Abstract
Sequential immunomagnetic isolation with 2 monoclonal antibodies was used to purify and characterize an undifferentiated mast cell in adult mouse bone marrow that had not been previously recognized. This cell represents 0.02% of the cells in the bone marrow, is CD34+, CD13+, and c-kit+, and does not express FcϵRI. However, by polymerase chain reaction (PCR) the cell contains message for the α and β subunits of FcϵRI, mast cell–specific proteases, and carboxypeptidase A. Morphologically, this cell has a large nucleus, little cytoplasm, few cytoplasmic organelles, and no cytoplasmic granules. In vitro, in the presence of interleukin-3 (IL-3) and stem cell factor (SCF) these cells differentiate only into a granulated mast cell that now expresses CD13, c-kit, mast cell–specific gangliosides, FcϵRI, and binds immunoglobulin E (IgE). When injected into lethally irradiated mice, these cells are able to reconstitute the mast cell population in the spleen.
Introduction
Mast cells have long been known to play a pivotal role in inflammatory and allergic reactions.1,2 Recently, they have gained new importance as immunoregulatory cells with the discovery that they may be a major source of cytokines and chemokines. Despite their growing significance in normal and pathological conditions, much still remains to be learned about mast cell development and recruitment. Like blood cells, mast cells are derived from pluripotent hematopoietic stem cells, but unlike blood cells they are thought to leave the bone marrow as progenitors and migrate to peripheral sites where they complete their maturation. It is the microenvironment surrounding the mast cells that determines their mature phenotype.3-6 However, an undifferentiated mast cell precursor has yet to be identified or characterized in the bone marrow due to the lack of mast cell–specific markers.
In the present study, an undifferentiated mast cell from adult mouse bone marrow was isolated and characterized using 2 monoclonal antibodies, mAb-AA4 and mAb-BGD6. While mAb-AA47 recognizes 2 derivatives of the ganglioside GD1b8 that are unique to the surface of rodent mast cells,9-11 mAb-BGD6 binds to the surface of RBL-2H3 mast cells at sites unrelated to FcϵRI. Both mAb-AA4 and mAb-BGD6 bind to granulated mast cells in all stages of maturation in the bone marrow, but mAb-BGD6 also binds to a very immature cell that is not recognized by mAb-AA4. The difference in binding patterns between mAb-AA4 and mAb-BGD6 has been used in a subtractive isolation method to isolate and characterize an undifferentiated cell (AA4-/BGD6+) from adult mouse bone marrow that gives rise only to mast cells.
Materials and methods
Animals
Young (8 to 12 weeks) male and female BALB/c mice (Harlan Sprague-Dawley, Indianapolis, IN; Bioterio, Faculdade de Medecina de Ribeirão Preto–Universidade de São Paulo (FMRP-USP), Ribeirão Preto, Brazil) were used. Animals were housed and experiments were conducted according to National Institutes of Health (NIH) and FMRP-USP protocols and guidelines.
Cells
Bone marrow from the femurs of mice was removed as previously described.9 Peritoneal cells were obtained using the method described by Mendonca et al.12 In some experiments the adherent cells were separated from nonadherent cells by resuspending the cells and allowing them to settle for 1 hour onto uncoated coverslips. The rat mast cell line, RBL-2H3, was grown as monolayers as described previously.13
Antibodies
The monoclonal antibody mAb-BGD6, an immunoglobulin G1 (IgG1), was raised against the cell surface of RBL-2H3 cells.7 mAb-BC4, which binds to and immunoprecipitates the α subunit of FcϵRI7 and a pool of monoclonal antibodies (mAbs) to the β subunit of FcϵRI,14 was raised in mice. mAb-AA4, mAb-BGD6, or normal mouse IgG were conjugated to tosylactivated Dynabeads (Dynal, Lake Success, NY) as previously described.15 A rat mAb antimouse IgE was purchased from Biosource International (Camarillo, CA). The rat mAb antimouse CD11b (Mac-1) was purchased from Abcam (Cambridge, MA). The following rat mAbs were purchased from PharMingen (San Diego, CA): antimouse c-kit (CD117), antimouse CD34, antimouse CD13, antimouse Ly-6g/Ly6c, antimouse CD45/B220, antimouse CD16/CD32 (FcBlock), antimouse Thy-1 (CD90), antimouse CD40, and antimouse Sca-1.
Immunoprecipitation
The surface of RBL-2H3 cells was biotinylated or radioiodinated, the cells (2.5 × 107/mL) solubilized, and the proteins immunoprecipitated with Sepharose 4B beads (Amersham Biosciences, Uppsala, Sweden) coupled to NMG, mAb-BC4, or mAb-BGD6, separated on 4% to 12% NuPAGE gels, transferred to Immobilon-P membranes (Millipore, Bedford, MA), used for autoradiography or blotted with horseradish peroxidase (HRP)–streptavidin (Pierce Biotechnology, Rockford, IL), and detected by chemiluminescence (New England Nuclear, Boston, MA).
Cell counts
The total number of bone marrow cells was determined by counting the cells using a hemocytometer. The percentage of mast cells present in the bone marrow was determined in a separate set of experiments by counting, in a hemocytometer, the positive and negative cells after immunostaining with mAb-BGD6, mAb-AA4, or anti-IgE. A minimum of 1000 cells per antibody were counted in each of 25 experiments. The percentage of mast cells was also determined by incubating suspensions of bone marrow cells with beads conjugated with mAb-AA4 or mAb-BGD6. Aliquots of cells were removed, and the number of cells either free or attached to mAb-AA4–conjugated beads or mAb-BGD6–conjugated beads was counted in a hemocytometer. One thousand cells per antibody were counted in each of 50 experiments. Both methods gave identical results. The percentage of cells positive with mAb-BGD6 or attached to the mAb-BGD6–conjugated magnetic beads was taken as the mast cell population. The percentage of AA4-/BGD6+ mast cells was determined by counting the number of cells from the starting cell suspension that attached to mAb-BGD6 beads after clearing twice with mAb-AA4 beads. A total of 500 cells attached to mAb-BGD6 beads were counted for each of 41 experiments. The results were confirmed by immunostaining suspensions of bone marrow cells with both mAb-AA4 and mAb-BGD6. For cell separation experiments the number of cells in the total bone marrow suspension was counted using a hemocytometer, and the number of mast cells present was calculated based on the percentage of mast cells in the total bone marrow population as described in this section (2.6% ± 0.5%). The number of AA4-/BGD6+ mast cells present in the total bone marrow suspension was also calculated using the percentage (0.02% ± 0.007%) obtained as described in this section. Flow cytometric analysis was performed as previously described9 using fluorescein isothiocyanate (FITC)–conjugated mAb-AA4 or mAb-BGD6 (2.5 μg/mL).
