Abstract
The clinical course of chronic lymphocytic leukemia (CLL) differs significantly between patients with mutated (M-CLL) and unmutated (U-CLL) immunoglobulin (Ig) variable heavy-chain (VH) genes, implying a role for B-cell receptor (BCR) signaling in the pathogenesis of this disease. We have now investigated activation of downstream BCR signaling pathways in U-CLL and M-CLL B cells using soluble anti-IgM (sol-IgM) and immobilized anti-IgM (imm-IgM) antibodies as models for antigenic stimulation. Ligation of the BCR with sol-IgM induced incomplete responses in both CLL subsets, resembling the pattern described for tolerant B cells. This response was characterized by transient phosphorylation of extracellular signal-related kinase (ERK) and Akt (protein kinase B [PKB]), lack of activation of c-JUN NH2-terminal kinase (JNK) and p38 mitogen-activated protein kinase (MAPK), and variable activation of phospholipase Cγ2 (PLCγ2) and nuclear factor-κB (NF-κB). Stimulation with imm-IgM elicited a more complete BCR signal and significantly prolonged phosphorylation of ERK and Akt, indicating persistent or repetitive BCR signaling. Moreover, this type of stimulation increased the levels of the antiapoptotic protein myeloid cell leukemia-1 (Mcl-1) and protected from chemotherapy-induced apoptosis, whereas induction of apoptosis and down-regulation of Mcl-1 was observed following stimulation with sol-IgM. These data demonstrate that only sustained BCR signaling can promote survival of CLL B cells and indicate that the main difference between CLL with mutated and unmutated VH genes may reside in the availability of such stimulation. (Blood. 2005;105:4820-4827)
Introduction
B-cell chronic lymphocytic leukemia (CLL) is the most common adult leukemia in the Western hemisphere. It is characterized by the progressive accumulation of mature monoclonal CD5+ B cells, which typically express low levels of surface immunoglobulin M (IgM) or IgM/IgD. The clinical course and outcome of CLL is variable. Some patients progress rapidly and die early in spite of therapy, whereas others exhibit a stable disease and a normal life span.1 Recent studies have indicated that the B-cell receptor (BCR) is a major determinant of the clinical course in CLL.2-5 The surface immunoglobulin (Ig) component of the BCR in patients with progressive disease is usually encoded by unmutated variable heavy-chain (VH) region genes and associates with the T-cell-specific protein tyrosine kinase zeta-associated protein 70 (ZAP-70).3-9 In contrast, CLL B cells from patients with stable disease usually express IgM encoded by mutated Ig VH genes and do not express ZAP-70. Moreover, analysis of early signaling events induced by IgM ligation indicate that the 2 subsets may also differ in their capacity to transmit BCR-derived stimuli.9-11
The BCR is a key signaling molecule that triggers pathways that can induce B-cell proliferation, survival, differentiation, anergy, and apoptosis (for a review, see Niiro and Clark12 ). In normal B cells, stimulation of the BCR leads to phosphorylation of immuno-receptor tyrosine-based activation motifs within the cytoplasmic tails of Igα and Igβ. The protein tyrosine kinase Syk is subsequently recruited to these motifs and becomes activated, initiating activation of the crucial effector enzymes phosphatidyl 3-kinase (PI3K) and phospholipase Cγ2 (PLCγ2). PI3K generates the key second messenger phosphatidylinositol-3,4,5-triphosphate, which recruits other BCR signaling molecules to the membrane and activates the downstream kinase Akt. The PI3K/Akt pathway plays an important role in promoting B-cell survival and protecting against BCR-induced cell death by inducing the expression of antiapoptotic proteins, such as myeloid cell leukemia-1 (Mcl-1) and X-linked inhibitor of apoptosis protein (XIAP), and inactivating cellular targets involved in the induction of apoptosis, such as BAD (Bcl2 antagonist of cell death) and caspase-9.13-16 Activation of PLCγ2 leads to the release of intracellular Ca2+ and activation of protein kinase C (PKC), which are crucial for the activation of mitogen-activated protein kinases (MAPKs), such as extracellular signal-regulated kinase (ERK), c-JUN NH2-terminal kinase (JNK), and p38 MAPK, and transcription factors, including nuclear factor-κB (NF-κB) and nuclear factor of activated T cells (NFAT). The balance of these signaling molecules subsequently determines B-cell fate. However, in terms of their individual activity, NF-κB appears to play a prominent role in the survival of antigen-stimulated B cells by inducing the expression of several antiapoptotic proteins, such as B-cell leukemia/lymphoma 2 (Bcl-2), Bcl-xL, and Bfl-1/A1.17-19 The role of the 3 MAPKs is less clear, although their activation in B cells correlates often with proliferation and apoptosis.20-22 Nevertheless, activation of all of these pathways is required for a complete immunogenic response to antigen stimulation (for a review, see Jun and Goodnow23 ). Suboptimal antigen stimulation, such as occurs with poorly cross-linking soluble autoantigens, can induce tolerogenic signaling that is characterized by the sole activation of ERK and NFAT.24
The PI3K/Akt, NF-κB, and ERK pathways can be activated in CLL B cells by a number of stimuli, including CD154 (CD40 ligand), phorbol ester, interleukin-4, stromal cell-derived factor 1, and human albumin, with consequent reduction in spontaneous or chemotherapy-induced apoptosis.25-29 Some data are also available regarding the activation of these pathways in CLL B cells after BCR stimulation. In one study, BCR engagement with anti-IgM F(ab′)2 fragments activated NF-κB and PI3K/Akt kinase; however, no data were presented regarding the VH gene mutational status of the investigated cases.30 Moreover, apoptosis inhibition was observed in all tested samples, which contrasts with other studies that have noted induction of apoptosis in CLL B cells that express the CD38 antigen.31,32 The latter cases usually account for more than 40% of all CLLs and more often belong to the CLL subset with unmutated VH genes.5 Considering the different clinical and biologic behavior of CLL with mutated and unmutated VH genes, we decided to investigate the activation of downstream BCR signaling pathways and the cellular response to BCR stimulation in CLL cells with defined VH gene mutational status. Moreover, since the nature of the antigen can affect BCR signaling, we used both soluble and immobilized anti-IgM antibodies to mimic T-cell-dependent and type 2 T-cell-independent antigens, respectively.
Patients, materials, and methods
Patients and cell samples
Blood samples were collected from patients who satisfied standard morphologic and immunophenotypic criteria for B-cell CLL,33 after obtaining informed consent according to the Declaration of Helsinki and approval for the study from the institutional human research committee at the Catholic University Medical School in Rome. The patients were either untreated or had not received chemotherapy or steroids for a period of at least 4 months prior to the investigation. Peripheral blood mononuclear cells (PBMCs) were separated by Ficoll gradient centrifugation (Amersham Biosciences, Uppsala, Sweden), and samples containing at least 85% CD5+CD19+ CLL B cells were further investigated.
