Abstract
The association of ethnicity with the incidence of graft-versus-host disease (GVHD) and other clinical outcomes after transplantation is controversial. We compared the results of HLA-identical sibling bone marrow transplantations for leukemia, performed between 1990 and 1999, among different ethnic populations, including 562 Japanese, 829 white Americans, 71 African Americans, 195 Scandinavians, and 95 Irish. Results for adults and children were analyzed separately. Multivariate analyses of adult patients showed that white Americans, African Americans, and Irish cohorts were at significantly higher risk for acute GVHD than Japanese or Scandinavian cohorts (relative risk [RR] = 1.77, P < .001; RR = 1.84, P < .006; RR = 2.22, P < .001, respectively). White Americans, African Americans, and Irish, but not Scandinavians, were at significantly higher risk for early (within 3 months of transplantation) transplant-related mortality (TRM) compared with Japanese (RR = 2.99, P < .001; RR = 5.88, P < .001; RR = 2.66, P < .009, respectively). No differences in the risk for chronic GVHD, relapse, and overall survival were noted. In the pediatric cohort (limited to Japanese and white Americans), white Americans were at significantly higher risk for acute (RR = 1.93; P = .04) and chronic (RR = 3.16; P = .002) GVHD. No differences in other clinical outcomes were noted. Our findings suggest that ethnicity may influence the risk for GVHD, though overall survival rates after transplantation remain similar.
Introduction
There is considerable interest in identifying genetically determined factors other than HLA that correlate with immune-mediated outcomes of allogeneic bone marrow transplantation (BMT). These genetic factors include the extensive, but poorly characterized, minor histocompatibility antigen (mHag) system involving hundreds of gene polymorphisms throughout the genome.1 A growing number of cytokine gene polymorphisms are now correlated with transplantation outcomes.2,3 Genetic diversity of transplant-related genes is likely to correlate with the diversity of the gene pool in a particular human population. Gene pool diversity, in turn, reflects population mixing. Populations (referred to hereafter as island populations) that have remained geographically isolated for significant periods of time are likely to have less genetic diversity than populations experiencing recent and multiple immigrations. The genetic homogeneity of island populations is likely to be greatest where the founder population was smallest. We hypothesized that transplantation outcome in specific global locations could be affected by the genetic characteristics of the local transplant population, such that transplantation centers treating island populations might experience transplantation outcomes different from those of centers treating genetically diverse populations.
Reports from various Asian countries suggest that risks for acute and chronic graft-versus-host disease (GVHD) are lower in those countries than in North America and Europe. It is speculated that the lower diversity of histocompatibility antigens in some ethnic groups, particularly those of island populations with restricted migration patterns, may account for this.3-5 However, many other factors may affect real and apparent risks for GVHD, including cytokine gene polymorphisms and differences in other clinical variables such as age and transplantation regimens. Data directly comparing risks for GVHD and other transplantation outcomes, adjusting for these potentially confounding factors, are lacking. Using data from the International Bone Marrow Transplant Registry (IBMTR), the Japan Adult Leukemia Study Group (JALSG), and the Japan Society for Hematopoietic Cell Transplantation (JSHCT), we compared outcomes after HLA-identical sibling BMT for acute or chronic leukemia in 3 island populations (Japan, Scandinavia, and southern Ireland) with outcomes in the genetically diverse population of the United States.
Materials and methods
Data sources
The IBMTR is a voluntary working group of more than 400 transplantation centers worldwide that contribute detailed data on consecutive allogeneic hematopoietic stem cell transplantations to a statistical center at the Health Policy Institute of the Medical College of Wisconsin in Milwaukee. Based on data collected in the Centers for Disease Control Hospital Surveys and the United States Government Accounting Office and worldwide surveys of transplantation activity, approximately 40% of allogeneic transplantations worldwide are registered with the IBMTR. Participating centers are required to register all transplantations consecutively; compliance is monitored by on-site audits. Patients are monitored longitudinally, with yearly follow-up. Computerized checks for errors, physician reviews of submitted data, and on-site audits of participating centers ensure data quality. IBMTR observational studies are conducted with a waiver of informed consent and are in compliance with Health Insurance Portability and Accountability Act (HIPAA) regulations, as determined by the Institutional Review Board and the Privacy Officer of the Medical College of Wisconsin.
The IBMTR collects data at 2 levels: registration and research. Registration data include disease type; age, sex, and pretransplantation performance status of patient; disease stage and responsiveness to chemotherapy; date of diagnosis; donor and graft type (bone marrow–derived or blood-derived stem cells); high-dose conditioning regimen; posttransplantation engraftment; GVHD; disease recurrence and patient survival; development of new malignancy; and cause of death. Requests for data on disease or death for registered patients are at 1-year intervals. All IBMTR teams contribute registration data on all patients. Research data, including comprehensive clinical information before and after transplantation, are collected on subsets of registered patients selected using a weighted randomization scheme. JALSG and JSHCT collected additional data for this study from Japanese transplantation centers not participating in the IBMTR.
The JSHCT is an academic society for stem cell transplantation; its data center, located in Nagoya, collects data on transplantations performed in Japan. Patients are followed up yearly. The JALSG is a voluntary cooperative study group of 196 hematology centers in Japan that treat more than 40% of Japanese adult leukemia patients. It has a central office at Hamamatsu University School of Medicine, a Data Management Center at Nagasaki University Graduate School of Medicine, and a Web Registration Center at Kanazawa University Graduate School of Medicine. JALSG participated in this study as a group using data obtained from JSHCT. Data were checked by computer and by physicians, and accuracy was reconfirmed by the institution before it was submitted to the IBMTR Statistical Center, where all analyses were performed.
Selection of study population
Island populations were selected using the following criteria: (1) availability of data for 50 or more HLA-identical sibling transplants per ethnic cohort; (2) historical records indicating 1000 or more years of settlement by the present day population, with minimal incursions by immigrants. Cohorts coming from Japan, southern Ireland, and Scandinavia (Sweden, Norway, Denmark) fulfilled these criteria. These ethnic cohorts were compared with white and African American populations in North America reported to the IBMTR. Analyses were restricted to patients who underwent transplantation for acute lymphoblastic leukemia (ALL), acute myelogenous leukemia (AML), or chronic myelogenous leukemia (CML) between 1990 and 1999. The population was further restricted to patients undergoing first transplantation, in first complete remission or in first chronic phase at the time of transplantation, and receiving a non–T-cell–depleted bone marrow graft from an HLA-identical sibling and administered methotrexate and cyclosporine, with or without other drugs, for GVHD prophylaxis.
Historical background of study population
Most of the Japanese population (78 million) is believed to have derived from a small founder population that migrated from a single region of China in approximately 5000 BC. No major immigration has occurred in more than 1000 years. This population is thus genetically homogeneous.6 The southern Irish population originated from an early postglacial migration of western Europeans some 20 000 years ago; the size of the founder population, though unknown, was probably small. The next series of migrations occurred by Celtic tribes from approximately 2000 to 1000 BC. Thereafter, with the exception of Viking settlements, the Irish population remained genetically isolated from large European migrations in the post-Roman period. Today's southern Irish population (4 million) represents the descendants of an estimated 0.25 million persons surviving in the country after the potato famines of the mid-19th century.7 Scandinavians originated from a series of northwesterly migrations of central European populations approximately 1000 years ago into a sparsely populated land. The founder population size is unknown but was likely small. The present day population has experienced little recent immigration.8 The mixed population of North Americans (270 million) represents a genetic admixture in the last 250 years mainly of diverse Europeans, American Indians, Hispanics, and African Americans. We studied 2 subgroups: NorthAmerican white persons, representing mixed European ancestry, and African Americans, whose African origin implies a well-described and extremely wide genetic diversity.