Cell separation
The cells were isolated according to the method of Jamur et al.15 Briefly, the suspension of bone marrow cells was washed twice by centrifugation (27g) in Iscove medium containing 2% bovine serum albumin (BSA) and 5 μg/mL NMG. The cell suspension of unfractionated bone marrow was incubated for 5 to 10 minutes at 16°C with mAb-AA4–coated beads. Following this incubation the mast cells bound to magnetic beads were removed using a magnetic particle concentrator (MPC) (Dynal). This step was repeated once. The AA4-/BGD6+ mast cells were then isolated by incubating the suspension of cells depleted of granulated mast cells with mAb-BGD6–coated beads (3 × 106 beads per 106 AA4-/BGD6+ mast cells). The beads with the bound AA4-/BGD6+ mast cells were isolated using the MPC and washed 4 times with Iscove medium containing 2% BSA. For experiments to isolate granulated mast cells, the unfractionated bone marrow was incubated first with magnetic beads conjugated to NMG and then with mAb-AA4–conjugated magnetic beads.
Cell culture
After isolation, unfractionated bone marrow cells, AA4+ mast cells, BGD6+ mast cells, or AA4-/BGD6+ mast cells were cultured, 3.3 × 104 cells per milliliter, in Iscove medium supplemented with antibiotics (penicillin, 100 U/mL; streptomycin, 100 μg/mL), antimycotic (amphotericin B, 0.25 μg/mL; Life Technologies, Rockville, MD), 0.05 mM β-mercaptoethanol, 10% fetal calf serum, 100 ng/mL recombinant stem cell factor (SCF) (Biosource International), and 20 ng/mL recombinant interleukin-3 (IL-3) (Biosource International). A total of 0.5 mL complete medium was added per flask every 5 days. Isolated cells were not removed from the magnetic beads. For depletion experiments, the unfractionated bone marrow was depleted 3 times with magnetic beads conjugated to NMG, mAb-AA4, or mAb-BGD6 or 2 times with mAb-AA4– and 1 time with mAb-BGD6–conjugated beads. The bone marrow cells remaining after depletion were cultured at 106/mL. At weekly intervals, nonadherent cells were removed from the flasks, centrifuged (28g; 5 minutes), resuspended in fresh medium, and replated at 106/mL. For experiments to test the effect of growth factors, the AA4-/BGD6+ mast cells were cultured with each of the hematopoietic cytokines, recombinant granulocyte-macrophage colony-stimulating factor (GM-CSF; 1 ng/mL; Biosource International), macrophage colony-stimulating factor (M-CSF; 25 U/mL; Biosource International), granulocyte colony-stimulating factor (G-CSF; 25 U/mL; Boehringer Mannheim, Mannheim, Germany), and erythropoietin (EPO; 2 U/mL; Laboratório Químico Bergamo, São Paulo, Brazil) without the addition of SCF or IL-3. The cells were also cultured with SCF or IL-3 along with each of the hematopoietic cytokines separately or with both SCF and IL-3 in combination with each of the hematopoietic cytokines. The RBL-2H3 cells were maintained in Eagle minimum essential medium (EMEM) supplemented with 15% fetal calf serum, penicillin, and streptomycin as previously described.13,16
β-hexosaminidase release
After 3 weeks in culture, AA4+/BGD6+ mast cells as well as the AA4-/BGD6+ mast cells were sensitized for 16 hours with IgE antitrinitrophenol (anti-TNP) at a concentration of 1:500 in culture medium. After 16 hours, the cells were washed 2 times with 100 μL Tyrode buffer (137 mM NaCl, 2.7 mM KCl, 12 mM NaHCO3, 0.37 mM NaH2PO4, 0.1 mM MgCl2, 1.3 mM CaCl2, 10 mM HEPES [N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid], pH 7.3) supplemented with 0.1% BSA and 0.01% gelatin. A total of 1.5 × 105 cells per assay were incubated in 100 μL Tyrode buffer with dinitrophenol–human serum albumin (DNP-HSA) for 30 minutes at 37°C (0.01 μg/mL to 1 μg/mL; 48 molecules of DNP per molecule of HSA; Sigma-Aldrich, St Louis, MO). A total of 0.25 μg/mL DNP-HSA gave maximal release of β-hexosaminidase. After incubation, the samples were centrifuged, the supernatants removed, and the cells solubilized in 1% Triton X-100 in Tyrode buffer, and 25 μL of the sample was added to an equal volume of 8 mM p-nitrophenyl-n-acetyl-β-D-glucosaminide (NAG; Sigma-Aldrich) in citrate–citric acid buffer, pH 4.5. The samples were incubated for 30 minutes at 37°C, and the reaction was stopped by the addition of 0.2 M NaCl, 0.2 M NaOH in 0.2 M glycine, pH 10.0. The samples were read at 405 nm in an Elisa Power Wave X Plate Reader (Bio-Tek Instruments, Winooski, VT). All assays were run in triplicate.
Microscopy
Cells were rinsed in phosphate-buffered saline (PBS) and placed on coverslips coated with Cell Tak (Becton Dickinson Labware, Bedford, MA). Some samples were fixed and stained for 20 minutes with toluidine blue (4% formaldehyde, 0.1% toluidine blue, and 1% acetic acid, pH 2.8) or alcian blue (1% alcian blue in 0.1 N hydrochloric acid). For fluorescence microscopy, frozen sections were placed on glass slides or cells were plated onto Cell Tak–coated coverslips. For some experiments cells were cultured for 24 hours prior to use with 2.5 μg/mL mouse IgE. Most samples were rinsed 3 times in PBS and fixed and permeabilized with 2% formaldehyde (Electron Microscopy Sciences, Fort Washington, PA) in methanol for 5 minutes at -20°C. Cells immunostained with anti–Ly-6G/Ly-6C and anti-CD45R/B220 were fixed and permeabilized with acetone for 5 minutes at - 20°C. Cells immunostained with anti-CD11b were fixed in 2% formaldehyde in PBS. The cells were then rinsed in PBS followed by PBS containing 0.1 M glycine, and nonspecific binding was blocked for 30 minutes at room temperature with FcBlock (1 μg/mL) and/or donkey antimouse IgG (5 μg/mL) diluted in PBS containing 1% BSA. The samples were then incubated with mAb-AA4 (5 μg/mL) directly conjugated to FITC, mAb-BGD6 (20 μg/mL), anti-FcϵRI β subunit (10 μg/mL), anti-IgE (10 μg/mL), anti–c-kit (15 μg/mL), anti-CD34 (15 μg/mL), anti-CD13 directly conjugated to FITC (15 μg/mL), anti–Thy-1 (15 μg/mL), anti-CD40 (10 μg/mL), anti–Ly-6G/Ly-6C (1:25), anti-CD11b (1:300), anti-CD45R/B220 (1:25), or anti–Sca-1 (5 μg/mL) for 1 hour at room temperature. Following incubation, the cells were rinsed thoroughly in PBS and the samples, except those incubated with mAb-AA4 and anti-CD13, incubated with secondary F(ab′)2 antibody conjugated to FITC (Jackson ImmunoResearch Labs, West Grove, PA). All cells were then rinsed and the coverslips mounted with Fluoromount-G (Electron Microscopy Sciences). Samples were observed by bright field (Olympus BX50, Olympus America, Melville, NY), phase contrast (Nikon Eclipse 800, Nikon USA, Melville, NY), fluorescence (Nikon Eclipse 800), or scanning confocal microscopy (Leica TCS-NT, Leica Microsystems, Heidelberg, Germany). Bright field, phase contrast, and fluorescence images were collected using a Nikon DXM 1200 digital camera (Nikon USA). Unfractionated bone marrow was used as a positive control, and cells incubated without primary antibody served as negative controls for immunolabeling experiments. Samples for transmission electron microscopy were prepared as previously described,17 and examined with a Phillips 108 electron microscope (FEI, Einhoven, The Netherlands). All images were prepared for publication using Adobe Photoshop 7.0 (Adobe Systems, San Jose, CA).