Cell culture
Freshly isolated CLL B cells were resuspended at a density of 0.8 to 1.2 × 107 cells/mL in RPMI-1640 medium supplemented with 10% heat-inactivated fetal bovine serum, 100 U/mL penicillin, 0.1 mg/mL streptomycin, 2 mM l-glutamine, and 1 mM sodium pyruvate (Invitrogen, Carlsbad, CA) and cultured at 37°C in a humidified atmosphere containing 5% CO2. BCR stimulation was performed with 5 μg/mL goat F(ab′)2 anti-human IgM, 10 μg/mL goat anti-human IgM, 10 μg/mL control goat IgG (all from Southern Biotechnology Associates, Birmingham, AL), or 1 × 107/mL Dynabeads M-450 (Dynal Biotech, Oslo, Norway) coated with 10 μg goat anti-human IgM. The coating procedure was done according to the manufacturer's instructions (Dynal Biotech). In some experiments, the following reagents were included in the culture media: 1 μM phorbol 12-myristate 13-acetate (PMA; Sigma-Aldrich, St Louis, MO), 2 μM BAY-11 (Calbiochem, La Jolla, CA), 10 μM LY294002, and 10 μM U0126 (both from New England BioLabs, Beverly, MA), 20 μM fludarabine (Schering, Berlin, Germany), and 20 μM methylpred-nisolone (MPS; Aventis Pharma, Milano, Italy).
Immunoblot analysis
Cell pellets were lysed in ice-cold 1% nonidet P-40 (NP-40) lysis buffer (10 mM Tris [tris(hydroxymethyl)aminomethane-HCl, pH 7.4], 5 mM EDTA [ethylenediaminetetraacetic acid], 150 mM NaCl, 0.1% sodium dodecyl sulfate [SDS], 0.1% sodium deoxycholate) containing 1:40 dilution of protease inhibitor cocktail for mammalian cells and 1:50 dilution of phosphatase inhibitor cocktail 2 (both from Sigma-Aldrich). The protein concentration of each cell lysate was determined with the RC DC Protein Assay (Bio-Rad Laboratories, Hercules, CA). The protein samples were separated by 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred on Immobilon-P polyvinilidene difluoride membranes (Millipore, Bedford, MA). The amount of protein that was loaded ranged from 40 to 80 μg/lane, depending on the antibody that was subsequently used. Membranes were blotted at 4°C overnight with the following primary antibodies: phospho-Akt(Ser473), phospho-PLCγ2(Tyr1217), phospho-IKKα(Ser180)/IKKβ(Ser181), phospho-p44/p42 (ERK) MAPK(Thr202/Tyr204), JNK(Thr183/Tyr185), phospho-p38 MAP kinase(Thr180/Tyr182), Akt, IκB-α, IKKβ, SAPK/JNK, p44/42 (ERK) MAP kinase, poly(adenosine diphosphate [ADP]-ribose) polymerase (PARP), Bcl-2, Bcl-xL, (all from New England BioLabs), Mcl-1 (Santa Cruz Biotechnology, Santa Cruz, CA), and β-actin (Sigma-Aldrich). Immunodetection was done with anti-rabbit IgG horseradish peroxidase (HRP)-linked or anti-mouse IgG HRP-linked antibodies (both from New England BioLabs) and the ECL Plus enhanced-chemiluminescence detection system (Amersham Biosciences, Buckinghamshire, United Kingdom) with BioMax MR films (Eastman Kodak, Rochester, NY). To ascertain equivalent loading, the membranes that were probed with antibodies against phosphorylated proteins were stripped and reprobed with the corresponding antibodies against total protein. The membranes probed with antibodies against PARP, Bcl-2, Bcl-xL, and Mcl-1 were reprobed with anti-β-actin. Protein bands were quantified by laser densitometry and analyzed by ImageQuant software (Amersham Biosciences).
Analysis of cell viability and apoptosis
Induction of apoptosis was analyzed by double staining with fluorescein isothiocyanate (FITC)-conjugated annexin V and propidium iodide (PI) using the ApoAlert Annexin V kit (BD Biosciences, Palo Alto, Ca), according to the manufacturer's instructions. Briefly, 1 × 106 cells were washed once with phosphate-buffered saline (PBS) and resuspended in 200 μL binding buffer with 0.5 μg/mL annexin V-FITC and 2 μg/mL PI. After incubation for 10 minutes at room temperature in a light-protected area, the samples were analyzed on a FACSCalibur flow cytometer (Becton Dickinson, San Jose, CA). Data analysis was performed with CellQuest software (Becton Dickinson). Cell viability was measured as the percentage of annexin V and PI double-negative cells.
Ig VH gene-sequence analysis
Total cellular RNA was isolated from PBMCs using the Trizol reagent (Invitrogen) according to the manufacturer's instructions. The polymerase chain reaction (PCR) amplification, cloning, and sequencing of VH region genes have been described in detail elsewhere.34 Briefly, RNA was reverse transcribed using random hexamers and then PCR amplified with a degenerate VH framework 1 (FWR1) primer in combination with Cμ, Cγ, and Cα reverse primers. Samples that failed to amplify with these combinations were amplified with a mixture of forward primers complementary to leader sequences of VH families 1 to 6. PCR products were purified with the QIAquick PCR purification kit (Qiagen, Valencia, CA) and either sequenced directly or cloned with the PCR cloningplus kit (Qiagen). Sequencing was done with the BigDye Terminator v3.1 Cycle Sequencing kit and ABI 3100 genetic analyzer (Applied Biosystems, Foster City, CA). Candidate germ-line genes were assigned by searching the VBASE directory. Percentage homology was calculated by counting the number of mutations between the 5′ end of FWR1 and the 3′ end of FWR3. Sequences with less than 2% differences from germ-line VH sequences were considered unmutated.
Statistical analysis
Correlations between the percentage of apoptotic cells after incubation with imm-IgM, sol-IgM, F(ab′)2 anti-IgM, fludarabine, MPS, and the various combinations were evaluated using the paired τ test. The unpaired τ test was used to compare the mean percentages of unmutated VH gene CLL (U-CLL) and mutated VH gene CLL (M-CLL) cells bound to imm-IgM. Statistical analysis was performed using the SPSS for Windows program version 12 (SPSS, ERKrath, Germany).