Study end points
Primary outcomes were the cumulative incidences of grades 2 to 4 acute GVHD for patients surviving more than 21 days, with evidence of engraftment and chronic GVHD in patients surviving more than 90 days with evidence of engraftment. Other clinical outcomes evaluated included overall survival, leukemia-free survival (LFS, survival without leukemia after transplantation), leukemia relapse, and treatment-related mortality (TRM, death in continuous complete remission after transplantation).
Statistical analyses
Separate analyses were performed for adults (20 years of age and older) and children (younger than 20 years). The study categorized ethnic populations into the following groups: Japanese, white American, African American, Scandinavian, and Irish. Tables 1 (adult) and 2 (children) show the characteristics of each population according to ethnic group. Because of the small numbers of available subjects, analyses of the pediatric cohort were limited to Japanese and white American groups. Univariate probabilities of LFS and survival were calculated using the Kaplan-Meier estimator; the log-rank test was used for univariate comparisons. For LFS, patients were considered to have experienced treatment failure at the time of relapse or death from any cause; patients alive in continuous complete remission were censored at the last follow-up evaluation. For survival, death from any cause was considered an event; surviving patients were censored at the last follow-up evaluation. Probabilities of acute or chronic GVHD, TRM, and leukemia relapse were calculated using cumulative incidence curves to accommodate competing risks.9 For acute and chronic GVHD, death from any cause was considered the competing event; patients surviving without GVHD were censored at last follow-up. For relapse, TRM was the competing event; patients alive and in remission were censored at the last follow-up evaluation. For TRM, recurrent leukemia was the competing event; patients alive in remission were censored at the last follow-up evaluation.
Characteristics of patients aged 20 years and older
. | No. eval . | Japanese . | No. eval . | White American . | No. eval . | African American . | No. eval . | Scandinavian . | No. eval . | Irish . | P . |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Disease type, no. (%) | 562 | < .001 | |||||||||
| AML | 179 (32) | 829 | 256 (31) | 71 | 18 (26) | 192 | 72 (37) | 95 | 35 (37) | ||
| ALL | 136 (24) | 88 (11) | 1 (1) | 26 (14) | 17 (18) | ||||||
| CML | 247 (44) | 485 (58) | 52 (73) | 94 (49) | 43 (45) | ||||||
| Donor-patient sex match, no (%) | 521 | .39 | |||||||||
| M-M | 170 (33) | 828 | 232 (28) | 71 | 25 (35) | 191 | 69 (36) | 95 | 30 (32) | ||
| M-F | 117 (22) | 198 (24) | 14 (20) | 45 (24) | 27 (28) | ||||||
| F-M | 137 (26) | 221 (27) | 17 (24) | 37 (19) | 24 (25) | ||||||
| F-F | 97 (19) | 177 (21) | 15 (21) | 40 (21) | 14 (15) | ||||||
| Donor-patient CMV status, no (%) | 562 | < .001 | |||||||||
| Both negative | 40 (7) | 829 | 236 (28) | 71 | 5 (7) | 192 | 27 (14) | 95 | 53 (56) | ||
| At least 1 positive | 296 (53) | 561 (68) | 65 (92) | 160 (83) | 40 (42) | ||||||
| Inconclusive/unknown | 226 (40) | 32 (4) | 1 (1) | 5 (3) | 2 (2) | ||||||
| Conditioning regimen, no (%) | 562 | < .001 | |||||||||
| BuCy ± other | 244 (43) | 829 | 376 (45) | 1 | 41 (58) | 192 | 53 (28) | 95 | 53 (56) | ||
| TBICy ± other | 250 (45) | 238 (29) | 21 (29) | 122 (64) | 40 (42) | ||||||
| TBI ± other | 50 (9) | 124 (15) | 4 (6) | 4 (2) | 0 | ||||||
| Other | 18 (3) | 91 (11) | 5 (7) | 13 (8) | 2 (2) | ||||||
| Patient age, y, median (range) | 562 | 36 (20-59) | 829 | 40 (20-63) | 71 | 39 (21-60) | 192 | 41 (20-59) | 95 | 36 (20-56) | < .001 |
| 21-40 | 354 (63) | 406 (49) | 40 (56) | 89 (46) | 59 (62) | < .001 | |||||
| Older than 40 | 208 (37) | 423 (51) | 31 (44) | 103 (54) | 36 (38) | NA | |||||
| WBC count at diagnosis, × 109/L | 477 | 29 (0-965) | 790 | 58 (0-900) | 70 | 74 (1-516) | 186 | 40 (0-500) | 89 | 50 (1-547) | < .001 |
| Karnofsky score, 90%-100%, no. (%) | ND | 823 | 597 (73) | 71 | 61 (86) | 191 | 155 (81) | 93 | 57 (61) | .003 | |
| Year of treatment, mo, median (range) | 562 | 95 (90-99) | 829 | 94 (90-99) | 71 | 94 (90-99) | 192 | 93 (90-99) | 95 | 96 (90-99) | < .001 |
| NA | |||||||||||
| 1990-1994 | 258 (46) | 455 (55) | 40 (56) | 126 (66) | 40 (42) | < .001 | |||||
| 1995-1999 | 304 (54) | 374 (45) | 31 (44) | 66 (34) | 55 (58) | ||||||
| Time from diagnosis to treatment, mo, median (range) | 550 | 7.3 (0.2-69) | 829 | 5.0 (1.3-224) | 71 | 7.3 (2.1-89) | 190 | 6.0 (2.3-48) | 94 | 10.4 (4.7-94) | < .001 |
| Follow-up time among patients who survived, mo, median (range) | 372 | 65 (5-130) | 530 | 48 (2-138) | 42 | 33 (3-123) | 135 | 60 (4-120) | 66 | 42 (3-114) | < .001 |
. | No. eval . | Japanese . | No. eval . | White American . | No. eval . | African American . | No. eval . | Scandinavian . | No. eval . | Irish . | P . |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Disease type, no. (%) | 562 | < .001 | |||||||||
| AML | 179 (32) | 829 | 256 (31) | 71 | 18 (26) | 192 | 72 (37) | 95 | 35 (37) | ||
| ALL | 136 (24) | 88 (11) | 1 (1) | 26 (14) | 17 (18) | ||||||
| CML | 247 (44) | 485 (58) | 52 (73) | 94 (49) | 43 (45) | ||||||
| Donor-patient sex match, no (%) | 521 | .39 | |||||||||