RT-PCR
RNA was extracted from mouse bone marrow cells, and reverse transcriptase–polymerase chain reaction (RT-PCR) was performed as previously described.9 The following primer sets were used: 5′-TGCCCAGCTGTGCCTAGCAC-3′ and 5′-CCACCTGCCTAAGATAGCCC-3′ for FcϵRIα; 5′-GTGCTGTTTGTTTTGTCTG-3′and 5′-GTTTCCCCTTTGTCTTCC-3′ for FcϵRIβ; 5′-CTTCAACTTTATCCCACC-3′ and 5′-CACAAAACCTGCACTATTC-3′ for mouse mast cell protease-5; 5′-TCACACACCCCGACTTCTAC-3′ and 5′-CTTCATTCCCAGCACACAG-3′ for mouse mast cell protease-7; 5′-ACACAGGATCGAATGTGGAG-3′ and 5′-TAATGCAGGACTTCATGAGC-3′ for mouse carboxypeptidase A; 5′-CATCCATCCAGCACAATCAG-3′ and 5′-AACACTCCAGAATCGTCAACTG-3′ for mouse c-kit; 5′-GGCGGACTGTTACTGAGCTGCG-3′ and 5′-AGAAGCAATGCTGTCACCTTCCCC-3′ for actin. Amplification was done for 30 seconds at 94°C, 30 seconds at 55°C, and 1 minute at 72°C for 35 cycles in a 25 μL reaction using Platinum Taq Polymerase (Invitrogen, Rockville, MD). The PCR products were analyzed on 2.5% agarose gels.
Mast cell reconstitution in irradiated mice
Female BALB/c mice, 8 to 12 weeks old, were housed in a pathogen-free environment, and 1 week prior to irradiation, their drinking water was replaced with sterile, acidifed water, pH 2.5, containing 2 mg/mL neomycin sulfate. The mice were lethally irradiated with 2 separate doses of 450 cGy using a cobalt-60 source with an interval of 3 hours between doses. Forty-eight hours after irradiation, mice were injected via the tail vein with unfractionated bone marrow, isolated AA4+ mast cells, or AA4-/BGD6+ mast cells (1.5 × 105 cells in 100 μL PBS). Control mice received 100 μL PBS. A minimum of 5 mice were used for each experimental condition. Twelve days after injection, the spleens were removed and examined macroscopically using a Leica MZFLIII stereomicroscope (Leica Microsystems, Wetzlar, Germany). For histologic examination, the spleens were fixed in 4% paraformaldehyde in PBS and embedded in Paraplast (Oxford Labware, St Louis, MO); 7 μm transversal serial sections were cut. Alternate sections were stained with 0.1% toludine blue in 4% formaldehyde plus 1% acetic acid, pH 2.8, or hematoxylin and eosin. The area of each section was determined using Image-Pro Plus, v 4.5.1 (Media Cybernetics, Silver Spring, MD). The number of metachromatic mast cells in each section was then determined. A minimum of 150 sections representing 3 blocks per spleen from each of 5 mice were examined. Results are expressed as the number of metachromatic mast cells ± SD. All quantification was done blind.
Results
Antibody characterization
By immunostaining, mAb-BGD6 bound to the cell surface of RBL-2H3 cells and to rat peritoneal mast cells. By fluorescence-activated cell sorter (FACS) analysis of rat peritoneal cells, mAb-BGD6 bound only to mast cells. Because mAb-AA4 and mAb-BGD6 were raised against the surface of a rat mast cell line, the cross-reactivity and specificity of these antibodies for mouse mast cells was confirmed by immunostaining 18 different tissues from mice: adrenal gland, brain, duodenum, epididymis, heart, kidney, lacrimal gland, liver, lung, parotid gland, pancreas, skeletal muscle, skin, sublingual gland, submandibular gland, tongue, testis, and thymus. Following immunostaining the mast cells were further identified by staining the sections with either alcian blue or toluidine blue. mAb-AA4 and mAb-BGD6 immunolabeled only mast cells in all of the tissues examined. No other cell type was labeled, and all controls were negative. Additionally, smears of whole peripheral blood and cells from peritoneal washings were immunostained with mAb-AA4 and mAb-BGD6. In the peripheral blood, no cells were stained and, as expected,18 no granulated mast cells could be identified with toluidine blue staining. In peritoneal washings, only mast cells were immunostained and all other cell types were negative. None of the control preparations were immunolabeled.
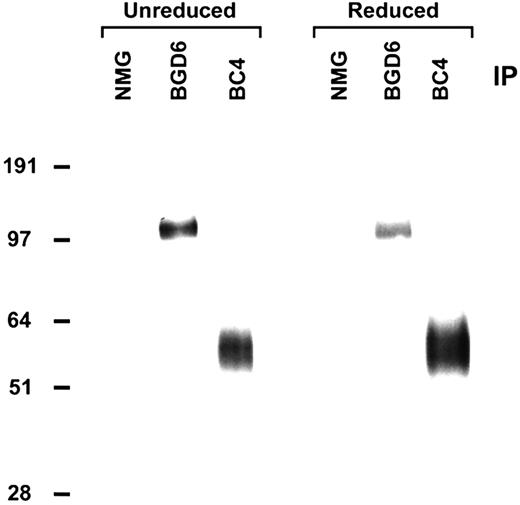
Immunoprecipitation of cell surface–labeled RBL-2H3 cells with mAb-BGD6 showed an approximately 110-kDa protein under both reduced and nonreduced conditions (Figure 1). By radioiodination a band of approximately 40-60 kDa was also seen. These bands are distinct from FcϵRI by immunoclearing experiments. In further studies mAb-BGD6 did not inhibit IgE binding and, in histamine release studies, it did not activate cells for release or inhibit IgE-induced degranulation. Therefore, mAb-BGD6 binds to a mast cell–specific cell-surface protein that is distinct from FcϵRI.