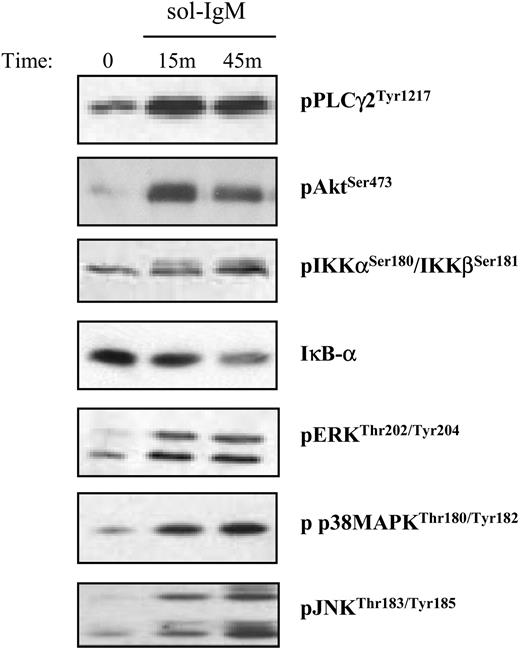
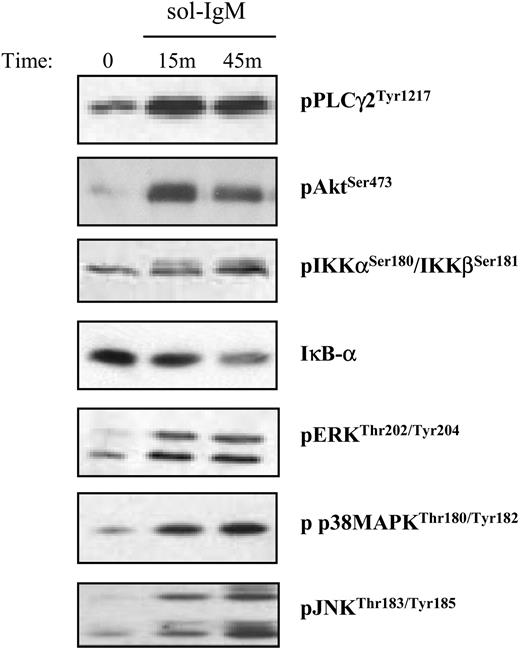
Activation of downstream BCR signaling pathways after stimulation with sol-IgM. CLL B cells were stimulated with sol-IgM for the indicated times, lysed, and immunoblotted as described in “Patients, materials, and methods.” Immunoblots show examples of typical positive cases.
Activation of downstream BCR signaling pathways after stimulation with sol-IgM. CLL B cells were stimulated with sol-IgM for the indicated times, lysed, and immunoblotted as described in “Patients, materials, and methods.” Immunoblots show examples of typical positive cases.
Results
Activation of downstream BCR signaling pathways in U-CLL and M-CLL B cells
Activation of downstream BCR signaling pathways was investigated in freshly isolated leukemic B cells from 41 IgM+ patients with CLL (16 U-CLLs and 25 M-CLLs) and 1 IgG+ CLL that served as a negative control. BCR stimulation was performed at several different time points using soluble goat anti-human IgM antibodies or F(ab′)2 fragments, which were used in some experiments to exclude nonspecific stimulation through Fc receptors. Moreover, incubation with control polyclonal goat IgG did not show activation of any signaling pathway. Activation of PLCγ2, Akt, and the 3 MAPKs was investigated using antibodies against phosphorylated PLCγ2, Akt, ERK, JNK, and p38 MAPK, while activation of NF-κB was analyzed using antibodies against phosphorylated IKKα/β and/or total IκB-α.19 Samples were considered positive if there was an increase in the amount of phosphorylated protein or a decrease in the amount of total IκB-α. Typical examples of positive cases are shown in Figure 1.
Phosphorylation of Akt and ERK was observed in 100% of the unmutated and 75% of the mutated VH gene CLLs, indicating that some BCR signaling occurs in most cases from both CLL subsets (Table 1 and Table S1, which is available on the Blood website; see the Supplemental Tables link at the top of the online article). However, the BCR response was incomplete in most cases, considering that JNK activation was observed in only 13% of the unmutated and 6% of the mutated CLLs. Similar results were obtained for p38 MAPK, which was constitutively phosphorylated and in most cases underwent a decrease, rather than an increase, in signal intensity upon stimulation. Some differences were observed between the 2 CLL subsets in terms of activation of PLCγ2 and NF-κB. PLCγ2 was more frequently activated in the U-CLL cases (86%), but it was also activated in a large fraction of the M-CLL cases (50%), whereas phosphorylation of IKKβ and/or degradation of IκB-α was observed in 47% of the unmutated and 13% of the mutated CLLs.
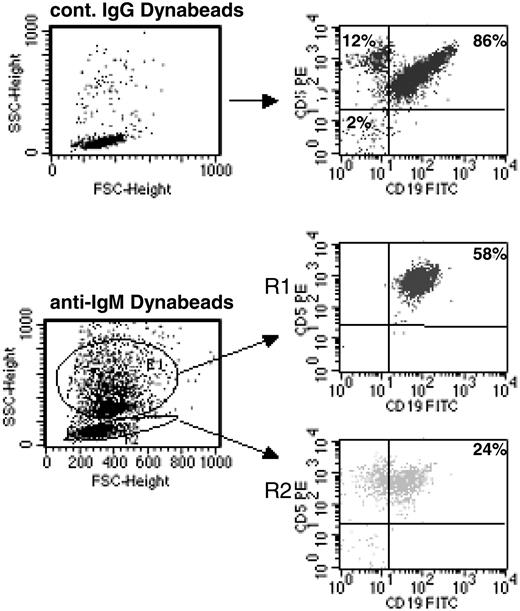
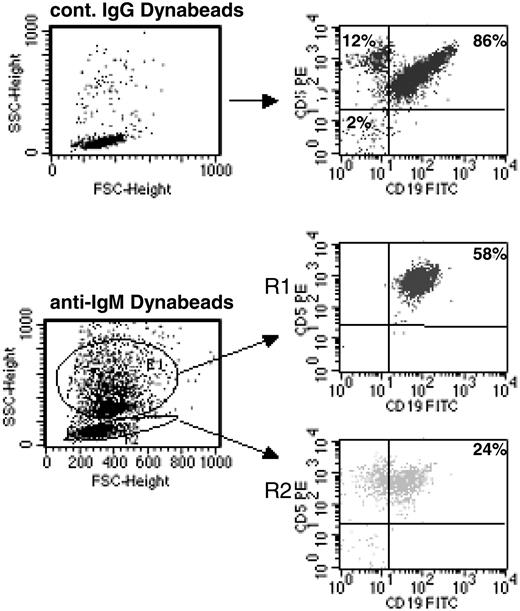
Continuous BCR stimulation of CLL B cells by imm-IgM. CLL B cells were analyzed by flow cytometry after 48 hours in culture with Dynabeads coated with control goat IgG (top panels) or anti-human IgM (bottom panels). Cells bound with Dynabeads have a different side scatter (SSC) and are present in region R1; unbound cells remain in R2. The percentage of CD5+CD19+ CLL B cells is indicated for each region. FSC indicates forward scatter.
