI) score.27
The study complied with all the requirements of the Declaration of Helsinki and its current amendments, and was conducted in accordance with Good Clinical Practice guidelines. All patients gave written informed consent. The protocol and accompanying materials were approved by the relevant institutional review board for each participating center. The study was overseen by an independent Data and Safety Monitoring Committee (DSMC).
Treatment
Patients treated with CVP received a combination of 750 mg/m2 cyclophosphamide intravenously on day 1; 1.4 mg/m2 of vincristine, up to a maximal dose of 2 mg intravenously, on day 1; and 40 mg/m2 of prednisone per day orally on days 1 to 5. Patients treated with R-CVP also received 375 mg/m2 of rituximab intravenously on day 1 of each therapy cycle. If a rituximab-induced infusion reaction occurred, therapy was interrupted and all symptoms had to resolve before rituximab was continued, or CVP started. Patients in both groups were treated every 21 days for a maximum of 8 cycles. Dosages of cytotoxic drugs were reduced if grades 2 to 4 neurological or grade 3 or 4 hematological toxicity occurred.
Trial assessments
Tumor response and progression were determined using standard criteria.28 The primary end point was time to treatment failure (TTF), defined as the time between randomization and any one of the following events: progressive disease (PD), relapse after response, institution of new antilymphoma treatment (NLT); stable disease after cycle 4 (SD4), or death by any cause. Stable disease after cycle 4 was considered a “treatment failure” event by the independent DSMC, who believed that patients with stable disease would be more likely to continue the same therapy in the R-CVP arm but would be more likely to start a new treatment in the CVP arm. Time to progression (TTP), defined as the interval between randomization and progression, relapse after response, or death from any cause, was also analyzed, as this is the most commonly used measure to define the efficacy of new treatments in clinical trials in these patients. Patients who reported SD4 or NLT as their first treatment-failure event were also followed until PD, relapse, or death occurred.
Additional efficacy parameters included response rates, overall survival (time between randomization and death), duration of response (interval between response and relapse or death), time to next antilymphoma treatment or death (period between randomization and the start of any new treatment or death), and disease-free survival (time between complete response and relapse or death).
Adverse events were graded according to NCI Common Toxicity Criteria and reported in detail.29
Statistical analysis
Sample size was calculated using the primary end point, TTF. A median TTF of 18 months was assumed for patients with advanced low-grade lymphoma treated with CVP.30,31 We calculated that 318 patients, randomized 1:1 between the 2 treatment groups recruited over 2 years and followed for a minimum of 3 years, would provide 85% power at a 2-tailed significance level of 5% to detect an anticipated 50% increase in the median TTF (ie, from 18 to 27 months) in patients treated with R-CVP compared with those who received CVP alone.
An interim analysis was performed after eighteen months follow-up when 189 patients had experienced a treatment failure event as defined above. At this point, since the predefined stopping criteria had been met, the DSMC recommended that the final analysis be performed. We here present the results of an updated analysis with a median follow-up of 30 months.
All analyses were conducted on an intention-to-treat basis—patients treated with second-line therapy were analyzed and are presented here according to their initially assigned treatment.
TTF and TTP were analyzed using the log-rank test; results were expressed as Kaplan-Meier plots. A multivariate Cox regression analysis was performed to assess the effects of treatment and the various baseline prognostic factors on TTP. Response rates in each treatment group were compared using chi-squared tests. Duration of response, time to new antilymphoma therapy, overall survival, and disease-free survival were analyzed with log-rank tests. Analyses of efficacy and safety included all randomized and treated patients and followed the intention-to-treat principle (ie, patients were analyzed according to their randomized treatment). All P values are 2-tailed. Statistical analyses were performed with SAS software (SAS Institute, Cary, NC) by George Stein (SFR Ltd., Basel, Switzerland).
Results
Patients' characteristics
This study was conducted at 47 sites in Australia, Belgium, Brazil, Canada, France, Israel, Poland, Portugal, Spain, Switzerland, and the United Kingdom. A total of 322 patients were enrolled between 2000 and 2002. One patient assigned to the CVP group did not receive any trial medication because this patient withdrew consent; 321 patients were included in the final analysis (159 CVP; 162 R-CVP).