mAb-BGD6 immunoprecipitates an approximately 110-kDa protein under both reduced and nonreduced conditions. RBL-2H3 cells were surface labeled with biotin and immunoprecipitated (IP) with Sepharose beads coupled with NMG, mAb-BGD6, or the anti-FcϵRI mAb-BC4. After extensive washing the immunoprecipitated proteins were detected by blotting with HRP-streptavidin. The 110-kDa protein immunoprecipitated by mAb-BGD6 is distinct from the α subunit of FcϵRI immunoprecipitated by mAb-BC4.
mAb-BGD6 immunoprecipitates an approximately 110-kDa protein under both reduced and nonreduced conditions. RBL-2H3 cells were surface labeled with biotin and immunoprecipitated (IP) with Sepharose beads coupled with NMG, mAb-BGD6, or the anti-FcϵRI mAb-BC4. After extensive washing the immunoprecipitated proteins were detected by blotting with HRP-streptavidin. The 110-kDa protein immunoprecipitated by mAb-BGD6 is distinct from the α subunit of FcϵRI immunoprecipitated by mAb-BC4.
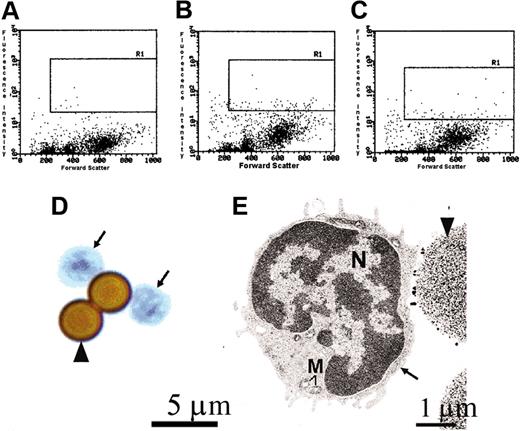
A pure population of AA4-/BGD6+ mast cells can be isolated from the mast cells present in bone marrow. The AA4-/BGD6+ mast cells are a homogeneous population of cells. Cells representative of the freshly isolated AA4-/BGD6+ cell population are shown here. In this representative experiment from 12 experiments, the flow profiles show that the AA4+ cells constitute 2.58% of the cells in the bone marrow while the BGD6+ cells make up 2.51% of the cells in the bone marrow. R1 indicates gated area. (A) Bone marrow cells labeled with mouse IgG-FITC. (B) Bone marrow cells labeled with mAb-AA4–FITC. (C) Bone marrow cells labeled with mAb-BGD6–FITC. (D) These undifferentiated cells have large nuclei and little cytoplasm (arrows). Cells were stained with toluidine blue (arrowhead, mAb-BGD6–conjugated magnetic bead); magnification, × 2000. Image was collected with an Olympus BX50 microscope (Olympus America) equipped with a 60× UPLANFL objective (N.A. 1.25). (E) By electron microscopy, these cells (arrow) have a large concave nucleus (N), little cytoplasm, and no cytoplasmic granules (M indicates mitochondria; arrowhead, mAb-BGD6–conjugated magnetic bead); magnification, × 6600.
A pure population of AA4-/BGD6+ mast cells can be isolated from the mast cells present in bone marrow. The AA4-/BGD6+ mast cells are a homogeneous population of cells. Cells representative of the freshly isolated AA4-/BGD6+ cell population are shown here. In this representative experiment from 12 experiments, the flow profiles show that the AA4+ cells constitute 2.58% of the cells in the bone marrow while the BGD6+ cells make up 2.51% of the cells in the bone marrow. R1 indicates gated area. (A) Bone marrow cells labeled with mouse IgG-FITC. (B) Bone marrow cells labeled with mAb-AA4–FITC. (C) Bone marrow cells labeled with mAb-BGD6–FITC. (D) These undifferentiated cells have large nuclei and little cytoplasm (arrows). Cells were stained with toluidine blue (arrowhead, mAb-BGD6–conjugated magnetic bead); magnification, × 2000. Image was collected with an Olympus BX50 microscope (Olympus America) equipped with a 60× UPLANFL objective (N.A. 1.25). (E) By electron microscopy, these cells (arrow) have a large concave nucleus (N), little cytoplasm, and no cytoplasmic granules (M indicates mitochondria; arrowhead, mAb-BGD6–conjugated magnetic bead); magnification, × 6600.
Immunomagnetic isolation of AA4-/BGD6+ mast cells
Only 2.6% ± 0.5% (X ± SD in 25 different experiments) of the cells in the bone marrow were immunolabeled with mAb-AA4 or mAb-BGD6. These results were confirmed by FACS analysis (Figure 2A-C). Electron microscopy of unfractionated bone marrow showed that both mAb-AA4 and mAb-BGD6 immunolabeled granulated mast cells in all stages of maturation.9 However, by toluidine blue staining less than 0.1% of the AA4+ or BGD6+ cells contained metachromatic granules and could be recognized as mast cells in conventional preparations, suggesting that these cells that do not stain with toluidine blue are immature mast cells that are not well granulated. Additionally, a very small population of undifferentiated mast cells was immunolabeled with mAb-BGD6 but not with mAb-AA4. Because the AA4-/BGD6+ mast cells are rare, sequential immunomagnetic isolation was used to obtain these cells in sufficient quantity to characterize. Suspensions of cells from the bone marrow were first incubated with mAb-AA4–conjugated magnetic beads to remove the granulated mast cells. The cell suspension depleted of granulated mast cells was then incubated with mAb-BGD6–conjugated magnetic beads. The cells that bound to the mAb-BGD6–conjugated magnetic beads constituted 0.02% ± 0.007% (X ± SD in 41 different experiments) of the cells in the bone marrow, which represents an approximately 5000-fold purification of the AA4-/BGD6+ mast cells. These cells are a morphologically homogeneous population of small, 3.2 ± 0.2 μm diameter cells. If the cell suspension that had been depleted of granulated mast cells was incubated with magnetic beads conjugated to NMG or mAb-AA4 instead of mAb-BGD6, no cells were recovered.
Characterization of AA4-/BGD6+ mast cells
By light microscopy, the AA4-/BGD6+ mast cells appeared as a homogeneous population of undifferentiated cells with a concave nucleus, little cytoplasm, and containing no metachromatic granules (Figure 2D). By electron microscopy, a pure population of small undifferentiated cells containing scant cytoplasm, few organelles, and no cytoplasmic granules (Figure 2E) was seen. The cell surface was covered with short microvilli.
The surface phenotype of the isolated cells (Table 1) confirms their identity as an undifferentiated mast cell rather than a basophil, because basophils do not express c-kit or CD13.5,19 Like human mast cell precursors,20 the mouse AA4-/BGD6+ mast cells also express CD34, c-kit, and CD13. However, unlike granulated mast cells, they do not express the β subunit of FcϵRI, bind IgE, or have the gangliosides recognized by mAb-AA4 present on their surface. They also do not express surface markers for granulocytes or monocytes (Ly6G/Ly6C), macrophages and their precursors (CD11b), T cells (Thy-1), B cells (CD40 and CD45R/B220), or hematopoietic progenitor cells (Sca-1).