Continuous BCR stimulation of CLL B cells by imm-IgM. CLL B cells were analyzed by flow cytometry after 48 hours in culture with Dynabeads coated with control goat IgG (top panels) or anti-human IgM (bottom panels). Cells bound with Dynabeads have a different side scatter (SSC) and are present in region R1; unbound cells remain in R2. The percentage of CD5+CD19+ CLL B cells is indicated for each region. FSC indicates forward scatter.
We next investigated whether a different response would be obtained after stimulation with immobilized anti-IgM antibodies, which have been used as a model for T-independent type 2 antigens.35,36 Immobilized anti-IgM antibodies have a higher ligand valency and do not downmodulate surface immunoglobulin (sIg), thus allowing persistent and/or repetitive BCR signaling.35 As shown in Figure 2, more than 50% of the CD5+CD19+ CLL B cells are bound with anti-IgM-coated polystyrene beads after 48 hours in culture, indicating that they are triggered in a sustained or repetitive manner. The specificity of binding was confirmed by analyzing 2 sIgG+ CLL cases and by using beads coated with control goat IgG. These experiments showed that the percentage of nonspecifically bound cells was less than 2%. The percentage of bound cells was not significantly different between the U-CLL and M-CLL cases (U-CLL: mean, 55%; range, 36%-80%; M-CLL: mean, 50%; range, 30%-75%; P = .33).
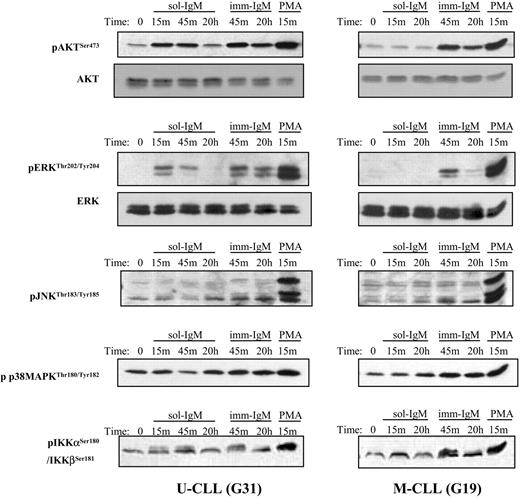
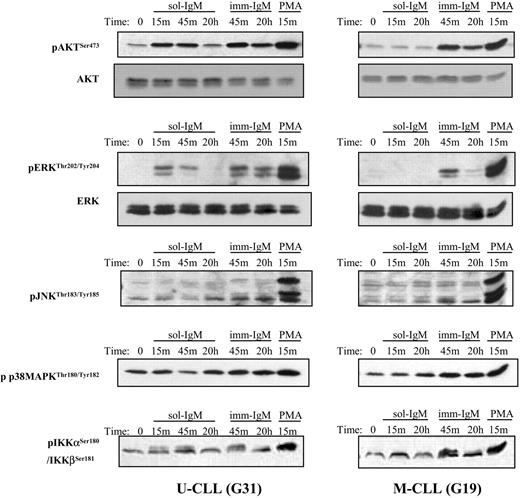
The response elicited by BCR stimulation with imm-IgM was more complete, characterized by activation of ERK, Akt, PLCγ2, and NF-κB in the majority of cases from both CLL subsets (Figure 3; Table 2; and Table S2). Moreover, weak activation of JNK and p38 MAPK was also observed in some cases. However, the most striking difference was related to the duration of the signal. Whereas phosphorylation of ERK and Akt was transient after stimulation with sol-IgM, achieving maximum levels after 10 to 15 minutes and disappearing within the first hour, it remained strongly detectable even after 20 hours of stimulation with imm-IgM (Figure 3). Sustained activation of these signaling molecules after BCR stimulation with imm-IgM was observed in leukemic cells from both CLL subsets. Thus, these data indicate that T-cell-dependent and T-cell-independent antigens can induce qualitatively and quantitatively different responses in CLL B cells.
Activation of AKT, ERK, IKKα/β, JNK, and p38 MAPK after BCR stimulation with sol- and imm-IgM. Immunoblot analysis is shown for one CLL case with unmutated (G31) and one case with mutated (G19) VH genes. Stimulation was done with sol-IgM for 15 minutes, 45 minutes, and 20 hours, and with imm-IgM for 45 minutes and 20 hours, as described in “Patients, materials, and methods.” Stimulation with PMA (1 μM) was used as a positive control. The antibodies against the phosphorylated and total proteins used for immunoblotting are indicated on the left.
Activation of AKT, ERK, IKKα/β, JNK, and p38 MAPK after BCR stimulation with sol- and imm-IgM. Immunoblot analysis is shown for one CLL case with unmutated (G31) and one case with mutated (G19) VH genes. Stimulation was done with sol-IgM for 15 minutes, 45 minutes, and 20 hours, and with imm-IgM for 45 minutes and 20 hours, as described in “Patients, materials, and methods.” Stimulation with PMA (1 μM) was used as a positive control. The antibodies against the phosphorylated and total proteins used for immunoblotting are indicated on the left.
