Abstract
Chronic graft-versus-host disease (cGVHD) is an important determinant of long-term morbidity and mortality in allogeneic stem cell transplantation patients. Because cGVHD has clinical, histologic, and laboratory findings of autoimmune diseases and anti–B-cell therapy has shown efficacy in autoimmune diseases, we hypothesized that monoclonal anti-CD20 antibody therapy might improve patients with cGVHD. We treated 5 men and 1 woman with therapy-refractory extensive cGVHD with anti-CD20 monoclonal antibody. Intravenous infusion was given at a weekly dose of 375 mg/m2 for 4 weeks. In case of incomplete clinical response, additional courses of 4 weeks were given. Five patients responded to treatment with marked clinical, biochemical, and histologic improvement. One patient failed to respond. Anti-CD20 monoclonal antibody seems to be effective in cGVHD. A controlled trial is mandatory to confirm these results. The outcome of this study suggests a participating role of B cells in the pathogenesis of cGVHD.
Introduction
Chronic graft-versus-host disease (cGVHD) is the most important determinant of long-term morbidity1-5 in patients undergoing allogeneic stem cell transplantation (SCT). Several investigational treatments have been performed in immunosuppressive therapy-refractory cGVHD,6-8 but only few patients have responded to such treatments. Especially patients with sclerodermoid cGVHD urgently need new treatment approaches.
Anti-CD20 antibody (rituximab) is a chimeric mouse-human immunoglobulin G (IgG) κ antibody, mediating B-cell lysis by complement-dependent cytotoxicity, antibody-dependent cellular cytotoxicity, and induction of apoptosis. It was developed for the treatment of B-cell malignancies but also has efficacy in the treatment of a variety of autoimmune diseases,9-18 like systemic sclerosis. Chronic GVHD has characteristics not only of immunodeficiency but also of autoimmunity.19 Although B cells are hardly present in the affected tissues in cGVHD,20 most patients with cGVHD have evidence of B-cell activation with the presence of autoantibodies to several intracellular and cell surface proteins.19,21,22 Importantly, a recent report of a genetic mouse model for human systemic sclerosis demonstrated that chronic B-cell activation leads to sclerosis together with autoantibodies, wherein augmented interleukin-6 (IL-6) production by activated B cells might be involved in the pathogenesis.23
A recent report on anti–B-cell therapy with anti-CD20 for thrombocytopenia in a single patient with cGVHD showed that apart from the recovery of thrombocytopenia, the severe xerostomia of the patient also responded.24 We therefore hypothesized that anti–B-cell therapy might be effective in patients with extensive cGVHD that was refractory to a variety of immunosuppressive treatments, including steroids.
Study design
Patients
Six patients suffering from extensive cGVHD and refractory to at least 3 lines of immunosuppressive therapy were treated with monoclonal anti-CD20 antibody. The first line of treatment was prednisone. Subsequent treatments included a variety of immunosuppressive drugs, such as cyclosporine, mycophenolate mofetil, clofazimine, azathioprine, thalidomide, hydrochloroquine, ultraviolet B radiation, and psoralen–ultraviolet A radiation (PUVA), generally combined with prednisone. Patients had received these immunosuppressive drugs for a median of 30 months (range, 12-110 months) when therapy with monoclonal anti-CD20 antibody was started. In 3 patients (nos. 1, 3, and 4) all immunosuppressive therapy was discontinued (at least 4 weeks before anti-CD20 antibody therapy was started) because of inefficiency of treatment together with their side effects. Patient nos. 2, 5, and 6 received low-dose prednisone combined with cyclosporine (2 patients) or together with mycophenolate mofetil (1 patient). After therapy with anti-CD20, prednisone and cyclosporine could be stopped within 6 months in all 3 patients. Patient no. 6 still needed mycophenolate mofetil, which could be stopped after the second course of anti-CD20. Two patients had lichenoid changes (patient nos. 1 and 2), 2 patients had sclerotic changes (patient nos. 5 and 6), and 2 patients had a combination of both (patient nos. 3 and 4). Patients were treated according to transplantation and rituximab protocols that were approved by the local institutional review board and all patients provided informed consent.