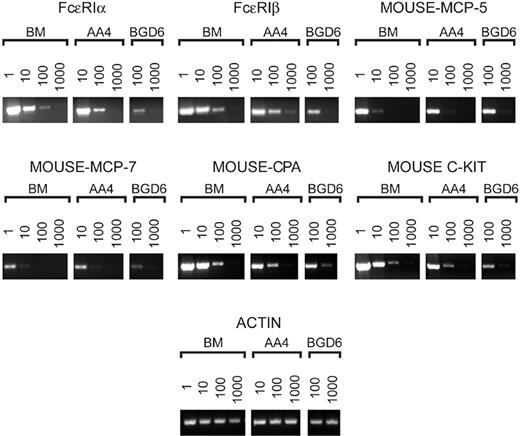
Although these cells do not yet express FcϵRI, the PCR (Figure 3) of the isolated cells demonstrated that the cells do contain message for the α and β subunits of FcϵRI. Additionally, these cells contained message for mast cell–specific proteases even though cytoplasmic granules could not be detected by light or electron microscopy. Message for these same proteins was also observed in the granulated mast cells isolated with mAb-AA4. Therefore, the PCR analysis provides further evidence that the AA4-/BGD6+ cells are indeed mast cells.
Culture of AA4-/BGD6+ cells
To confirm that the isolated undifferentiated mast cells would develop into granulated mast cells, the cells were cultured with various hematopoietic cytokines (M-CSF, G-CSF, GM-CSF, EPO) as well as with IL-3 and SCF either singly or in combination. The AA4-/BGD6+ cells showed an absolute requirement for the presence of both IL-3 and SCF. When one or more other hematopoietic cytokines were added to the culture medium along with IL-3 and SCF, only mast cells were seen. Cell growth was not supported. IL-3, SCF, M-CSF, G-CSF, GM-CSF, or EPO were added individually to the culture medium. Cell growth also was not observed with any combination of these hematopoietic cytokines. When the AA4-/BGD6+ mast cells were cultured in the presence of one or more of the hematopoietic cytokines along with SCF, there was no cell growth. However, when the unfractionated bone marrow was cultured in the presence of the hematopoietic cytokines, growth of the appropriate cell type was observed. The AA4-/BGD6+ mast cells required approximately 2 weeks in culture before there was an appreciable increase in the cell number. However, at 2 weeks the growth rate increased dramatically, and by 6 weeks in culture the cell number had increased approximately 60-fold (Figure 4). A similar growth rate was seen when the unfractionated bone marrow was cultured in the presence of SCF and IL-3, but there was no lag in growth and there was an approximately 70-fold increase in cell number by 6 weeks. In contrast, when the granulated mast cells—isolated with mAb-AA4— were cultured, they increased approximately 5-fold by 3 weeks, but the cell number then declined, indicating the lack of renewable precursors in this population. Furthermore, the AA4-/BGD6+ mast cells formed mast cell colonies when grown in methylcellulose with SCF and IL-3.
By semiquantitative RT-PCR, freshly isolated AA4-/BGD6+ mast cells contain message for the α and β subunits of FcϵRI, mast cell–specific proteases, and carboxypeptidase A. The cDNA prepared from the same number of either total bone marrow cells (BM) or cells bound to mAb-AA4 beads (AA4) or cells bound to mAb-BGD6 beads after clearing with mAb-AA4 beads (BGD6) was serially diluted as indicated and amplified. The products were analyzed on 2.5% agarose gels. The RT-PCR confirms that the cells isolated from the bone marrow with mAb-AA4 beads are mast cells and that the undifferentiated mast cells (BGD6) contain message for FcϵRI, mast cell–specific proteases, and c-kit. FcϵRIα indicates α subunit of the high-affinity IgE receptor; FcϵRIβ, β subunit of the high-affinity IgE receptor; mouse-MCP-5, mouse mast cell protease-5; mouse-MCP-7, mouse mast cell protease-7; mouse-CPA, mouse carboxypeptidase A.
By semiquantitative RT-PCR, freshly isolated AA4-/BGD6+ mast cells contain message for the α and β subunits of FcϵRI, mast cell–specific proteases, and carboxypeptidase A. The cDNA prepared from the same number of either total bone marrow cells (BM) or cells bound to mAb-AA4 beads (AA4) or cells bound to mAb-BGD6 beads after clearing with mAb-AA4 beads (BGD6) was serially diluted as indicated and amplified. The products were analyzed on 2.5% agarose gels. The RT-PCR confirms that the cells isolated from the bone marrow with mAb-AA4 beads are mast cells and that the undifferentiated mast cells (BGD6) contain message for FcϵRI, mast cell–specific proteases, and c-kit. FcϵRIα indicates α subunit of the high-affinity IgE receptor; FcϵRIβ, β subunit of the high-affinity IgE receptor; mouse-MCP-5, mouse mast cell protease-5; mouse-MCP-7, mouse mast cell protease-7; mouse-CPA, mouse carboxypeptidase A.
Growth characteristics of AA4+ and AA4-/BGD6+ mast cells following immunomagnetic isolation. Total bone marrow cells (BM; ♦), AA4+ (▪), and AA4-/BGD6+ (▴) isolated cells were placed in culture at 3.3 × 104/mL. Cells were fed every 5 days as described in “Materials and methods” and the total viable cell number determined at the indicated times. When freshly isolated AA4-/BGD6+ cells are cultured in the presence of IL-3 and SCF, there is a lag of approximately 2 weeks before the cell number increases. After 6 weeks there has been a 60-fold increase in the cell number. The unfractionated bone marrow exhibits similar growth kinetics, with the exception of the lag at the initiation of the culture, increasing 70-fold at 6 weeks. The isolated AA4+ cells have different growth kinetics, expanding only 5-fold at 3 weeks with the cell numbers declining after this time, indicating the lack of a renewable precursor in this population. Data are X ± SD from 10 cultures.
Growth characteristics of AA4+ and AA4-/BGD6+ mast cells following immunomagnetic isolation. Total bone marrow cells (BM; ♦), AA4+ (▪), and AA4-/BGD6+ (▴) isolated cells were placed in culture at 3.3 × 104/mL. Cells were fed every 5 days as described in “Materials and methods” and the total viable cell number determined at the indicated times. When freshly isolated AA4-/BGD6+ cells are cultured in the presence of IL-3 and SCF, there is a lag of approximately 2 weeks before the cell number increases. After 6 weeks there has been a 60-fold increase in the cell number. The unfractionated bone marrow exhibits similar growth kinetics, with the exception of the lag at the initiation of the culture, increasing 70-fold at 6 weeks. The isolated AA4+ cells have different growth kinetics, expanding only 5-fold at 3 weeks with the cell numbers declining after this time, indicating the lack of a renewable precursor in this population. Data are X ± SD from 10 cultures.