| M-M | 170 (33) | 828 | 232 (28) | 71 | 25 (35) | 191 | 69 (36) | 95 | 30 (32) | ||
| M-F | 117 (22) | 198 (24) | 14 (20) | 45 (24) | 27 (28) | ||||||
| F-M | 137 (26) | 221 (27) | 17 (24) | 37 (19) | 24 (25) | ||||||
| F-F | 97 (19) | 177 (21) | 15 (21) | 40 (21) | 14 (15) | ||||||
| Donor-patient CMV status, no (%) | 562 | < .001 | |||||||||
| Both negative | 40 (7) | 829 | 236 (28) | 71 | 5 (7) | 192 | 27 (14) | 95 | 53 (56) | ||
| At least 1 positive | 296 (53) | 561 (68) | 65 (92) | 160 (83) | 40 (42) | ||||||
| Inconclusive/unknown | 226 (40) | 32 (4) | 1 (1) | 5 (3) | 2 (2) | ||||||
| Conditioning regimen, no (%) | 562 | < .001 | |||||||||
| BuCy ± other | 244 (43) | 829 | 376 (45) | 1 | 41 (58) | 192 | 53 (28) | 95 | 53 (56) | ||
| TBICy ± other | 250 (45) | 238 (29) | 21 (29) | 122 (64) | 40 (42) | ||||||
| TBI ± other | 50 (9) | 124 (15) | 4 (6) | 4 (2) | 0 | ||||||
| Other | 18 (3) | 91 (11) | 5 (7) | 13 (8) | 2 (2) | ||||||
| Patient age, y, median (range) | 562 | 36 (20-59) | 829 | 40 (20-63) | 71 | 39 (21-60) | 192 | 41 (20-59) | 95 | 36 (20-56) | < .001 |
| 21-40 | 354 (63) | 406 (49) | 40 (56) | 89 (46) | 59 (62) | < .001 | |||||
| Older than 40 | 208 (37) | 423 (51) | 31 (44) | 103 (54) | 36 (38) | NA | |||||
| WBC count at diagnosis, × 109/L | 477 | 29 (0-965) | 790 | 58 (0-900) | 70 | 74 (1-516) | 186 | 40 (0-500) | 89 | 50 (1-547) | < .001 |
| Karnofsky score, 90%-100%, no. (%) | ND | 823 | 597 (73) | 71 | 61 (86) | 191 | 155 (81) | 93 | 57 (61) | .003 | |
| Year of treatment, mo, median (range) | 562 | 95 (90-99) | 829 | 94 (90-99) | 71 | 94 (90-99) | 192 | 93 (90-99) | 95 | 96 (90-99) | < .001 |
| NA | |||||||||||
| 1990-1994 | 258 (46) | 455 (55) | 40 (56) | 126 (66) | 40 (42) | < .001 | |||||
| 1995-1999 | 304 (54) | 374 (45) | 31 (44) | 66 (34) | 55 (58) | ||||||
| Time from diagnosis to treatment, mo, median (range) | 550 | 7.3 (0.2-69) | 829 | 5.0 (1.3-224) | 71 | 7.3 (2.1-89) | 190 | 6.0 (2.3-48) | 94 | 10.4 (4.7-94) | < .001 |
| Follow-up time among patients who survived, mo, median (range) | 372 | 65 (5-130) | 530 | 48 (2-138) | 42 | 33 (3-123) | 135 | 60 (4-120) | 66 | 42 (3-114) | < .001 |
Eval indicates evaluated; M, male; F, female; NA, not applicable; and ND, not done.
Characteristics of patients younger than 20 years of age
. | No. eval . | Japanese . | No. eval. . | White American . | P . |
|---|---|---|---|---|---|
| Disease type, no (%) | .04 | ||||
| AML | 156 | 66 (42) | 144 | 78 (54) | |
| ALL | 67 (43) | 42 (29) | |||
| CML | 23 (15) | 24 (17) | |||
| Donor-patient sex match, no (%) | .80 | ||||
| M-M | 144 | 37 (26) | 142 | 42 (30) | |
| M-F | 39 (27) | 32 (22) | |||
| F-M | 43 (30) | 43 (30) | |||
| F-F | 25 (17) | 25 (28) | |||
| Donor-patient CMV status, no (%) | .01 | ||||
| Both negative | 76 | 25 (33) | 143 | 73 (51) | |
| At least 1 positive | 51 (67) | 67 (47) | |||
| Inconclusive/not tested | 0 | 3 (2) | |||
| Conditioning regimen, no (%) | < .001 | ||||
| BuCy ± other | 156 | 49 (32) | 144 | 60 (42) | |
| TBICy ± other | 64 (41) | 37 (26) | |||
| TBI ± other | 13 (8) | 36 (25) | |||
| Other | 30 (19) | 11 (7) | |||
| Patient age, y, median (range) | 156 | 16 (1-20) | 144 | 13 (0-20) | .001 |
| 0-10 | 42 (27) | 55 (38) | .04 | ||
| Older than 10 | 114 (73) | 89 (62) | NA | ||
| WBC count at diagnosis, × 109/L | 143 | 23 (1-541) | 138 | 28 (0-923) | .30 |
| Karnofsky score, 90%-100%, no (%) | ND | 144 | 127 (88) | NA | |
| Year of treatment | 156 | 94 (90-99) | 144 | 94 (90-99) | |
| 1990-1994 | 81 (52) | 73 (51) | .93 | ||
| 1995-1999 | 75 (48) | 71 (49) | .83 | ||
| Time from diagnosis to treatment, mo | 154 | 7.1 (1.4-37.9) | 143 | 3.8 (1.2-62.0) | < .001 |
| Follow-up time among patients who survived, mo | 109 | 71 (8-138) | 101 | 37 (3-127) | < .001 |
. | No. eval . | Japanese . | No. eval. . | White American . | P . |
|---|---|---|---|---|---|
| Disease type, no (%) | .04 | ||||
| AML | 156 | 66 (42) | 144 | 78 (54) | |
| ALL | 67 (43) | 42 (29) | |||
| CML | 23 (15) | 24 (17) | |||
| Donor-patient sex match, no (%) | .80 | ||||
| M-M | 144 | 37 (26) | 142 | 42 (30) | |
| M-F | 39 (27) | 32 (22) | |||
| F-M | 43 (30) | 43 (30) | |||
| F-F | 25 (17) | 25 (28) | |||
| Donor-patient CMV status, no (%) | .01 | ||||
| Both negative | 76 | 25 (33) | 143 | 73 (51) | |
| At least 1 positive | 51 (67) | 67 (47) | |||
| Inconclusive/not tested | 0 | 3 (2) | |||
| Conditioning regimen, no (%) | < .001 | ||||
| BuCy ± other | 156 | 49 (32) | 144 | 60 (42) | |
| TBICy ± other | 64 (41) | 37 (26) | |||
| TBI ± other | 13 (8) | 36 (25) | |||
| Other | 30 (19) | 11 (7) | |||
| Patient age, y, median (range) | 156 | 16 (1-20) | 144 | 13 (0-20) | .001 |
| 0-10 | 42 (27) | 55 (38) | .04 | ||
| Older than 10 | 114 (73) | 89 (62) | NA | ||
| WBC count at diagnosis, × 109/L | 143 | 23 (1-541) | 138 | 28 (0-923) | .30 |
| Karnofsky score, 90%-100%, no (%) | ND | 144 | 127 (88) | NA | |
| Year of treatment | 156 | 94 (90-99) | 144 | 94 (90-99) | |
| 1990-1994 | 81 (52) | 73 (51) | .93 | ||
| 1995-1999 | 75 (48) | 71 (49) | .83 | ||
| Time from diagnosis to treatment, mo | 154 | 7.1 (1.4-37.9) | 143 | 3.8 (1.2-62.0) | < .001 |
| Follow-up time among patients who survived, mo | 109 | 71 (8-138) | 101 | 37 (3-127) | < .001 |
ND indicates no data; NA, not applicable.