Treatment
The patients were treated with monoclonal anti-CD20 (rituximab [MabThera], Roche, Milan, Italy). Treatment consisted of weekly intravenous infusions, 375 mg/m2, given for 4 weeks. In case of incomplete clinical response within 12 weeks, a second (or third) treatment of 4 weeks was given.
Variables analyzed
To measure the effect of the therapy, we used clinical, histologic, and laboratory parameters. The clinical outcome was based on the subjective feelings of the patient and the macroscopically visible changes of the skin and oral cavity 1 week before treatment and 8 to 12 weeks after therapy. A 3-mm punch biopsy of the skin of the upper arm was performed before treatment and 8 weeks after the last dose of rituximab was given. The skin biopsies were fixated in formalin, and the slides were stained with hematoxylin and eosin (H&E), Verhoeff stain, Sirius red, CD3, CD4, CD8, CD19, CD20, and CD68. Before and 4 and 8 weeks after treatment, peripheral blood T cells (CD3), B cells (CD19), and natural killer (NK) cells (CD16, CD56) were measured. Furthermore, immunoglobulin levels, monoclonal immunoglobulins, autoantibodies, and liver functions were studied
Results and discussion
We observed marked improvement of the skin and oral cavity lesions in 5 of 6 patients with therapy-refractory extensive cGVHD treated with monoclonal anti-CD20 antibody, whereas liver function abnormalities also recovered in 2 of 5 patients (nos. 1 and 3). In all 4 patients with lichenoid changes of the skin (patient nos. 1, 2, 3, and 4), the itching and redness improved. Hair growth restored almost completely in both patients with hair loss (nos. 4 and 6). In 3 of the 4 patients with sclerodermoid changes (patient nos. 3, 4, 5, and 6), the superficial sclerosis improved. The decrease in thickness of collagen bundles was associated with a decrease in the amount of superficial macrophages in those patients (nos. 3, 4, and 6). In none of our patients was improvement of the deep sclerosis seen. In 1 patient (no. 6) the sclerosis-associated dyspnea and dysphagia disappeared completely. Patient no. 5 showed no response after therapy. Patient nos. 3, 4, and 6 had incomplete responses; therefore, they were treated with a second and third course of monoclonal anti-CD20 antibody. In patient no. 4, further improvement of the sclerosis was reached after the second course. The third course in this patient and the second and third course in patient nos. 3 and 6 were not successful anymore. The improvement obtained after the first (and second) course of anti-CD20 still remains in all responding patients. At the time of this writing, all 6 patients were alive, and 4 of 6 patients were not using any immunosuppressive therapy. Two patients (nos. 2 and 5), both with multiple myeloma, had recurrences of their disease (respectively, 20 months and 16 months after anti-CD20 was given) and presently receive (immunosuppressive) treatment for their relapse.
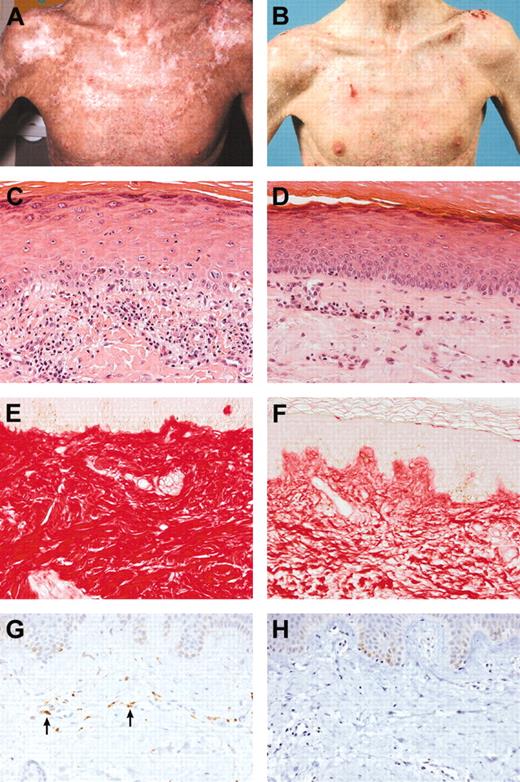
Table 1 presents patient data. The clinical scores of both the patients and the clinician and the outcome of the liver function tests and autoantibodies are demonstrated in Table 2. Table 3 shows a summary of the histopathological findings. Figure 1 shows the clinical improvement of patient no. 4, the epidermal changes of patient no. 1, and the improvement of the sclerosis and disappearing of the macrophages of patient no. 6.