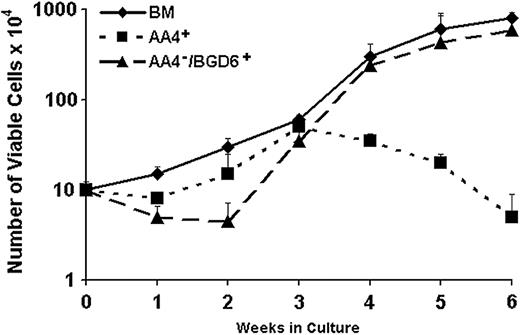
Cultures were also prepared with bone marrow cells that had been depleted with mAb-AA4 and/or mAb-BGD6. Depletion of the total bone marrow with only mAb-AA4 resulted in a slight reduction in the total number of mast cells after 3 weeks in culture, indicating that mAb-AA4 does not remove undifferentiated mast cells or the pluripotent hematopoietic stem cells. However, when the total bone marrow was depleted of mast cells with mAb-BGD6 alone or with both mAb-AA4 and mAb-BGD6, there was a significant decrease in the total number of mast cells after 3 weeks in culture (Figure 5), demonstrating that the AA4-/BGD6+ mast cells had been removed. These results also suggest that the multipotent hematopoietic progenitors that give rise to the mast cell lineage-committed cells are not removed and are mAb-BGD6 negative.
Morphologic and functional characterization of cultured AA4-/BGD6+ mast cells
The morphology of the cultured AA4-/BGD6+ mast cells was also consistent with their identification as mast cells. When the isolated cells were first placed in culture, they formed small nonadherent colonies that frequently arose from a cell still attached to a magnetic bead. With time in culture, the colonies dispersed but the cells continued to grow in suspension. After 4 weeks in culture, by light microscopy 30% ± 3% (X ± SD, n = 7) of the cells contained metachromatic granules (Figure 6A), but by electron microscopy all of the cells were granulated (Figure 6B). Moreover, all of the cells displayed a morphology typical for cultured bone marrow–derived mast cells. The cells had a large lobated nucleus, a cytoplasm with a well-developed Golgi apparatus, and numerous cytoplasmic granules of varying density. The cell surface was covered with fine microvilli.
Immunostaining (Table 2) of the cultured AA4-/BGD6+ mast cells demonstrated that after 2 weeks in culture these cells had developed a surface phenotype nearly identical to that of freshly isolated granulated mast cells (Table 1) with the exception of CD34, which was still high in the AA4-/BGD6+ mast cells. This phenotype did not change appreciably with further time in culture except for the expression of CD34 that dropped to the levels seen in the granulated mast cells. Interestingly, after 2 weeks in vitro cultures of the unfractionated bone marrow still contained other cell types, with only 85% to 90% of the cells expressing mast cell–specific markers. At 4 weeks in culture, the AA4-/BGD6+ mast cells now expressed the high-affinity IgE receptor, bound IgE, were positive for c-kit, and contained the mast cell–specific gangliosides on their surface. In addition, the percentage of cells expressing CD34 had dropped close to that seen in the freshly isolated granulated mast cells.
Growth characteristics of mast cell–depleted populations of cells following immunomagnetic isolation. Nonfractionated bone marrow cells (BM) or cells depleted with immunomagnetic beads conjugated with normal mouse IgG (NMG), mAb-AA4, mAb-BGD6, or sequentially with both mAb-AA4 and mAb-BGD6 were placed in culture at 106/mL with IL-3 and SCF. The total number of viable cells was determined after 3 weeks' culture in 15 different experiments. When the bone marrow cells are depleted of AA4-/BGD6+ mast cells by sequential incubation with mAb-AA4–conjugated beads followed by mAb-BGD6–conjugated beads or by mAb-BGD6–conjugated beads alone, there is a highly significant reduction (P < .001 by analysis of variance [ANOVA]) in the total number of cells after 3 weeks in culture when compared with cells from unfractionated bone marrow (BM). There is a slight but significant (P < .01) reduction in the total number of cells when the bone marrow cells are depleted only of granulated mast cells with mAb-AA4–conjugated beads. Incubation of the bone marrow cells with beads conjugated to NMG had no effect on the total number of cells seen at 3 weeks. These results indicate that the AA4-/BGD6+ mast cells are responsible for a significant number of the mast cells seen in cultures of unfractionated bone marrow but that a pluripotent hematopoietic stem cell remains after the AA4-/BGD6+ mast cells are removed. Error bars indicate standard deviation.
Growth characteristics of mast cell–depleted populations of cells following immunomagnetic isolation. Nonfractionated bone marrow cells (BM) or cells depleted with immunomagnetic beads conjugated with normal mouse IgG (NMG), mAb-AA4, mAb-BGD6, or sequentially with both mAb-AA4 and mAb-BGD6 were placed in culture at 106/mL with IL-3 and SCF. The total number of viable cells was determined after 3 weeks' culture in 15 different experiments. When the bone marrow cells are depleted of AA4-/BGD6+ mast cells by sequential incubation with mAb-AA4–conjugated beads followed by mAb-BGD6–conjugated beads or by mAb-BGD6–conjugated beads alone, there is a highly significant reduction (P < .001 by analysis of variance [ANOVA]) in the total number of cells after 3 weeks in culture when compared with cells from unfractionated bone marrow (BM). There is a slight but significant (P < .01) reduction in the total number of cells when the bone marrow cells are depleted only of granulated mast cells with mAb-AA4–conjugated beads. Incubation of the bone marrow cells with beads conjugated to NMG had no effect on the total number of cells seen at 3 weeks. These results indicate that the AA4-/BGD6+ mast cells are responsible for a significant number of the mast cells seen in cultures of unfractionated bone marrow but that a pluripotent hematopoietic stem cell remains after the AA4-/BGD6+ mast cells are removed. Error bars indicate standard deviation.
The AA4-/BGD6+ mast cells differentiate into mature mast cells in vitro. (A) Light microscopy of cells from cultures of AA4-/BGD6+ mast cells. After 4 weeks in culture many of the cells now contain granules (arrows) when stained with toluidine blue; magnification, × 750. Additionally, an occasional granulated cell can be seen still attached to an mAb-BGD6–conjugated magnetic bead (inset, arrow-head; magnification, × 880). Image was collected with an Olympus BX50 microscope equipped with a 60× UPLANFL objective (N.A. 1.25). (B) Electron microscopy of cells from cultures of AA4-/BGD6+ mast cells. By electron microscopy at 4 weeks, the cells from the culture of mast cell precursors have a morphology typical of cultured bone marrow–derived mast cells. Their surface is covered with thin microvilli (small arrow), and the cytoplasm contains a well-developed Golgi apparatus (G), numerous mitochondria (M), and cytoplasmic granules (large arrows) of varying density. N indicates nucleus. Magnification, × 4000.