Effects of sol-IgM and imm-IgM on CLL B-cell survival and levels of antiapoptotic proteins
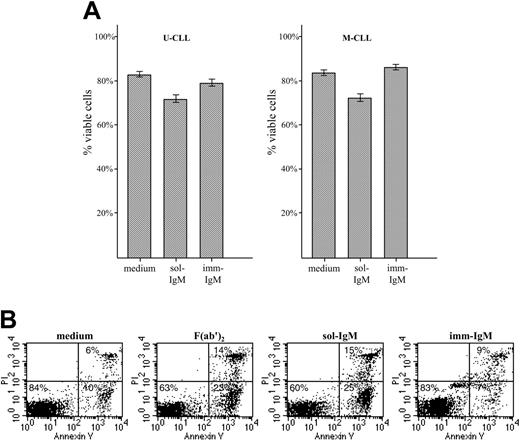
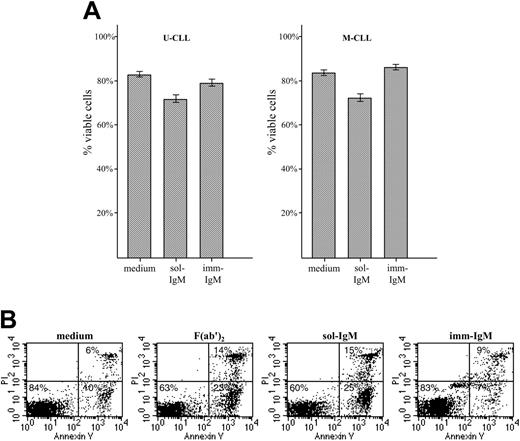
We next investigated the cellular response of U-CLL and M-CLL lymphocytes to stimulation with sol- and imm-IgM. As expected, neither type of stimulation induced significant levels of proliferation, which was investigated by 3H thymidine uptake and carboxy-fluorescein diacetate-succinimidyl ester (CFDA-SE) staining (data not shown). However, significant differences were observed in terms of survival, which was investigated by annexin V/PI staining of CLL B cells from 17 U-CLL and 20 M-CLL cases after 48 hours in culture with sol- or imm-IgM (Figure 4). Stimulation with sol-IgM induced apoptosis of CLL B cells from both VH subgroups. The mean percentage of viable U-CLL cells in the absence of stimulation was 81% (± 9.7% SD) versus 68% (± 14.1% SD) in the presence of sol-IgM (P < .001). Similarly, the mean percentage of viable M-CLL cells in medium only was 80% (± 11.5% SD) compared with 69% (± 16.4% SD) for sol-IgM (P < .001). The increase in apoptotic cells with respect to spontaneous apoptosis was less than 20% in the majority of cases (data for individual cases are shown in Table S3). However, 4 M-CLL and 4 U-CLL cases exhibited much higher levels of apoptotic cells, ranging from 20% to 40%. No difference was observed in the 8 cases that were stimulated with sol-IgM in the form of complete antibody molecules and F(ab′)2 fragments, confirming that apoptosis was induced as a consequence of BCR ligation. Stimulation with imm-IgM did not increase the percentage of cells undergoing apoptosis (U-CLL: 79% ± 9.9% SD, P = .067 with respect to medium; M-CLL: 86% ± 8.9% SD, P = .127 with respect to medium). Moreover, in a few cases the percentage of cells undergoing spontaneous apoptosis was slightly reduced (Table S3).
Survival of CLL B cells with unmutated and mutated VH genes after exposure to sol- and imm-IgM. (A) CLL B cells from patients with unmutated (n = 17, left panel) and mutated (n = 20, right panel) VH genes were cultured for 48 hours in the presence of sol-IgM (10 μg/mL complete antibody or 5 μg/mL F(ab′)2 fragments) or 10 μg/mL imm-IgM. Apoptosis was investigated by annexin-V FITC and PI double staining. Values represent the mean percentage of viable (double negative) cells; error bars indicate SEM. (B) Flow cytometry analysis of a representative case with unmutated VH genes (G83).
Survival of CLL B cells with unmutated and mutated VH genes after exposure to sol- and imm-IgM. (A) CLL B cells from patients with unmutated (n = 17, left panel) and mutated (n = 20, right panel) VH genes were cultured for 48 hours in the presence of sol-IgM (10 μg/mL complete antibody or 5 μg/mL F(ab′)2 fragments) or 10 μg/mL imm-IgM. Apoptosis was investigated by annexin-V FITC and PI double staining. Values represent the mean percentage of viable (double negative) cells; error bars indicate SEM. (B) Flow cytometry analysis of a representative case with unmutated VH genes (G83).
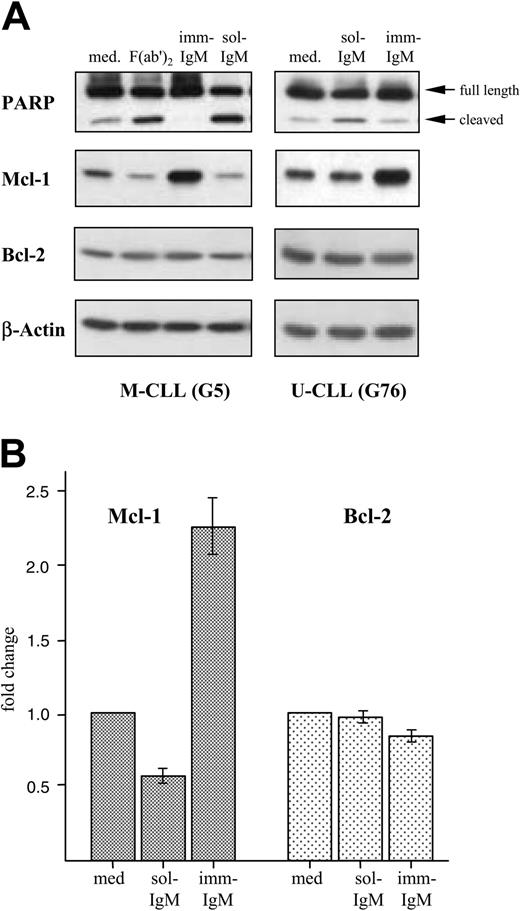
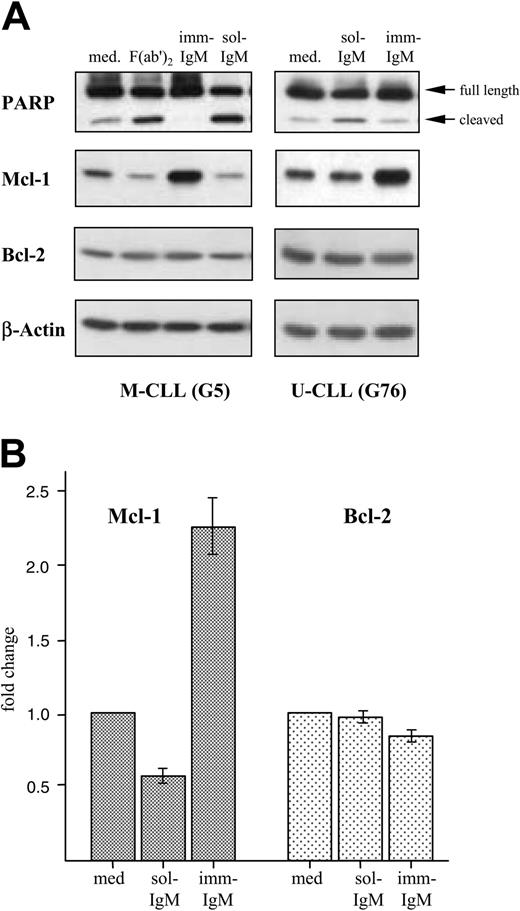
Induction of apoptosis by sol-IgM was confirmed by analyzing cleavage of PARP, which is commonly used as an indicator of the activity of caspase-3. As shown in Figure 5A, sol-IgM as complete molecules or F(ab′)2 fragments induced significant PARP cleavage. On the other hand, the amount of cleaved product was lower in CLL cells stimulated with imm-IgM than in unstimulated cells. This analysis was performed on leukemic B cells from 12 different cases (7 with mutated and 5 with unmutated VH genes) with similar results.