Comparisons between the Japanese cohort (reference group) and the other ethnic cohorts, while adjusting for other clinical covariates, were performed with multivariate Cox proportional hazards regression analysis.10 The assumption of proportional hazards was tested using a time-dependent covariate. Ethnicity was forced in all models. Other variables considered in the models are described in Tables 1 and 2. Forward stepwise variable selection at a .05 significance level was used to identify covariates other than ethnicity associated with outcome. Interactions between ethnicity and all covariates were tested before and after model building. Overall covariate effects were tested using the Wald test. Because of multiple comparisons, Bonferroni adjusted P values were applied to all multivariate models (ie, P < .005 was considered significant). All computations were executed using the procedure PHREG in the statistical package SAS (SAS Institute, Cary, NC).
Results
Tables 1 and 2 show characteristics of the adult and pediatric patients by ethnic group. The adult cohort consisted of 562 Japanese, 192 Scandinavians, 95 Irish, 71 African Americans, and 829 white Americans. The pediatric cohort consisted of 156 Japanese and 144 white Americans. Differences in patient-, disease-, and transplant-related characteristics are noted and were considered in the multivariate analyses of all outcomes studied.
GVHD and TRM in adults
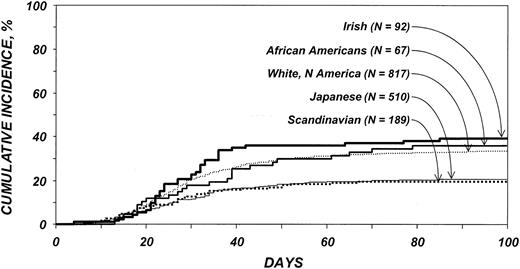
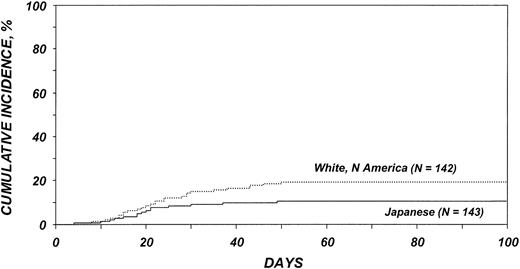
Cumulative incidences of grades 2 to 4 acute GVHD among patients older than 20 years are shown in Figure 1. All ethnic cohorts except the Scandinavians had significantly higher risks for acute GVHD than the Japanese (Tables 3, 4). Risks for acute GVHD were similar among white Americans, African Americans, and Irish. No other variable analyzed was significantly associated with acute GVHD. No statistically significant differences in the risk for chronic GVHD were detected among the ethnic cohorts.
Cumulative incidence of acute GVHD by ethnic group among patients 20 years and older. Bold solid line indicates Irish; medium solid line, African Americans; thin dotted line, white Americans; thin solid line, Japanese; and bold dotted line, Scandinavians.
Cumulative incidence of acute GVHD by ethnic group among patients 20 years and older. Bold solid line indicates Irish; medium solid line, African Americans; thin dotted line, white Americans; thin solid line, Japanese; and bold dotted line, Scandinavians.
Multivariate analysis of grades 2 to 4 acute GVHD for patients aged 20 years and older
Ethnic group . | N . | Relative risk (95% CI) . | P . |
|---|---|---|---|
| Japanese | 510 | 1.00 | NA |
| White American | 817 | 1.77 (1.42-2.21) | < .001† |
| African American | 67 | 1.84 (1.18-2.87) | .006† |
| Scandinavian | 189 | 0.94 (0.65-1.37) | .99 |
| Irish | 92 | 2.22 (1.53-3.23) | < .001† |
Ethnic group . | N . | Relative risk (95% CI) . | P . |
|---|---|---|---|
| Japanese | 510 | 1.00 | NA |
| White American | 817 | 1.77 (1.42-2.21) | < .001† |
| African American | 67 | 1.84 (1.18-2.87) | .006† |
| Scandinavian | 189 | 0.94 (0.65-1.37) | .99 |
| Irish | 92 | 2.22 (1.53-3.23) | < .001† |
Multivariate analysis of grades 2 to 4 acute GVHD for patients younger than 20 years of age
Variables . | N . | Relative risk (95% CI) . | P . |
|---|---|---|---|
| Ethnic group | |||
| Japanese | 143 | 1.00 | NA |
| White American | 142 | 1.93 (1.02-3.64) | .04 |
| Conditioning regimen | .01* | ||
| Bu-Cy ± other | 103 | 1.00 | NA |
| CY-TBI ± other | 143 | 2.38 (1.16-4.88) | .02 |
| Other | 39 | 0.29 (0.04-2.25) | .23 |
| Year of transplantation | |||
| 1990-1994 | 150 | 1.00 | NA |
| 1995-1999 | 135 | 2.53 (1.31-4.88) | .006 |
Variables . | N . | Relative risk (95% CI) . | P . |
|---|---|---|---|
| Ethnic group | |||
| Japanese | 143 | 1.00 | NA |
| White American | 142 | 1.93 (1.02-3.64) | .04 |
| Conditioning regimen | .01* | ||
| Bu-Cy ± other | 103 | 1.00 | NA |
| CY-TBI ± other | 143 | 2.38 (1.16-4.88) | .02 |
| Other | 39 | 0.29 (0.04-2.25) | .23 |
| Year of transplantation | |||
| 1990-1994 | 150 | 1.00 | NA |
| 1995-1999 | 135 | 2.53 (1.31-4.88) | .006 |
NA indicates not applicable.
See Table 1 for abbreviations.
2 degrees of freedom test.
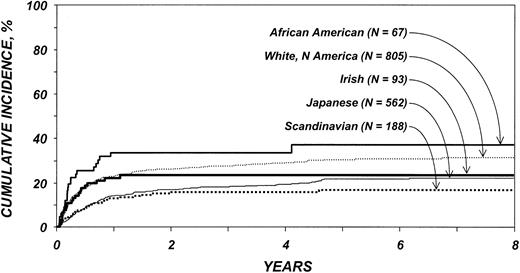
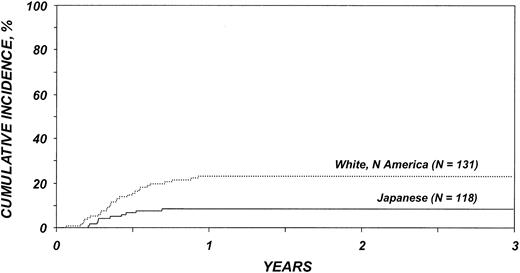
Cumulative incidences of TRM among patients older than 20 years are shown in Figure 2. The effect of ethnicity on TRM varied before and after 3 months after transplantation. White Americans, African Americans, and Irish had significantly higher risks for TRM than Japanese during the first 3 months after transplantation, whereas Scandinavians did not (Table 5). Among patients surviving 3 or more months after transplantation, no differences in TRM were seen. Other variables associated with higher TRM risk were patient age older than 40 years and presence of ALL.
Cumulative incidence of treatment-related mortality by ethnic group among patients 20 years and older. Lines represent populations as in Figure 1.
Cumulative incidence of treatment-related mortality by ethnic group among patients 20 years and older. Lines represent populations as in Figure 1.