There were no significant differences between the 2 groups in any of the baseline demographic, clinical, or pathologic characteristics (Table 1). Central review of pathology was performed on lymph node material from 90% of the patients: follicular lymphoma was confirmed in 95% of these cases. All cases entered in the study are included in this report. Eighty-nine percent of patients in the CVP arm and 86% in the R-CVP arm were randomized and treated within 6 months of first diagnosis. More than 80% of patients had a least 1 symptom requiring therapy according to local treatment guidelines (British National Lymphoma Investigation [BNLI], Groupe d'Etudes de Lymphoma Folliculaire [GELF], ECOG/South West Oncology Group [SWOG]) (Table 2). IPI scores were balanced across groups, with most patients (79%) having scores of 1 or 2 (Table 1); 15% of CVP patients and 13% of R-CVP patients had scores of 3 or 4. Although not part of the original inclusion criteria, 47% of patients randomized to CVP and 44% of those randomized to R-CVP had scores of 3 to 5 according to the Follicular Lymphoma International Prognostic Index (FLIPI), placing them in the poorest prognostic group.32
Treatment
The 8 scheduled cycles of chemotherapy were administered to 68% of patients treated with CVP and 85% of patients treated with R-CVP. This difference was due to fewer patients in the R-CVP arm being withdrawn from treatment because of an insufficient therapeutic response, mainly after cycle 4. The proportion of patients that received more than 90% of the planned dose of prednisolone and vincristine at each administered cycle was comparable between the treatment arms, but was slightly higher for cyclophosphamide in the CVP group (> 94% for CVP; > 85% for R-CVP). This was mainly due to dose modifications in the R-CVP group for NCI common toxicity criteria (CTC) grades 3 and 4 neutropenia. Ninety-six percent of patients received more than 90% of the planned dose of rituximab at each administered cycle.
Efficacy
Investigators assessed response rates using the Cheson et al28 guidelines. Up to 42 days after the end of trial treatment, 57% of patients treated with CVP were classified as responders (10% complete response [CR]/complete response, unconfirmed [Cru]), compared with 81% of patients treated with R-CVP (41% CR/CRu; P < .0001 for ORR and CR/CRu) (Table 3). In the CVP arm, 26 (62%) of 42 patients with stable disease after cycle 4 (SD4) continued with CVP; 19 of these completed 8 cycles and 9 patients had a response (CR or CRu or partial response [PR] up to 42 days after trial treatment end). In the R-CVP group, 17 (81%) of 21 patients with SD4 continued randomized treatment, 12 patients completed 8 cycles, and 7 patients responded (CR, CRu, or PR up to 42 days after trial treatment end).
Treatment with R-CVP significantly lengthened TTF. At a median follow-up of 30 months, median TTF was 7 months in the CVP arm and 27 months in the R-CVP arm (P < .0001; Table 3).
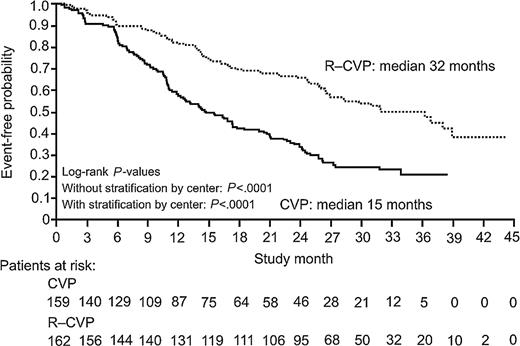
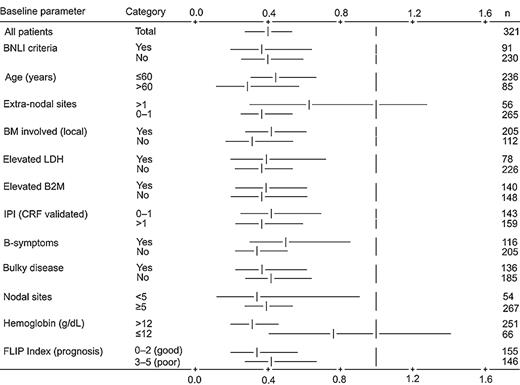
In the analysis of TTP 192 patients have now experienced disease progression, relapse, or death. The median TTP so defined is 15 months in the CVP group and 32 months in the R-CVP group, with 72% of CVP-treated patients experiencing an event versus 48% in the R-CVP group (P < .0001; Figure 1). An exploratory Cox regression analysis of TTP using baseline characteristics showed that the addition of rituximab to CVP reduced the risk of experiencing disease progression across all patient subgroups (age, IPI, lactate dehydrogenase [LDH], etc) compared with CVP (Figure 2).