We also investigated the effects of BCR stimulation on the levels of 3 antiapoptotic proteins that have been implicated in the pathogenesis of CLL. Stimulation with sol-IgM was associated with an approximately 50% reduction in the levels of Mcl-1, whereas imm-IgM induced an average 2.5-fold increase in the 10 investigated cases (Figure 5). The changes in Mcl-1 levels were similar in the 5 M-CLL and 5 U-CLL cases. The levels of Bcl-2 did not change significantly, whereas Bcl-xL was not detected.
In vitro chemosensitivity of CLL B cells stimulated with sol-IgM and imm-IgM
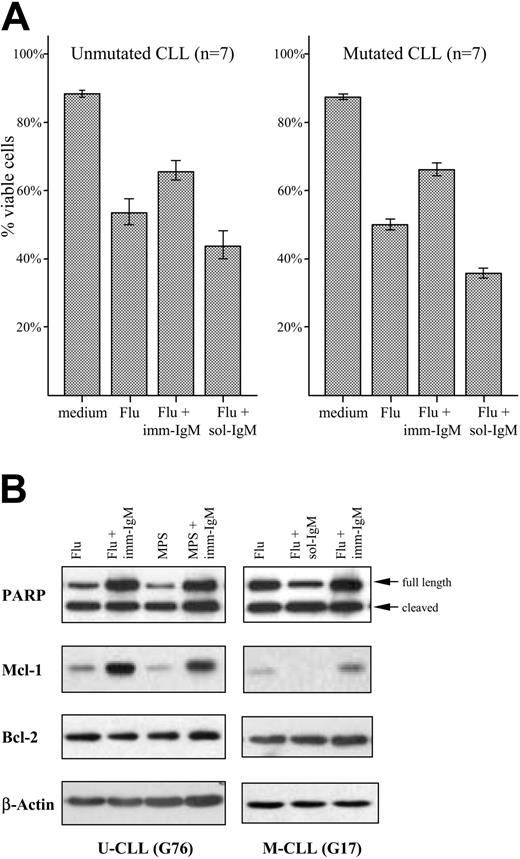
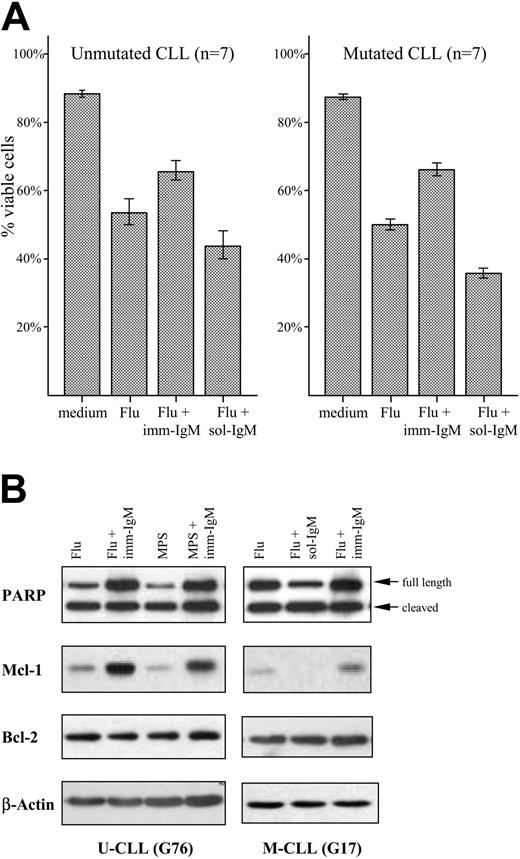
High expression of Mcl-1 in freshly isolated CLL B cells has been associated with lower response rates to chemotherapy.37 We therefore investigated whether stimulation with imm-IgM will protect CLL B cells from chemotherapy-induced apoptosis. The percentage of viable leukemic cells was determined by annexin V/PI staining in 7 unmutated and 7 mutated CLL samples after 48 hours in culture with 20 μM fludarabine. Fludarabine induced significant apoptosis in CLL cells from both subgroups, which was strongly inhibited by imm-IgM. As shown in Figure 6A and Table S4, the percentage of viable CLL cells increased from 53% ± 20% SD to 66% ± 14.7% SD in the U-CLL group (P = .024), and from 51% ± 8.3% SD to 67% ± 10.5% SD in the M-CLL group (P < .013). An opposite effect was observed with sol-IgM, which decreased the percentage of viable cells in both subgroups (U-CLL: 44% ± 21.3% SD, P = .024 with respect to fludarabine alone; M-CLL: 36% ± 7.6% SD, P < .001 with respect to fludarabine alone). Imm-IgM also afforded protection from MPS-induced apoptosis, albeit at a lower level (data not shown). Moreover, the amount of cleaved PARP was reduced in cells stimulated with imm-IgM and increased in cells stimulated with sol-IgM compared with cells that were incubated only with fludarabine or MPS (Figure 6B). Induction of PARP cleavage correlated inversely with the levels of Mcl-1, whereas again no significant change was observed in the levels of Bcl-2.
Opposing effects of sol-IgM and imm-IgM on PARP cleavage and Mcl-1 expression. (A) CLL B cells were stimulated for 40 hours with sol-IgM (10 μg/mL), sol-F(ab′)2 fragments (5 μg/mL), or imm-IgM (10 μg/mL). Protein extracts were analyzed by immunoblotting with antibodies against PARP, Mcl-1, Bcl-2, and β-actin. Two representative cases are shown. (B) Summary of changes in Mcl-1 (dark bars) and Bcl-2 (light bars) protein levels after 40-hour stimulation of CLL B cells with sol- and imm-IgM. For quantification, band intensities were first normalized to the respective actin signal and then calculated as fold change relative to unstimulated CLL B cells, which was set to 1.0. Values represent mean of 10 different cases (5 M-CLL, 5 U-CLL); error bars indicate SEM. Med indicates medium.
Opposing effects of sol-IgM and imm-IgM on PARP cleavage and Mcl-1 expression. (A) CLL B cells were stimulated for 40 hours with sol-IgM (10 μg/mL), sol-F(ab′)2 fragments (5 μg/mL), or imm-IgM (10 μg/mL). Protein extracts were analyzed by immunoblotting with antibodies against PARP, Mcl-1, Bcl-2, and β-actin. Two representative cases are shown. (B) Summary of changes in Mcl-1 (dark bars) and Bcl-2 (light bars) protein levels after 40-hour stimulation of CLL B cells with sol- and imm-IgM. For quantification, band intensities were first normalized to the respective actin signal and then calculated as fold change relative to unstimulated CLL B cells, which was set to 1.0. Values represent mean of 10 different cases (5 M-CLL, 5 U-CLL); error bars indicate SEM. Med indicates medium.
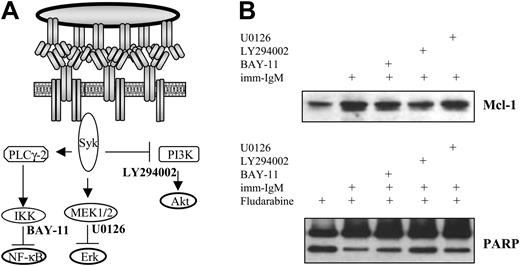
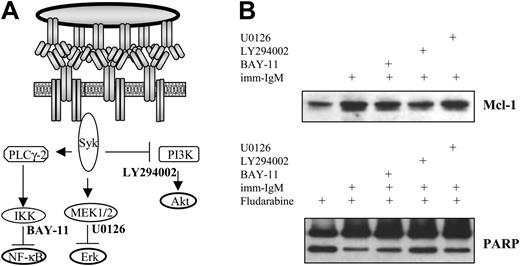
To investigate which of the 3 pathways activated by imm-IgM is responsible for induction of Mcl-1 and protection from fludarabine-induced apoptosis, we incubated CLL B cells with LY294002 (inhibitor of PI3K), U0126 (inhibitor of the mitogen-induced extracellular kinase 1/2 [MEK1/2] kinases, which activate ERK), and BAY-11 (inhibitor of IκB phosphorylation) prior to stimulation with imm-IgM and addition of fludarabine. Relatively low concentrations of each inhibitor were used, which have been shown to inhibit the individual pathways without exerting direct toxic effects on the CLL cells.16,28 As shown in Figure 7, only the PI3K inhibitor LY294002 significantly prevented induction of Mcl-1 and abrogated the inhibition of fludarabine-induced PARP cleavage by imm-IgM.
Protection of CLL B cells from fludarabine and MPS-induced apoptosis by imm-IgM. (A) Percentage of viable CLL cells determined by annexin V/PI staining after 48 hours in culture medium, in the presence of fludarabine, fludarabine and imm-IgM, or fludarabine and sol-IgM. Cells were preincubated for 3 hours with imm-IgM or sol-IgM prior to the addition of 20 μM fludarabine. Values represent mean of 7 U-CLL (left panel) and 7 M-CLL (right panel) cases. Error bars indicate SEM. F(ab′)2 fragments were used as sol-IgM in these experiments. (B) Cells were preincubated for 3 hours with sol-IgM-F(ab′)2 or imm-IgM prior to the addition of fludarabine (Flu, 20 μM) or MPS (20 μM). After 40 hours, cells were collected and analyzed for PARP cleavage and expression of Mcl-1 and Bcl-2 by immunoblotting. Membranes were stripped and reprobed with anti-β-actin to ensure equal protein loading. Of 8 experiments (5 M-CLL and 3 U-CLL cases), 2 representatives are presented.
Protection of CLL B cells from fludarabine and MPS-induced apoptosis by imm-IgM. (A) Percentage of viable CLL cells determined by annexin V/PI staining after 48 hours in culture medium, in the presence of fludarabine, fludarabine and imm-IgM, or fludarabine and sol-IgM. Cells were preincubated for 3 hours with imm-IgM or sol-IgM prior to the addition of 20 μM fludarabine. Values represent mean of 7 U-CLL (left panel) and 7 M-CLL (right panel) cases. Error bars indicate SEM. F(ab′)2 fragments were used as sol-IgM in these experiments. (B) Cells were preincubated for 3 hours with sol-IgM-F(ab′)2 or imm-IgM prior to the addition of fludarabine (Flu, 20 μM) or MPS (20 μM). After 40 hours, cells were collected and analyzed for PARP cleavage and expression of Mcl-1 and Bcl-2 by immunoblotting. Membranes were stripped and reprobed with anti-β-actin to ensure equal protein loading. Of 8 experiments (5 M-CLL and 3 U-CLL cases), 2 representatives are presented.
Discussion
A number of recent studies have indicated that signaling through the B-cell receptor may be largely responsible for the different clinical course and outcome in patients with CLL who have mutated and unmutated Ig VH genes. In particular, microarray gene expression analysis has revealed that the 2 subsets share a common profile, with differences in the expression of only a couple of hundred genes. Interestingly, approximately half of the differentially expressed genes are inducible in normal B cells by BCR stimulation, and these genes are expressed at higher levels in the unmutated cases.6 Furthermore, the protein tyrosine kinase ZAP-70, which is expressed predominantly in the unmutated CLLs, has recently been shown to associate with the BCR and to be involved in BCR signaling.9 Finally, certain differences have been observed between the 2 subsets regarding their capacity to transmit BCR-derived signals. In particular, CLL cells with unmutated VH genes show stronger activation of proximal BCR signaling molecules, such as Syk and PLCγ-2, whereas activation of these molecules is usually weaker or absent in the mutated cases.9,10,38 Based on these data, a concept has emerged that the different clinical and biologic behavior of the 2 CLL subsets may reflect a different history of antigen encounter, which in the case of M-CLL has rendered the leukemic cells tolerant, but has not affected the capacity of the U-CLL BCR to respond to subsequent stimulations.
Inhibition of the PI3K/Akt pathway prevents induction of Mcl-1 and abrogates the protective effect of imm-IgM on fludarabine-induced PARP cleavage. (A) Schematic representation of the pathways activated by imm-IgM and their respective inhibitors. (B) Cells were preincubated for 2 hours with the IKK inhibitor BAY-11 (2 μM), PI3K inhibitor LY294002 (10 μM), or ERK inhibitor U0126 (10 μM) prior to the addition of imm-IgM. Fludarabine (20 μM) was added 3 hours later. Cells were collected after 40 hours and analyzed for Mcl-1 expression and PARP cleavage by immunoblotting. The experiment was performed with CLL cells from 3 different cases (2 with mutated and 1 with unmutated VH genes), with similar results. One representative experiment is shown (case G31, U-CLL).
Inhibition of the PI3K/Akt pathway prevents induction of Mcl-1 and abrogates the protective effect of imm-IgM on fludarabine-induced PARP cleavage. (A) Schematic representation of the pathways activated by imm-IgM and their respective inhibitors. (B) Cells were preincubated for 2 hours with the IKK inhibitor BAY-11 (2 μM), PI3K inhibitor LY294002 (10 μM), or ERK inhibitor U0126 (10 μM) prior to the addition of imm-IgM. Fludarabine (20 μM) was added 3 hours later. Cells were collected after 40 hours and analyzed for Mcl-1 expression and PARP cleavage by immunoblotting. The experiment was performed with CLL cells from 3 different cases (2 with mutated and 1 with unmutated VH genes), with similar results. One representative experiment is shown (case G31, U-CLL).