Multivariate analysis of TRM for patients 20 and older
Variables . | N . | Relative risk (95% CI) . | P . |
|---|---|---|---|
| 0-3 mo after transplantation* | < .001† | ||
| Japanese | 562 | 1.00 | NA |
| White American | 805 | 2.99 (1.93-4.64) | < .001‡ |
| African American | 67 | 5.88 (3.08-11.20) | < .001‡ |
| Scandinavian | 188 | 1.03 (0.48-2.21) | .99‡ |
| Irish | 93 | 2.66 (1.28-5.54) | .009‡ |
| More than 3 mo after transplantation | .26† | ||
| Japanese | 524 | 1.00 | NA |
| White American | 683 | 1.09 (0.83-1.44) | .99‡ |
| African American | 51 | 1.08 (0.52-2.24) | .99‡ |
| Scandinavian | 176 | 0.64 (0.40-1.03) | .68‡ |
| Irish | 80 | 0.90 (0.48-1.68) | .99‡ |
| Age at transplantation, y | |||
| 20-40 | 935 | 1.00 | NA |
| Older than 40 | 780 | 1.79 (1.46-2.18) | < .001 |
| Disease type | .005§ | ||
| CML | 891 | 1.00 | NA |
| ALL | 268 | 1.51 (1.14-1.99) | .004 |
| AML | 556 | 0.94 (0.75-1.18) | .59 |
Variables . | N . | Relative risk (95% CI) . | P . |
|---|---|---|---|
| 0-3 mo after transplantation* | < .001† | ||
| Japanese | 562 | 1.00 | NA |
| White American | 805 | 2.99 (1.93-4.64) | < .001‡ |
| African American | 67 | 5.88 (3.08-11.20) | < .001‡ |
| Scandinavian | 188 | 1.03 (0.48-2.21) | .99‡ |
| Irish | 93 | 2.66 (1.28-5.54) | .009‡ |
| More than 3 mo after transplantation | .26† | ||
| Japanese | 524 | 1.00 | NA |
| White American | 683 | 1.09 (0.83-1.44) | .99‡ |
| African American | 51 | 1.08 (0.52-2.24) | .99‡ |
| Scandinavian | 176 | 0.64 (0.40-1.03) | .68‡ |
| Irish | 80 | 0.90 (0.48-1.68) | .99‡ |
| Age at transplantation, y | |||
| 20-40 | 935 | 1.00 | NA |
| Older than 40 | 780 | 1.79 (1.46-2.18) | < .001 |
| Disease type | .005§ | ||
| CML | 891 | 1.00 | NA |
| ALL | 268 | 1.51 (1.14-1.99) | .004 |
| AML | 556 | 0.94 (0.75-1.18) | .59 |
NA indicates not applicable.
Other significant comparisons:
Time-dependent covariate, such that risk for TRM varied before and 3 months after transplantation.
4 degrees of freedom test.
Adjusted for 10 multiple comparisons.
2 degrees of freedom test.
Relapse, survival, and LFS in adults
There were no differences among the ethnic groups in the risk for relapse (data not shown). Factors associated with relapse were disease type (patients with ALL or AML were at higher risk for relapse [RR 2.90, P < .0001; RR 1.51, P = .008, respectively] than those with CML) and year of transplantation (transplantations performed from 1995 to 1999 involved lower risk [RR 0.76, P = .04] than did those performed from 1990 to 1994).
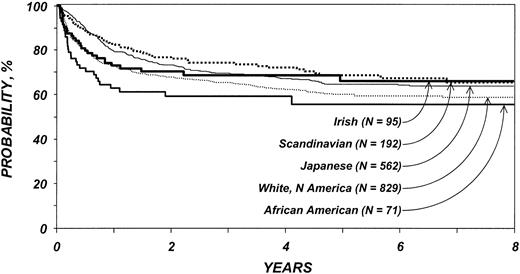
The effect of ethnicity on treatment failure (the inverse of LFS) and overall survival varied with time after transplantation. White Americans and African Americans had significantly higher risks for treatment failure than Japanese patients in the first 3 months after transplantation; Irish patients had marginally increased risk (Table 6). Scandinavian patients did not differ significantly from Japanese patients in the first 3 months. White American and African American patients were at significantly higher risk for treatment failure in the first 3 months than Scandinavian patients. No other differences among ethnic cohorts were seen. Subsequent risks for treatment failure were similar for all ethnic cohorts among patients surviving in remission at least 3 months after transplantation. Higher risks for treatment failure were also associated with patient age older than 40 years, presence of ALL, and transplantation before 1995.
Multivariate analysis of treatment failure for patients 20 and older
Variables . | N . | Relative risk (95% CI) . | P . |
|---|---|---|---|
| 0-3 mo after transplantation* | < .001† | ||
| Japanese | 562 | 1.00 | NA |
| White American | 805 | 2.42 (1.68-3.49) | < .001‡ |
| African American | 67 | 4.47 (2.49-8.05) | < .001‡ |
| Scandinavian | 188 | 0.92 (0.48-1.76) | .99‡ |
| Irish | 93 | 1.97 (1.01-3.86) | .48‡ |
| More than 3 mo after transplantation | .34† | ||
| Japanese | 524 | 1.00 | NA |
| White American | 683 | 0.88 (0.72-1.09) | .99‡ |
| African American | 51 | 0.87 (0.48-1.56) | .99‡ |
| Scandinavian | 176 | 0.74 (0.54-1.01) | .61‡ |
| Irish | 80 | 0.76 (0.47-1.22) | .99‡ |
| Age at transplantation, y | |||
| 20-40 | 935 | 1.00 | NA |
| Older than 40 | 780 | 1.44 (1.23-1.68) | < .001 |
| Disease type | < .001§ | ||
| CML | 891 | 1.00 | NA |
| ALL | 268 | 1.96 (1.59-2.42) | < .001 |
| AML | 556 | 1.11 (0.93-1.33) | .26 |
| Year of transplantation | |||
| 1990-1994 | 901 | 1.00 | NA |
| 1995-1999 | 814 | 0.81 (0.69-0.95) | .01 |
Variables . | N . | Relative risk (95% CI) . | P . |
|---|---|---|---|
| 0-3 mo after transplantation* | < .001† | ||
| Japanese | 562 | 1.00 | NA |
| White American | 805 | 2.42 (1.68-3.49) | < .001‡ |
| African American | 67 | 4.47 (2.49-8.05) | < .001‡ |
| Scandinavian | 188 | 0.92 (0.48-1.76) | .99‡ |
| Irish | 93 | 1.97 (1.01-3.86) | .48‡ |
| More than 3 mo after transplantation | .34† | ||
| Japanese | 524 | 1.00 | NA |
| White American | 683 | 0.88 (0.72-1.09) | .99‡ |
| African American | 51 | 0.87 (0.48-1.56) | .99‡ |
| Scandinavian | 176 | 0.74 (0.54-1.01) | .61‡ |
| Irish | 80 | 0.76 (0.47-1.22) | .99‡ |
| Age at transplantation, y | |||
| 20-40 | 935 | 1.00 | NA |
| Older than 40 | 780 | 1.44 (1.23-1.68) | < .001 |
| Disease type | < .001§ | ||
| CML | 891 | 1.00 | NA |
| ALL | 268 | 1.96 (1.59-2.42) | < .001 |
| AML | 556 | 1.11 (0.93-1.33) | .26 |
| Year of transplantation | |||
| 1990-1994 | 901 | 1.00 | NA |
| 1995-1999 | 814 | 0.81 (0.69-0.95) | .01 |
NA indicates not applicable.