The AA4-/BGD6+ mast cells differentiate into mature mast cells in vitro. (A) Light microscopy of cells from cultures of AA4-/BGD6+ mast cells. After 4 weeks in culture many of the cells now contain granules (arrows) when stained with toluidine blue; magnification, × 750. Additionally, an occasional granulated cell can be seen still attached to an mAb-BGD6–conjugated magnetic bead (inset, arrow-head; magnification, × 880). Image was collected with an Olympus BX50 microscope equipped with a 60× UPLANFL objective (N.A. 1.25). (B) Electron microscopy of cells from cultures of AA4-/BGD6+ mast cells. By electron microscopy at 4 weeks, the cells from the culture of mast cell precursors have a morphology typical of cultured bone marrow–derived mast cells. Their surface is covered with thin microvilli (small arrow), and the cytoplasm contains a well-developed Golgi apparatus (G), numerous mitochondria (M), and cytoplasmic granules (large arrows) of varying density. N indicates nucleus. Magnification, × 4000.
Because the cultured AA4-/BGD6+ mast cells express FcϵRI on their surface as well as bind IgE, it was of interest to determine if the cells could be stimulated to release their granule contents. Cells that had been in culture for 3 weeks were sensitized with antigen-specific IgE and stimulated with antigen for 30 minutes. The cells from the AA4-/BGD6+ mast cell cultures released 30% ± 0.85% of their β-hexosaminidase while those from the AA4+ mast cell cultures released 35% ± 0.54% of their β-hexosaminidase (X ± SD, n = 5). These results demonstrate that after 3 weeks in culture the AA4-/BGD6+ mast cells possess one of the principal functional characteristics of mast cells, degranulation through the FcϵRI.
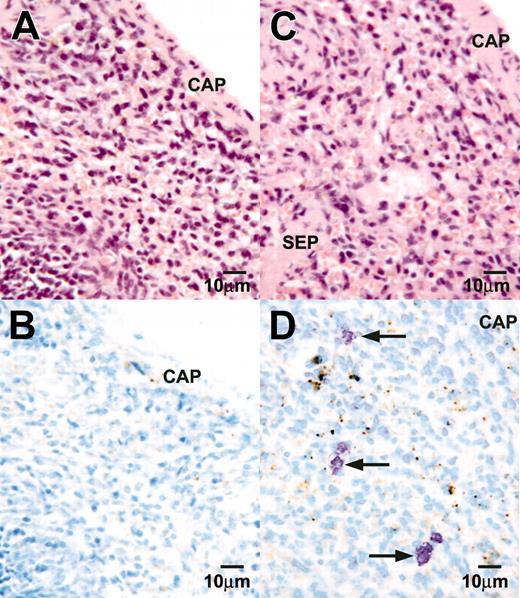
The AA4-/BGD6+ mast cells differentiate into mature mast cells in vivo. Light microscopy of a paraffin section of the spleen from a lethally irradiated mouse, hemotoxylin and eosin stained (A), or a serial section stained with toluidine blue (B). No mast cells can be seen. Light microscopy of a paraffin section of the spleen from a lethally irradiated mouse reconstituted with AA4-/BGD6+ mast cells stained with hemotoxylin and eosin (C) or toluidine blue (D). Mast cells with metachromatic granules (arrows) are preferentially present in the area adjacent to the capsule of the spleen. CAP indicates capsule; SEP, septum. Magnification, × 500. Image was collected with an Olympus BX50 microscope equipped with a 40× UPLANFL objective (N.A. 0.75).
The AA4-/BGD6+ mast cells differentiate into mature mast cells in vivo. Light microscopy of a paraffin section of the spleen from a lethally irradiated mouse, hemotoxylin and eosin stained (A), or a serial section stained with toluidine blue (B). No mast cells can be seen. Light microscopy of a paraffin section of the spleen from a lethally irradiated mouse reconstituted with AA4-/BGD6+ mast cells stained with hemotoxylin and eosin (C) or toluidine blue (D). Mast cells with metachromatic granules (arrows) are preferentially present in the area adjacent to the capsule of the spleen. CAP indicates capsule; SEP, septum. Magnification, × 500. Image was collected with an Olympus BX50 microscope equipped with a 40× UPLANFL objective (N.A. 0.75).
AA4-/BGD6+ mast cells are able to reconstitute the spleens of lethally irradiated mice
The ability of the undifferentiated AA4-/BGD6+ mast cells to differentiate in vivo was confirmed by injecting these cells into lethally irradiated mice. Twelve days after injection, the spleens were examined for the presence of metachromatic mast cells (Figure 7). In control, nonirradiated mice, mast cells numbered 10.25 ± 3.7/mm2; in irradiated, noninjected mice, 0.18 ± 0.32/mm2; in irradiated mice injected with unfractionated bone marrow, 4.15 ± 2.5/mm2; in irradiated mice injected with AA4+ mast cells, 8.3 ± 2.8/mm2; and in irradiated mice injected with AA4-/BGD6+ mast cells, 14 ± 2.9/mm2 (X ± SD, n = 5). The spleens from mice injected with unfractionated bone marrow presented 2 to 3 lymphocytic colonies on their surface per 105 cells injected. Mast cells were restricted to the connective tissue surrounding the lymphocytic colonies and to the connective tissue in the subcapsular area. No discrete mast cell colonies were observed. In contrast, after injection of AA4-/BGD6+ cells, no lymphocytic colonies were seen and the mast cells were preferentially distributed to the subcapsular region. After injection of the AA4+ cells, the mast cell distribution was similar to that seen with the AA4-/BGD6+ undifferentiated mast cells. The AA4+ mast cells also did not form colonies but were found primarily in the subcapsular region of the spleen. The AA4+ mast cells were less effective than the AA4-/BGD6+ mast cells in reconstituting the spleen, presumably due to a diminished capacity to proliferate, but colonies of other cell types were not observed. These results confirm that the AA4-/BGD6+ mast cells have the potential to differentiate into mature mast cells in vivo.
Discussion
In the present study, a homogeneous population of undifferentiated mast cells (AA4-/BGD6+) has been isolated from adult mouse bone marrow. These cells constitute only 0.02% of the cells in the bone marrow and may represent a committed mast cell precursor. Although they resemble a pluripotent hematopoietic stem cell,21 they grow only in the presence of both SCF and IL-3. The AA4-/BGD6+ mast cells express the surface proteins CD34, c-kit, and CD13 that when expressed jointly may be considered characteristic of mast cell precursors.20 By PCR the cells contain message for the α and β subunits of FcϵRI and mouse mast cell–specific proteases even though they do not express the FcϵRI.