Time to disease progression, relapse or death after a median follow-up of 30 months among 321 patients assigned to chemotherapy with CVP or with R-CVP. Solid line represents CVP; dotted line, R-CVP.
Time to disease progression, relapse or death after a median follow-up of 30 months among 321 patients assigned to chemotherapy with CVP or with R-CVP. Solid line represents CVP; dotted line, R-CVP.
Cox regression analysis for time to progression by baseline parameter. Vertical lines represent no treatment effect; risk ratio equals 1. Horizontal bars represent 95% confidence intervals for relevant category. Model includes stratification by center pool. BNLI indicates British National Lymphoma Investigation Group; BM, bone marrow; CRF, case report form; FLIP Index, Follicular Lymphoma International Prognostic Index; LDH, lactate dehydrogenase; B2M, β2 microglobulin; and IPI, International Prognostic Index.
Cox regression analysis for time to progression by baseline parameter. Vertical lines represent no treatment effect; risk ratio equals 1. Horizontal bars represent 95% confidence intervals for relevant category. Model includes stratification by center pool. BNLI indicates British National Lymphoma Investigation Group; BM, bone marrow; CRF, case report form; FLIP Index, Follicular Lymphoma International Prognostic Index; LDH, lactate dehydrogenase; B2M, β2 microglobulin; and IPI, International Prognostic Index.
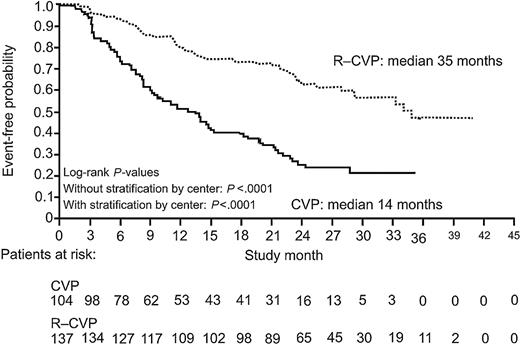
In patients achieving PR or CRu or CR, the median duration of response was 14 months in the CVP group versus 35 months in the R-CVP group at 30 months' median follow up (P < .0001; Figure 3). In patients achieving CR or CRu after initial therapy, the median DFS was 21 months for patients receiving CVP and has not yet been reached for patients in the R-CVP arm (P = .0009).
Duration of response (CR, CRu, PR) after a median follow-up of 30 months among 321 patients assigned to chemotherapy with CVP or with R-CVP. Lines represent groups as in Figure 1.
Duration of response (CR, CRu, PR) after a median follow-up of 30 months among 321 patients assigned to chemotherapy with CVP or with R-CVP. Lines represent groups as in Figure 1.
Median time to institution of new antilymphoma treatment or death was 12 months in the CVP group and has not yet been reached in the R-CVP patients (P < .0001). One hundred and fourteen patients have now received second-line therapy in the CVP arm. Of these, 32 were treated with rituximab alone or in combination with chemotherapy. In the R-CVP group, 63 patients have now received new treatment, of whom 6 were given rituximab or rituximab-containing therapy.
Forty-nine patients have died (28 CVP, 21 R-CVP): Kaplan-Meier estimates for overall survival at 30 months were 85% for CVP and 89% for R-CVP (log-rank test of overall survival duration, P = .22). Twenty-two (14%) patients have died from lymphoma in the CVP arm and 13 (8%) patients from lymphoma in the R-CVP arm (P = .088, log-rank test of overall survival duration; lymphoma-related death only).
Adverse effects
The proportion of patients that reported at least one adverse event was comparable between the CVP (95%) and R-CVP (97%) groups. Adverse events associated with the gastrointestinal and nervous systems as well as general disorders and administration-site reactions were the most commonly occurring types of events in both treatment groups. Differences between the groups with respect to the type and incidence of adverse events were mainly accounted for by events typically associated with rituximab monotherapy. Fatigue, neutropenia, and back pain were the most common severe adverse events and occurred at a slightly higher frequency in patients receiving R-CVP. Five patients experienced a total of 6 life-threatening events following R-CVP. No treatment-related deaths occurred.