Clinical and histopathological improvement. (A) Patient no. 4 before therapy. (B) Patient no. 4 after therapy. (C) Patient no. 1 before therapy (H&E stain; magnification × 200). Dyskeratotic cells in the epidermis with a mild lymphocytic infiltrate. (D) Patient no. 1 after therapy (H&E stain; magnification × 200). Improvement of the epidermal features. (E) Patient no. 6 before therapy (Sirius red stain; magnification × 200). Compact, severely thickened collagen in the superficial dermis. (F) Patient no. 6 after therapy (Sirius red stain; magnification × 200). The collagen is less compact. (G) Patient no. 6 before therapy (CD68 stain; magnification × 200). A few macrophages are present in the superficial dermis. The arrows in panel G indicate CD68-positive macrophages. (H) Patient no. 6 after therapy (CD68 stain; magnification × 200). The macrophages have disappeared. The microscope used was a Nikon Eclipse E800M with a Nikon Plan apo 20 × objective. The camera used was a digital Nikon FDX-35. Acquisition software and processing software were Nikon ACT-1 and Photoshop, respectively.
Clinical and histopathological improvement. (A) Patient no. 4 before therapy. (B) Patient no. 4 after therapy. (C) Patient no. 1 before therapy (H&E stain; magnification × 200). Dyskeratotic cells in the epidermis with a mild lymphocytic infiltrate. (D) Patient no. 1 after therapy (H&E stain; magnification × 200). Improvement of the epidermal features. (E) Patient no. 6 before therapy (Sirius red stain; magnification × 200). Compact, severely thickened collagen in the superficial dermis. (F) Patient no. 6 after therapy (Sirius red stain; magnification × 200). The collagen is less compact. (G) Patient no. 6 before therapy (CD68 stain; magnification × 200). A few macrophages are present in the superficial dermis. The arrows in panel G indicate CD68-positive macrophages. (H) Patient no. 6 after therapy (CD68 stain; magnification × 200). The macrophages have disappeared. The microscope used was a Nikon Eclipse E800M with a Nikon Plan apo 20 × objective. The camera used was a digital Nikon FDX-35. Acquisition software and processing software were Nikon ACT-1 and Photoshop, respectively.
In our patients, the anti-CD20 therapy had no effect on T cells, T-cell subsets, and NK cells but did eliminate all the B cells from the peripheral blood. Apart from some side effects during the antibody infusion, none of the patients experienced other side effects or infectious complications after anti-CD20 therapy. Although there was no effect on levels of immunoglobulins and numbers of M components in the peripheral blood, we found elimination of autoantibodies in 2 responding patients (before therapy 3 patients had autoantibodies). This finding suggests a possible role of autoantibodies in the pathogenesis of cGVHD. The possible role of macrophages in the pathogenesis of skin cGVHD is unknown, but the relation between the response of the skin in cGVHD to anti-CD20 therapy and the disappearance of the macrophages is intriguing. Furthermore, it is remarkable that the nonresponding patient did not have any macrophages in the biopsy before treatment.
The response to anti-CD20 monoclonal antibody in 5 of 6 patients with extensive cGVHD, being treated earlier unsuccessfully with many immunosuppressive drugs, suggests that anti-CD20 monoclonal antibody therapy might be applied earlier in the treatment of cGVHD—for example, after failure of steroids. A controlled trial is mandatory to confirm these results.
Prepublished online as Blood First Edition Paper, July 13, 2004; DOI 10.1182/blood-2004-05-1855.
Supported by Roche, the Netherlands.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.