Other significant comparisons:
Time-dependent covariate, such that risk for TRM varied before and 3 months after transplantation.
4 degrees of freedom test.
Adjusted for 10 multiple comparisons.
2 degrees of freedom test.
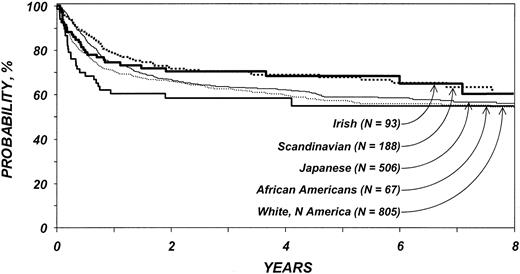
In the first 3 months after transplantation, white American, African American, and Irish patients were at significantly higher risk for overall mortality than Japanese patients but not Scandinavian patients (Table 7). Among patients surviving the first 3 months after transplantation, subsequent mortality risks were similar in all 5 ethnic groups. Other variables associated with mortality were age older than 40 years, disease type (patients with ALL or AML were at higher risk for mortality than those with CML), and year of transplantation (transplantations performed after 1994 put patients at lower risk for death). Probabilities of LFS and overall survival among patients older than 20 years of age are shown in Figures 3 and 4.
Multivariate analysis of overall mortality for patients 20 and older
Variables . | N . | Relative risk (95% CI) . | P . |
|---|---|---|---|
| 0-3 mo after transplantation* | < .001† | ||
| Japanese | 562 | 1.00 | NA |
| White American | 829 | 3.63 (2.35-5.60) | < .001‡ |
| African American | 71 | 7.23 (3.89-13.45) | < .001‡ |
| Scandinavian | 192 | 1.38 (0.69-2.76) | .99‡ |
| Irish | 95 | 3.26 (1.64-6.50) | .008‡ |
| More than 3 mo after transplantation | .63† | ||
| Japanese | 537 | 1.00 | NA |
| White American | 707 | 0.97 (0.78-1.20) | .99‡ |
| African American | 54 | 1.02 (0.57-0.85) | .99‡ |
| Scandinavian | 180 | 0.78 (0.56-1.09) | .99‡ |
| Irish | 81 | 0.83 (0.51-1.38) | .99‡ |
| Age at transplantation, y | |||
| 20-40 | 948 | 1.00 | NA |
| Older than 40 | 801 | 1.53 (1.29-1.80) | < .001 |
| Disease type | < .001§ | ||
| CML | 921 | 1.00 | NA |
| ALL | 268 | 2.26 (1.82-2.81) | < .001 |
| AML | 560 | 1.29 (1.07-1.55) | .008 |
| Year of transplantation | |||
| 1990-1994 | 919 | 1.00 | NA |
| 1995-1999 | 830 | 0.84 (0.71-0.99) | .037 |
Variables . | N . | Relative risk (95% CI) . | P . |
|---|---|---|---|
| 0-3 mo after transplantation* | < .001† | ||
| Japanese | 562 | 1.00 | NA |
| White American | 829 | 3.63 (2.35-5.60) | < .001‡ |
| African American | 71 | 7.23 (3.89-13.45) | < .001‡ |
| Scandinavian | 192 | 1.38 (0.69-2.76) | .99‡ |
| Irish | 95 | 3.26 (1.64-6.50) | .008‡ |
| More than 3 mo after transplantation | .63† | ||
| Japanese | 537 | 1.00 | NA |
| White American | 707 | 0.97 (0.78-1.20) | .99‡ |
| African American | 54 | 1.02 (0.57-0.85) | .99‡ |
| Scandinavian | 180 | 0.78 (0.56-1.09) | .99‡ |
| Irish | 81 | 0.83 (0.51-1.38) | .99‡ |
| Age at transplantation, y | |||
| 20-40 | 948 | 1.00 | NA |
| Older than 40 | 801 | 1.53 (1.29-1.80) | < .001 |
| Disease type | < .001§ | ||
| CML | 921 | 1.00 | NA |
| ALL | 268 | 2.26 (1.82-2.81) | < .001 |
| AML | 560 | 1.29 (1.07-1.55) | .008 |
| Year of transplantation | |||
| 1990-1994 | 919 | 1.00 | NA |
| 1995-1999 | 830 | 0.84 (0.71-0.99) | .037 |
NA indicates not applicable.
Other significant comparisons:
Time-dependent covariate, such that risk for TRM varied before and 3 months after transplantation.
4 degrees of freedom test.
Adjusted for 10 multiple comparisons.
2 degrees of freedom test.
Probability of leukemia-free survival by ethnic group among patients 20 years and older. Lines represent populations as in Figure 1.
Probability of leukemia-free survival by ethnic group among patients 20 years and older. Lines represent populations as in Figure 1.
Probability of survival by ethnic group among patients 20 years and older. Lines represent populations as in Figure 1.
Probability of survival by ethnic group among patients 20 years and older. Lines represent populations as in Figure 1.
Outcomes in patients younger than 20 years
Cumulative incidences of acute and chronic GVHD among patients younger than 20 years of age are shown in Figures 5 and 6. White American children were at significantly higher risk for acute (RR = 1.93; 95% confidence interval [CI], 1.02-3.64; P = .04) (Table 4) and chronic (RR = 3.16; 95% CI, 1.53-6.50; P = .002) GVHD than Japanese children. Patient age at transplantation, type of conditioning regimen, and year of transplantation were associated with risk for acute GVHD. Age at transplantation and donor-recipient sex match were associated with risks for chronic GVHD.
Cumulative incidence of acute GVHD by ethnic group among patients younger than 20 years. Thin dotted line indicates white persons from North America; thin solid line, Japanese.
Cumulative incidence of acute GVHD by ethnic group among patients younger than 20 years. Thin dotted line indicates white persons from North America; thin solid line, Japanese.
Cumulative incidence of chronic GVHD by ethnic group among patients younger than 20 years. Lines represent populations as in Figure 5.
Cumulative incidence of chronic GVHD by ethnic group among patients younger than 20 years. Lines represent populations as in Figure 5.
There was no significant difference between Japanese and white American children in the risk for TRM (RR = 1.78; 95% CI, 0.80-3.96; P = .16) or of relapse (RR = 0.89; 95% CI, 0.56-1.40; P = .61), nor were there significant differences between Japanese and white American children in risks for treatment failure (RR = 1.78; 95% CI, 0.80-3.96; P = .16) and overall survival (RR = 1.78; 95% CI, 0.80-3.96; P = .16).
Frequency distribution of HLA alleles
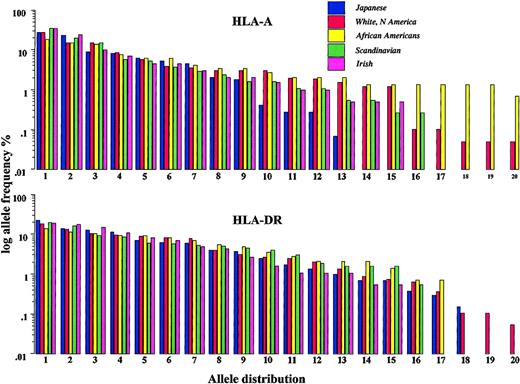
HLA-A, -B, and -DR frequencies are shown in Table 8. The white American, Scandinavian, and Irish populations were largely similar in their allele frequency, whereas the Japanese and African American populations differed substantially. Figure 7 shows the frequency distribution of the 20 most common HLA-A and -DR alleles in each population. There were fewer HLA-A alleles in the Japanese cohort than in the other ethnic groups. HLA-A alleles were more variable in the African American cohort.