More patients in the R-CVP group than in the CVP group experienced an adverse event within 24 hours of an infusion (71% vs 51%, respectively). Fourteen (9%) patients had a grade 3 or 4 rituximab infusion-related reaction, and 2 of these were withdrawn from study treatment. These data are consistent with previous studies of rituximab.22,33 The incidence of grade 3 or 4 neutropenia was higher during treatment with R-CVP (24%) compared with CVP (14%), but there was no difference between groups in the overall infection rate or incidence of neutropenic sepsis.
Discussion
In this randomized trial, adding rituximab to CVP chemotherapy in previously untreated patients with advanced follicular lymphoma results in major improvement in all clinical endpoints. At a median follow-up of 30 months, the addition of rituximab to a standard CVP regimen significantly lengthened time to treatment failure and more than doubled time to progression, with significantly improved response rates, duration of response, disease-free survival, and time to next antilymphoma treatment. It is also important to note that the median time to new antilymphoma treatment has not yet been reached in the R-CVP arm, suggesting that such patients gain long-term benefit from this simple 24-week regimen.
Rituximab plus CVP also significantly increased the duration of response, disease-free survival, and time to progression compared with that obtained in patients receiving CVP only. This is in contrast to the results seen with the addition of anthracyclines to other regimens or use of purine analogs, where increased response rates have not always translated into an increase in response duration or time to progression, even in responding patients.
Therapy with R-CVP was well tolerated, and produced a pattern of adverse events broadly similar to CVP alone, with the exception of some mild to moderate infusion-related reactions following rituximab administration and a higher incidence of grade 3 or 4 neutropenia, without an increased risk of infection. This may have biased the data against R-CVP, as patients in this group received lower doses of chemotherapy as a consequence of the stringent trial rules for dose reduction (see “Treatment”), which may not be applied in standard clinical practice.
The apparently shorter TTF (7 months) observed with CVP treatment in our study compared with the TTF achieved with the same regimen in other trials is a result of stable disease at cycle 4 being considered a treatment-failure event. When the TTP analysis evaluating disease progression, relapse after response, or death is considered, the results obtained in the CVP arm (median TTP 15 months) are comparable with other published data for this and other similar regimens in similar patient populations.15,31
A number of studies14,16,17,34 suggest that interferon may improve the outcome in patients with follicular lymphoma when added to anthracycline-containing regimens. However, the incremental toxicity conferred by the addition of interferon and anthracycline and the long treatment period (up to 18 months), has limited the uptake of this approach as primary therapy in many countries. The improvement in TTP observed with the addition of rituximab to CVP in our trial as a 6-month regimen appears similar to that seen in patients with comparable prognoses treated with more intensive combinations of interferon and anthracycline-containing regimens, but without the attendant toxicity.14,35 Furthermore these results have been achieved in a very poor prognostic group: patients recruited to this trial had a high tumor burden (Table 1), with 50% of patients having high FLIPI scores, a group in whom 5-year overall survival is only 53%.32
Further studies of rituximab in first-line therapy have recently been presented. Hainsworth et al36 reported follow-up data for patients with indolent lymphoma treated with prolonged first-line rituximab monotherapy. The initial overall response rate was 73%: at a median follow-up of 55 months, median progression-free survival is 37 months in the overall patient group and is 52 months in the subgroup of patients with follicular lymphoma. Over three-quarters (77%) of patients who experienced a complete response remain progression-free.36
Hochster and colleagues37 have recently presented the results of a large prospective randomized trial where patients with low-grade NHL achieving a response or stable disease after induction treatment with CVP were randomized to receive rituximab maintenance therapy (4 weekly infusions every 6 months) over 2 years or no further therapy. The study was terminated early after an interim analysis, due to significant superiority of the maintenance arm: after a median follow-up of 14 months, the Kaplan-Meier estimate for progression-free survival at 2 years was 74% of patients in the maintenance arm compared with 42% of patients receiving no further therapy (P = .0008).
Although alkylating agent-based therapies remain standard first-line therapy for follicular lymphoma in many centers, groups where anthracycline-based therapies are standard therapy are now also demonstrating the benefits of adding rituximab to a more intensive induction regimen. Hiddemann et al38 have recently presented preliminary data from a randomized study comparing rituximab plus CHOP versus CHOP alone in patients with untreated follicular lymphoma. Although the data are complicated by a second randomization to interferon or autologous stem-cell transplantation, the median progression-free survival obtained in the rituximab-CHOP arm of the study was significantly superior to that seen with CHOP alone, confirming the results of our study.
There is, thus, accumulating preliminary evidence from other large-scale multicenter randomized trials that the addition of rituximab to first-line therapy for follicular lymphoma significantly improves outcomes when compared with standard chemotherapy regimens of varying intensities. In this study the addition of rituximab to CVP demonstrated major improvements in all clinical end points with minimal additional side effects. R-CVP is a highly effective, short, and very low-toxicity regimen that may now be considered as a new standard regimen for the treatment of previously untreated patients with follicular NHL.
Appendix
The following people and institutions participated in this study: Data and Safety Monitoring Committee (DSMC)—M. Dreyling (Germany), C. Gisselbrecht (France), M. Herold (Germany), and M. Buyse (DSMC Statistician, Belgium). Study centers: Australia—Monash Medical Centre, Clayton (J. Catalano); Box Hill Hospital, Box Hill (J. McKendrick); Royal Perth Hospital, Perth (R. Herrmann); Liverpool Hospital, Liverpool (D. Rosenfeld); Westmead Hospital, Westmead (K. Bradstock); The Queen Elizabeth Hospital, Woodville (J. Norman); and Mater Adult Hospital, South Brisbane (K. Taylor); Belgium—Algemeen Ziekenhuis Middelheim, Antwerpen (R. De Bock); Institut Jules Bordet, Bruxelles (D. Bron); and Universitair Ziekenhuis, Gent (F. Offner); Brazil—Santa Casa de Misericórdia de São Paulo, São Paulo (C. Chiattone); Canada—Cross Cancer Institute, Edmonton (A. Belch); Ottawa Hospital, Ottawa (I. Bence-Bruckler); QE II Health Sciences Centre, Halifax (S. Robinson); Toronto-Sunnybrook Regional Cancer Centre, Toronto (K. Imrie); Royal Victoria Hospital, Montreal (C. Shustik); and Princess Margaret Hospital, Toronto (M. Crump); France—Clinique Victor Hugo, Le Mans (P. Solal-Celigny); and Centre Hospitalier de Versailles, Le Chesnay (S. Castaigne); Israel—Rabin Medical Center, Petach-Tikva (M. Shaklai); Poland—M. Sklodowska-Curie Memorial Institute, Warszawa (J. Walewski); Medical University of Lublin, Lublin (A. Dmoszynska); and Medical University of Wroclaw, Wroclaw (K. Kuliczkowski); Portugal—Hospital de St António dos Capuchos, Lisboa (J. Veiga); Hospitais da Universidade de Coimbra, Coimbra (F. Plácido); and Hospital Santa Maria, Lisboa (J. Raposo); Spain—Hospital La Fe de Valencia, Valencia (J. Gómez-Codina); Hospital Gregorio Marañón, Madrid (E. Flores); Hospital Clínico Universitario de Málaga, Málaga (A. Rueda); Complejo Hospitalario de Pontevedra, Pontevedra (M. Constenla); and Hospital Sant Joan, Tarragona (J. Gumá); Switzerland—Kantonsspital St. Gallen, St. Gallen (T. Cerny); and Institut für med. Onkologie, Bern (R. Zenhäusern); United Kingdom—Hospital Universitario de Canarias, La Laguna, Tenerife (N. Batista); Addenbrooke's Hospital, Cambridge (R. Marcus); Kings College Hospital, London (A. Pagliuca); University of Glasgow, Glasgow (T. Fitzsimons); Glasgow Infirmary, Glasgow (D. Dunlop); Leeds General Infirmary, Leeds (G. Morgan); Royal Victoria Infirmary, Newcastle upon Tyne (S. Proctor); John Radcliffe Hospital, Headington (C. Hatton); Royal Free Hospital, London (M. Potter); Royal Marsden Hospital, Sutton (D. Cunningham); Weston Park Hospital NHS Trust, Sheffield (B. Hancock); Southampton General Hospital, Southampton (P. Johnson); University College Hospital, Cardiff (C. Poynton); and St George's Hospital, London (R. Pettengell).
Prepublished online as Blood First Edition Paper, October 19, 2004; DOI 10.1182/blood-2004-08-3175.
Supported by grants from F. Hoffman-LaRoche Ltd. (R.M.).
A complete list of study participants appears in the “Appendix.”
An Inside Blood analysis of this article appears in the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
The authors gratefully acknowledge the valued assistance of the M39021 Study Management Team.