In this study, we have examined in detail the activation of downstream signaling pathways known to be differentially induced in tolerant and antigen-competent B cells. Studies in normal and transgenic mice have shown that BCR signaling in tolerant B cells results in the activation of ERK and NFAT, but no activation of Akt, NF-κB, JNK, and p38 MAPK, which are all required for a complete immunogenic response.23,24,39 The data presented in this study indicate that both CLL subsets have certain features of tolerant B cells, since JNK, p38 MAPK, and NF-κB were not activated in the majority of cases after engagement of the BCR with soluble anti-IgM antibodies. On the other hand, Akt and ERK were activated in 100% of the U-CLL and 75% of the M-CLL cases, indicating that the absence of strong Syk activation, observed in previous reports in the majority of M-CLL and a significant fraction of the U-CLL cases,10,38 does not completely preclude activation of some of the downstream BCR signaling pathways. Moreover, signal transduction could be partially restored even in the completely unresponsive M-CLL cases by BCR engagement with imm-IgM. This functional BCR deficiency is also reminiscent of tolerant B cells in which responsiveness can be restored if the antigen is presented in a multivalent membrane-bound form.23,40 Thus, these data indicate that CLL B cells generally exhibit a spectrum of incomplete responses after stimulation with soluble antigens, with relatively few differences between the 2 subsets, mainly related to the activation of PLCγ2 (86% of U-CLL and 50% of M-CLL cases) and NF-κB (47% of U-CLL and 13% of M-CLL cases). However, the differences in the frequency of activation of these signaling pathways appeared relatively narrow, and as such, insufficient to account for the significantly different clinical and biologic behavior of mutated and unmutated VH gene CLL. Furthermore, the NF-κB target genes Bcl-2 and Bcl-xL were not induced by BCR stimulation, and, most importantly, the final outcome of stimulation with sol-IgM was induction of apoptosis in both CLL subsets.
Stimulation with imm-IgM elicited a more complete BCR signal that was also characterized by significantly prolonged Akt and ERK activation. Sustained activation of these pathways was associated with induction of the antiapoptotic protein Mcl-1 and protection from chemotherapy-induced apoptosis in leukemic cells from both CLL subsets. On the contrary, stimulation with sol-IgM down-regulated Mcl-1, induced apoptosis, and augmented chemotherapy-induced apoptosis in most examined cases, regardless of their VH gene mutational status. These different responses indicate that the distinct biologic and clinical behavior of the M-CLL and U-CLL cases may be primarily determined by the antigen-specificity of their BCRs. Thus, CLL B cells with unmutated VH genes may predominantly display specificity for T-independent foreign antigens or membrane-bound autoantigens, which would provide them with survival signals by triggering their BCRs in a sustained or repetitive manner. In line with this possibility, studies in transgenic mice have shown that autoreactive B cells can be positively selected and expanded when they bind membrane autoantigens in peripheral tissues, whereas they are rendered tolerant if the same antigens are expressed in soluble form.41,42 Moreover, certain features of the U-CLL antibodies, such as their germ-line-encoded specificity, restricted V region repertoire, and frequent poly- and autoreactivity, are also features of natural autoantibodies that react with common carbohydrate cell-surface autoantigens and type 2 T-independent foreign antigens.2,43
Sustained signaling through the BCR induced Mcl-1, which is a Bcl-2 homolog that has a prominent role in preventing spontaneous apoptosis of normal B cells. A decline in Mcl-1 levels precedes spontaneous and chemotherapy-induced cell death in normal and CLL B lymphocytes, whereas its levels are increased by survival stimuli.44,45 High levels of Mcl-1 in freshly isolated CLL B cells have been strongly correlated with poor responses to fludarabine and chlorambucil chemotherapy.37 Moreover, protection against spontaneous or chemotherapy-induced apoptosis of CLL B cells by coculture with follicular dendritic cells is dependent on the induction of Mcl-1.46 Therefore, our observation that imm-IgM prevents fludarabine- and MPS-induced down-regulation of Mcl-1 and protects from apoptosis induced by these agents suggests that patients with U-CLL, whose B cells presumably receive sustained antigen stimulation in vivo, should be more resistant to treatment. Studies comparing responses to standard chemotherapy in patients with CLL with mutated and unmutated VH genes are still lacking, but comparison of the outcomes after autologous stem cell transplantation has shown a significantly higher percentage of recurrences and shorter responses in U-CLL patients, indicating that they are more resistant even to myeloablative chemotherapy.47
Inhibition of PI3K prevented induction of Mcl-1 and abrogated the protective effect of imm-IgM on chemotherapy-induced apoptosis, indicating that the PI3K/AKT pathway is a major link between the BCR and apoptosis resistance of CLL B cells. The significance of this pathway is also underscored by the development of a CLL-like disorder in mice transgenic for TCL-1, an oncogene that functions by augmenting the activity of AKT.48 Sustained activation of Akt is also a major requirement for the longevity of T cells after antigen encounter.49 The significance of sustained ERK activation after BCR stimulation is less clear, but this kinase also mediates survival and growth in various cell types.50,51 In CLL, activation of this MAPK by stromal cell-derived factor-1 has been associated with protection from spontaneous apoptosis.27 However, a specific inhibitor of ERK did not prevent up-regulation of Mcl-1 and did not abrogate the antiapoptotic effect of imm-IgM, indicating that this pathway may not be directly involved in BCR-induced leukemic cell survival.
The data presented in this study may also have therapeutic implications, since they indicate that interruption of BCR signaling will increase the susceptibility of CLL cells to chemotherapy-induced apoptosis. Inhibitors of various arms of the PI3K/Akt pathway are already in clinical use or development, and we are currently testing their potential to abrogate apoptosis resistance provided by sustained BCR signaling. Moreover, an important drawback for using anti-idiotypic immunotherapy in CLL has been the concern that anti-idiotypic antibodies may promote survival or growth of the leukemic clone. Our data strongly suggest that this type of treatment will have a beneficial effect, considering that soluble anti-IgM antibodies were capable of directly inducing apoptosis or increasing the sensitivity of CLL B cells to chemotherapy. The restricted VH and variable light chain (VL) region repertoire of leukemic cells with unmutated V genes indicates that anti-idiotypic immunotherapy may be a feasible approach in CLL.
In conclusion, this study demonstrates that the response of CLL B cells to BCR stimulation depends on the nature of the antigenic stimulus. Moreover, it provides compelling evidence that sustained BCR stimulation can exert a promoting effect on the expansion of the malignant clone, and indicates that the distinct clinical and biologic behavior of CLL with mutated and unmutated VH genes may be a consequence of the different specificity of their BCRs.
Prepublished online as Blood First Edition Paper, February 22, 2005; DOI 10.1182/blood-2004-07-2669.
The online version of the article contains a data supplement.
An Inside Blood analysis of this article appears in the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
The authors would like to thank Prof F. E. Baralle for the most valuable comments and for critical reading of the manuscript.