Ten most common gene frequencies, listed left to right in descending order of frequency.
HLA-A type | ||||||||||
| Japanese | 24 | 2 | 26 | 11 | 31 | 9 | 33 | 10 | 19 | 3 |
| White American | 2 | 1 | 3 | 24 | 11 | 29 | 32 | 26 | 31 | 28 |
| African American | 2 | 23 | 30 | 3 | 28 | 1 | 34 | 29 | 74 | 68 |
| Scandinavian | 2 | 3 | 1 | 24 | 11 | 28 | 32 | 9 | 31 | 25 |
| Irish | 2 | 1 | 3 | 11 | 28 | 29 | 24 | 26 | 32 | 25 |
| HLA-B type | ||||||||||
| Japanese | 61 | 52 | 35 | 51 | 54 | 44 | 7 | 46 | 48 | 60 |
| White American | 7 | 44 | 8 | 35 | 62 | 60 | 18 | 57 | 27 | 14 |
| African American | 53 | 70 | 7 | 44 | 42 | 45 | 35 | 52 | 8 | 18 |
| Scandinavian | 7 | 35 | 8 | 44 | 27 | 62 | 15 | 40 | 5 | 18 |
| Irish | 44 | 8 | 7 | 14 | 5 | 62 | 18 | 60 | 35 | 27 |
| HLA-DR type | ||||||||||
| Japanese | 4 | 9 | 2 | 8 | 15 | 6 | 1 | 12 | 14 | 13 |
| White American | 4 | 7 | 1 | 15 | 3 | 13 | 11 | 2 | 8 | 17 |
| African American | 11 | 1 | 3 | 15 | 7 | 13 | 2 | 8 | 18 | 6 |
| Scandinavian | 4 | 1 | 2 | 3 | 13 | 15 | 7 | 6 | 8 | 11 |
| Irish | 4 | 7 | 3 | 1 | 13 | 2 | 15 | 6 | 11 | 14 |
HLA-A type | ||||||||||
| Japanese | 24 | 2 | 26 | 11 | 31 | 9 | 33 | 10 | 19 | 3 |
| White American | 2 | 1 | 3 | 24 | 11 | 29 | 32 | 26 | 31 | 28 |
| African American | 2 | 23 | 30 | 3 | 28 | 1 | 34 | 29 | 74 | 68 |
| Scandinavian | 2 | 3 | 1 | 24 | 11 | 28 | 32 | 9 | 31 | 25 |
| Irish | 2 | 1 | 3 | 11 | 28 | 29 | 24 | 26 | 32 | 25 |
| HLA-B type | ||||||||||
| Japanese | 61 | 52 | 35 | 51 | 54 | 44 | 7 | 46 | 48 | 60 |
| White American | 7 | 44 | 8 | 35 | 62 | 60 | 18 | 57 | 27 | 14 |
| African American | 53 | 70 | 7 | 44 | 42 | 45 | 35 | 52 | 8 | 18 |
| Scandinavian | 7 | 35 | 8 | 44 | 27 | 62 | 15 | 40 | 5 | 18 |
| Irish | 44 | 8 | 7 | 14 | 5 | 62 | 18 | 60 | 35 | 27 |
| HLA-DR type | ||||||||||
| Japanese | 4 | 9 | 2 | 8 | 15 | 6 | 1 | 12 | 14 | 13 |
| White American | 4 | 7 | 1 | 15 | 3 | 13 | 11 | 2 | 8 | 17 |
| African American | 11 | 1 | 3 | 15 | 7 | 13 | 2 | 8 | 18 | 6 |
| Scandinavian | 4 | 1 | 2 | 3 | 13 | 15 | 7 | 6 | 8 | 11 |
| Irish | 4 | 7 | 3 | 1 | 13 | 2 | 15 | 6 | 11 | 14 |
Ranked frequencies of the 20 most common HLA-A (top) and HLA-DR (bottom) alleles of each population as a representation of the degree of genetic diversity within populations. Blue bars indicate Japanese; red bars, White Americans; yellow bars, African Americans; green bars, Scandinavians; and pink bars, Irish.
Ranked frequencies of the 20 most common HLA-A (top) and HLA-DR (bottom) alleles of each population as a representation of the degree of genetic diversity within populations. Blue bars indicate Japanese; red bars, White Americans; yellow bars, African Americans; green bars, Scandinavians; and pink bars, Irish.
Discussion
The present study suggests that transplant-related complications, among them GVHD, are less likely to occur in certain ethnic populations, such as Japanese and Scandinavians. There are plausible biologic rationales for such differences given the known differences in diversity of major and minor histocompatibility frequencies among ethnic groups. However, previous clinical evidence for such differences come from small heterogeneous studies involving 1 or 2 ethnic groups and multiple GVHD prophylaxis regimens and were uncontrolled for differences in other risk factors. We studied a large group of patients, all of whom had early leukemia and received non–T-cell depleted grafts with posttransplantation cyclosporine and methotrexate for GVHD prophylaxis, and we used multivariate regression to adjust for other prognostic factors.
Multivariate analysis of outcomes in adults shows that the white American and Irish cohorts had significantly higher risks for acute GVHD than the Japanese cohort, consistent with the observation made by Morishima et al3 that the incidence of GVHD is lower in Japanese recipients than in white recipients after HLA-identical sibling transplantation in the United States. Scandinavians, another potentially homogeneous population, are the exception, with a frequency of acute GVHD not significantly different from that of the Japanese. Our results show that the low incidence of acute GVHD after HLA-identical sibling BMT among Scandinavians is in concordance with previous reports, suggesting that the incidence and severity of GVHD after allogeneic BMT is influenced by the ethnic origin of patients and their donors; the incidence is lower in ethnic groups with less HLA polymorphism. However, a recent single-center Scandinavian study showed no difference in acute GVHD after HLA-identical sibling transplantation in Scandinavians compared with patients of other ethnic origins treated at the same center.11 Nevertheless, in that study, the Scandinavians were at lower risk for chronic GVHD—47% compared with 68% in other ethnic groups—a difference that was significant in multivariate analysis (P < .001). These data contrast with the findings of the present study, in which Scandinavians had a lower probability of acute GVHD than the American and Irish patients but a similar risk for chronic GVHD. An alternative explanation for the low probability of acute GVHD in Scandinavians may be that a major Swedish center in Huddinge treats grade 1 acute GVHD using high-dose corticosteroids.12 This preemptive use of corticosteroids may decrease the probability of grades 2 to 4 acute GVHD. Low incidences of acute GVHD in recipients of allogeneic transplants are reported from many Asian countries,3-5,13 though 2 reports from China and Hong Kong demonstrated similar rates of GVHD in Chinese and white patients after HLA-identical sibling BMT.14,15 These observations support the data presented, suggesting that the incidence of acute GVHD varies with ethnic background unpredictably. Additional comparisons of GVHD rates among Asian populations would be of interest in helping to separate genetic from environmental causes of transplantation outcome variations.