Immunologic methods have been used previously in an attempt to identify committed mast cell precursors, but unlike the antibodies used here, the antibodies used in earlier studies were not mast cell specific and also recognized other cell types, yielding mixed populations of cells with pluripotent growth potential.20,22-26 A subpopulation of human hematopoietic progenitors, CD34+, c-kit+, and CD13+, was sorted by FACS from bone marrow.20 However, unlike the cell isolated here, this population of cells gave rise to both mast cells and monocytes. A promastocyte has also been identified from fetal mouse blood.27 This cell is considerably different from the AA4-/BGD6+ mast cells. The promastocyte isolated from fetal mouse blood was c-kit positive, FcϵRI negative, and contained metachromatic granules, suggesting that it is a more differentiated cell.
The number of undifferentiated mast cells detected in the bone marrow with mAb-BGD6 in the present study, 200 per 106 cells, is in agreement with the number of mast cell precursors estimated from limiting dilution in vitro assays (291 per 106 cells,28 680 per 106 cells,29 and 924 per 106 mononuclear cells30 ) or from in vivo assays (100 per 106 cells31 ). The number of undifferentiated AA4-/BGD6+ mast cells isolated from the bone marrow in this study does not include pluripotent hematopoietic stem cells that have the potential to develop into mast cells. Further evidence that the AA4-/BGD6+ mast cells may be a mast cell precursor is provided by the fact that these cells grow and mature in vitro only in the presence of both SCF and IL-3, the major factors required to support growth and differentiation by cultured mast cells.32-38 Although the freshly isolated AA4-/BGD6+ mast cells do contain mRNA for the α and β subunits of FcϵRI, the receptor is not expressed on the surface of the freshly isolated cells, consistent with earlier reports on FcϵRI expression in rodent bone marrow–derived mast cell cultures.23,29,39,40 However, after time in culture the AA4-/BGD6+ mast cells do express FcϵRI, bind IgE, can be activated through FcϵRI, exhibit the mast cell–specific gangliosides on their surface, as well as contain metachromatic granules. Morphologic studies of rat bone marrow–derived mast cells have shown that the gangliosides recognized by mAb-AA4 are present only on the surface of granulated mast cells.9 Other reports suggest that during mast cell maturation, the appearance of FcϵRI on the cell surface occurs at the same time as the initiation of the formation of cytoplasmic granules.40 The appearance of FcϵRI and the gangliosides on the surface of the mast cells occurs prior to the acquisition of metachromasia at a time when by electron microscopy only 1 or 2 small cytoplasmic granules may be present in the cells.9 Morphologically, after 4 weeks in culture, the AA4-/BGD6+ mast cells appeared identical to cells described in previous studies of bone marrow cultured with SCF and IL-3.35,41
The ability of the AA4-/BGD6+ mast cells to reconstitute the spleens of lethally irradiated mice lends further support to the suggestion that these cells represent a population of committed mast cell precursors. Previous studies using unfractionated bone marrow to reconstitute the spleen with other types of cells have shown that the spleen is the first site where mature cells appear in lethally irradiated mice.42,43 In the present study, the injection of unfractionated bone marrow reconstituted the mast cells in the spleen as well as gave rise to surface colonies that, as has been shown before,44 are composed of lymphocytes. These lymphocytic colonies or colonies composed of other cell types were not observed following reconstitution with the AA4-/BGD6+ mast cells.
Prepublished online as Blood First Edition Paper, February 17, 2005; DOI 10.1182/blood-2004-02-0756.
Supported by grants from the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Coodenação de Aperfiçoamento de Pessoal de Nivel Superior (CAPES), Fundação de Apoio as Ensino, Pesquisae Assistência (FAEPA), and by Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP). A.C.G.G. was the recipient of an FAPESP fellowship.
Presented in abstract form at the 41st annual meeting of the American Society for Cell Biology, Washington, DC, December 10, 2001, and at the Fourth International Workshop on Signal Transduction in the Activation and Development of Mast Cells and Basophils, Bethesda, MD, November 29, 2001.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
The authors thank Maria Tereza Picinoto Maglia, Electron Microscopy Laboratory, FMRP, and Lynda Weedon and Linda Bowers, National Institute of Dental and Craniofacial Research (NIDCR), NIH, for technical assistance; Alexandra Rosa Vieira Dias, FMRP, and Patricia Vianna Bonini Pauma, Hemocentro de Ribeirão Preto (HCFMRP), for assistance with the FACS analysis; and Dr William D. Swaim, Director, Cellular Imaging Core Facility, NIDCR, NIH, for his assistance in preparing the micrographs.

![Figure 5. Growth characteristics of mast cell–depleted populations of cells following immunomagnetic isolation. Nonfractionated bone marrow cells (BM) or cells depleted with immunomagnetic beads conjugated with normal mouse IgG (NMG), mAb-AA4, mAb-BGD6, or sequentially with both mAb-AA4 and mAb-BGD6 were placed in culture at 106/mL with IL-3 and SCF. The total number of viable cells was determined after 3 weeks' culture in 15 different experiments. When the bone marrow cells are depleted of AA4-/BGD6+ mast cells by sequential incubation with mAb-AA4–conjugated beads followed by mAb-BGD6–conjugated beads or by mAb-BGD6–conjugated beads alone, there is a highly significant reduction (P < .001 by analysis of variance [ANOVA]) in the total number of cells after 3 weeks in culture when compared with cells from unfractionated bone marrow (BM). There is a slight but significant (P < .01) reduction in the total number of cells when the bone marrow cells are depleted only of granulated mast cells with mAb-AA4–conjugated beads. Incubation of the bone marrow cells with beads conjugated to NMG had no effect on the total number of cells seen at 3 weeks. These results indicate that the AA4-/BGD6+ mast cells are responsible for a significant number of the mast cells seen in cultures of unfractionated bone marrow but that a pluripotent hematopoietic stem cell remains after the AA4-/BGD6+ mast cells are removed. Error bars indicate standard deviation.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/105/11/10.1182_blood-2004-02-0756/6/m_zh80110579100005.jpeg?Expires=1769520676&Signature=WBKtYFg9YxVfRTGYYFGiXkHgjgVuOqE78qNRHwjXsVwH6f9WkogJNBu0q--kKraXGvVhm0hXfjXwqCFeKAGQ~0UqiUJh9Sk1EKBuBgF6OrFYLzp8KzwIfxvqF9q4wuaWj9TEz2l~lRORwv06KFmJJgS2T4EudbYfgGHLutl5iQvrrOUxOtCnjXJ4Vg4yl7bWWA7xpEc4kLdwOU9WM0EMyWHCiymaaQb9sAZUbC-pCK6FCxuBhgBGX--OfFCJwHfRTq9GhDwES7cysSzL5jl7S8rIs8JRaibyjkCGYJ5RMLE2tGp9BnguTSLHO~f~02wwzpq0W-DoehtaEoHH6ihxfw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)