We did not observe a difference in the risk for chronic GVHD between Japanese and other ethnic cohorts. As in previously published reports,15-17 the risk factors associated with chronic GVHD were disease type, age at transplantation, and donor-recipient sex match. We did find a lower risk for early TRM in Japanese and Scandinavian transplant recipients. This may be a result of the lower incidence of acute GVHD in these 2 ethnic groups. No differences were noted in risks for later TRM (more than 3 months after transplantation), relapse, treatment failure (relapse or death), and overall survival. Multivariate analyses of children were limited to a comparison between Japanese and white American patients because of the small sample size. The higher risk for acute GVHD in the white American cohort was comparable to that seen in adults. However, again there were no differences in relapse, TRM, and treatment failure. We cannot exclude the possibility of bias caused by disparities in patient management that might have influenced the results of the study.
Genetic factors may influence transplantation outcome in 2 ways. First, more homogeneous populations may have smaller repertoires of minor histocompatibility antigens, leading to lower GVHD incidence. Second, each population may have a different preponderance of genes that affect immune reconstitution and transplantation outcome, such as cytokine gene polymorphisms2 and certain HLA types that are favorable or unfavorable for GVHD occurrence.18-21 Figure 7 demonstrates the diversity and representation of HLA-A among the groups studied. The degree of HLA diversity largely reflects the presumed degree of genetic isolation or diversity of the groups studied, with the Japanese population having the most restricted HLA representation, the African American population having the most diverse, and the other groups occupying an intermediate position. This pattern of HLA diversity may be a surrogate for diversity in minor histocompatibility antigens. These antigens, which are increasingly being defined,1 are polymorphic antigens inherited separately from HLA with the potential to affect the development of GVHD after HLA-identical sibling transplantation. There was also a difference in the representation of the most common HLA genes among the populations (Table 8), with the well-described preponderance of HLA-A24, HLA-B61, and HLA-B52 in the Japanese population and the preponderance of HLA-A23, HLA-B53, HLA-B70, and HLA-DR11 in the African American population. Certain HLA alleles are associated with decreased risk for GVHD.1 This may be attributed to the effect of major histocompatibility complex–linked genes that regulate tumor necrosis factor-α (TNF-α) and other mediators of inflammation. Thus, the differences in preponderant HLA types in different populations could affect outcome through linkage to other genes. Such an explanation seems more likely to explain the disparity between the Scandinavian, the Irish, and the North American white populations, all of whom have the same degree of HLA diversity but dissimilar risk for acute GVHD. It is important to consider nongenetic explanations for the differences observed. These include differences in the diagnosis and treatment of GVHD in different countries, dietary factors, and socioeconomic or cultural factors that may affect the access to and the delivery of optimal posttransplantation care. The diagnosis of GVHD is notoriously difficult to standardize. Therefore, it is possible that, despite the detailed reporting guidelines used by the IBMTR, consistent biases of reporting exist among countries. True differences in GVHD would be expected to translate into differences in the corresponding graft-versus-leukemia (GVL) effect, yet the survival rates of all populations were similar. This leaves open the question of whether the diagnostic criteria for GVHD were comparably applied. Furthermore, the early treatment of grade 1 GVHD in many Scandinavian patients is an obvious bias that could have caused the lower incidence of grades 2 to 4 GVHD and subsequent chronic GVHD. If this explanation is correct, it implies that preemptive management may overcome a genetically determined predisposition to GVHD.
In conclusion, we found less acute GVHD and less early TRM in Japanese and Scandinavian populations than in white American, African American, and Irish populations. Clearly, we can provide no single hypothesis to explain differences in GVHD. Rather, the data suggest several hypotheses. First, the HLA diversity/minor antigen surrogate hypotheses fit the Japanese population difference but are not supported by the Scandinavian data. Second, the pattern of HLA representation/surrogate for cytokine gene polymorphisms could explain all differences. Third, nongenetic explanations should be considered. These include, but are not limited to, the following possibilities: dietary and environmental differences, differences in GVHD diagnosis, and differences in success of GVHD management. One characteristic dietary feature of the Japanese population is the high consumption of fish.22,23 As a consequence, Japanese have higher levels of eicosapentaenoic acid and fatty acid with immunomodulatory properties that may reduce the severity of GVHD. Nevertheless, these findings provide a basis for prospective studies to better understand differences in outcome in genetically well-defined populations.
Prepublished online as Blood First Edition Paper, October 14, 2004; DOI 10.1182/blood-2004-06-2385.
Supported by Grant-in-Aid for Cancer (no. 9-2) from the Ministry of Health, Labor and Welfare, Japan; Public Health Service grant U24-CA76518 from the National Cancer Institute, the National Institute of Allergy and Infectious Diseases, and the National Heart, Lung and Blood Institute; Agency for Healthcare Research and Quality; and grants from Allianz Life/Life Trac; American Red Cross; American Society of Clinical Oncology; Amgen, Inc; Anonymous; Aventis Pharmaceuticals; Baxter Healthcare Corp; Baxter Oncology; Berlex Laboratories, Inc; Blue Cross and Blue Shield Association; The Lynde and Harry Bradley Foundation; Bristol Myers Squibb Oncology; BRT Laboratories, Inc; Cedarlane Laboratories Ltd; Cell Pathways; Cell Therapeutics, Inc; CelMed Biosciences; Centocor, Inc; Cubist Pharmaceuticals; Darwin Medical Communications, Ltd; Dynal Biotech ASA; Edwards Lifesciences RMI; Endo Pharmaceuticals, Inc; Enzon Pharmaceuticals, Inc; ESP Pharma; Excess, Inc; Fujisawa Healthcare, Inc; Gambro BCT, Inc; GlaxoSmithKline, Inc; Human Genome Sciences; ICN Pharmaceuticals, Inc; ILEX Oncology; Kirin Brewery Company; Ligand Pharmaceuticals, Inc; Eli Lilly and Company; Nada and Herbert P. Mahler Charities; Merck & Company; Millennium Pharmaceuticals; Miller Pharmacal Group; Milliman USA, Inc; Miltenyi Biotec; The Irving I. Moskowitz Foundation; National Marrow Donor Program; NeoRx Corporation; Novartis Pharmaceuticals, Inc; Novo Nordisk Pharmaceuticals; Ortho Biotech, Inc; Osiris Therapeutics, Inc; PacifiCare Health Systems; Pall Medical; Pfizer U.S. Pharmaceuticals; Pharmametrics; Pharmion Corp; Protein Design Labs; QOL Medical; Roche Laboratories; SangStat Medical; Schering AG; StemCyte, Inc; StemCell Technologies, Inc; Stemco Biomedical; StemSoft Software, Inc; SuperGen, Inc; Sysmex; THERAKOS, a Johnson & Johnson Company; University of Colorado Cord Blood Bank; Upside Endeavors; ViaCell, Inc; ViaCor Biotechnologies; WB Saunders Mosby Churchill; Wellpoint Health Network and Zymogenetics, Inc.
An Inside Blood analysis of this article appears in the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Ryuzo Ohno, chairman of JALSG, for critical advice and helpful discussion. We thank the 64 members of JSHCT and JALSG who voluntarily participated in this study.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal