Abstract
Abnormal tissue factor (TF) expression has been demonstrated on blood monocytes and circulating endothelial cells in humans with sickle cell anemia. We have now studied sickle transgenic mice to help define the biology of endothelial TF expression in sickle disease. Using immunostaining of tissue sections, we find that this is confined almost exclusively to the pulmonary veins. About 15% and 13% of these exhibit TF-positive endothelium in the wild-type normal mouse and the normal human hemoglobin (HbA)–expressing control transgenic mouse, respectively. The mild sickle mouse is indistinguishable from normal (∼ 14% positive), but TF expression is significantly elevated in the moderate and severe mouse models of sickle disease (∼ 29% and ∼ 41% positive, respectively). Exposure of the mild sickle mouse to hypoxia for 3 hours, followed by reoxygenation, converted its TF expression phenotype to that of the severe sickle mouse (∼ 36% positive). Pretreatment with lovastatin eliminated excessive expression of TF in the posthypoxic mild sickle mouse (∼ 16% positive) and in the more severe mouse at ambient air (∼ 21% positive). In addition to identifying tissue expression of endothelial TF in the sickle lung, these studies implicate reperfusion injury physiology in its expression and suggest a rationale for use of statins in sickle disease.
Introduction
The vascular pathobiology of sickle cell disease includes a coagulopathy, as evidenced by activation of plasma factors1 and platelets2 ; increased factor VII turnover3 ; and increased levels of prothrombin F1.2 fragment,4 thrombin-antithrombin complexes,3 and D-dimers.3,5 The most evident clinical consequence of this activation is the ischemic stroke, a frequent complication of sickle cell anemia that tends to involve thrombosis over an area of underlying vessel wall abnormality.6,7 In addition, thrombosis has been found to accompany pulmonary arterial vasculopathy in sickle disease.8 It is less certain if coagulation activation plays other roles in this disease, although generated thrombin itself is a significant endothelial perturbant9 and could contribute to the abnormal endothelial activation that characterizes this disease.10
The underlying basis for this coagulation activation likely derives from the inflammatory state inherent in sickle disease,10 a feature that is shared by sickle transgenic mice.11-14 Based on studies of such mice, we earlier proposed that the proximate stimulus for genesis of this state lies in “reperfusion injury” physiology, in which vascular (and organ) damage derives from oxidative stress and incitement of inflammation after cessation of an obstruction (ie, during reoxygenation).13,14 Regardless of proximate cause, the state of coagulation activation presumably must be initiated by expression of tissue factor (TF), the trigger of the coagulation system.15 In fact, TF is abnormally expressed on blood monocytes from sickle disease subjects,16 as well as on circulating endothelial cells,17 that were studied as a (presumed) surrogate marker for status of the vessel wall endothelium. Therefore, we have used sickle transgenic mice to further investigate endothelial expression of TF. Here we demonstrate that sickle mice do express endothelial TF on the pulmonary veins and that this is augmented by hypoxia/reoxygenation (H/R) and can be inhibited by lovastatin.
Materials and methods
Mice
We studied 7 types of mice, all of which were raised and housed in our institution's specific pathogen-free facility. Animals were studied at 3 to 4 months of age or 3 to 4 months after marrow transplantation. Mouse genotypes were confirmed by isoelectric focusing and, when necessary, real-time polymerase chain reaction for transgene number. All mouse studies were done under supervision of our Institutional Animal Care and Use Committee (IACUC).
Three types of mice, all on a C57Bl/6 background, were raised on standard mouse chow. These include (1) wild-type control mice that are referred to here as “Nor” for normal; (2) NY1DD mice that were originally generated by Fabry et al18 are homozygous for deletion of murine beta major globin and they carry a single copy of linked transgenes for human α and βS globins (αHβS); and (3) S+S-Antilles mice that are homozygous for the murine beta deletion and carry 2 transgenes, αHβS and αHβS-Antilles.19
In contrast, 4 other types of mice have a mixed genetic background.20 The BERK mouse with the most severe phenotype is homozygous for knockout for both alpha and beta murine globins and carries a single copy of a αHβS transgene.20 We also examined BERK-type mice that differ because they are heterozygous for the murine beta knockout (hBERK). These had either one copy (hBERK1) or 2 copies (hBERK2) of the transgene. All BERK-type mice were raised on a high-fat diet (from onset of maternal gestation until one week after weaning) to enhance fecundity. In addition, we studied control transgenic animals, raised on standard chow, that have a mixed genetic background similar to the BERK-type sickle animals: mice with complete knockout of murine globins that have a transgene (αHβA) leading to expression of normal human hemoglobin (HbA) instead of sickle hemoglobin20 ; we designate these controls as HbA-BERK.
Of these animals, NY1DD mice have mild phenotype,18 S+S-Antilles mice have an intermediate phenotype,19 and BERK mice have the most severe phenotype.20 For certain experiments we used some hBERK1 animals, which also have a fairly high degree of severity,21 for the practical reason that they could more easily be obtained in the numbers necessary for these studies. The surprisingly high severity of the phenotype of the hBERK1 animals is probably because they have very low whole-blood oxygen affinity (P50 ∼ 57 mm Hg21 ) and consequent trouble with oxygen loading, which augments their sickling tendency.
Antibody to murine TF
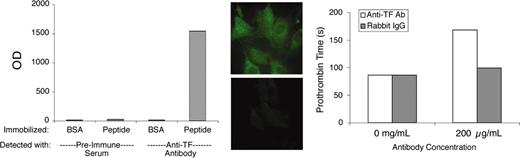
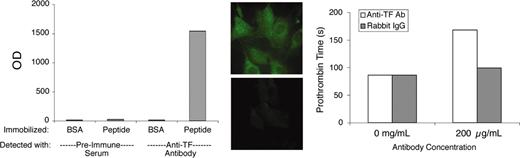
This study employed a previously described preparation of antibody to the extracellular domain of murine TF.22 Polyclonal antibodies of the immunoglobulin G (IgG) class were enriched from rabbit antisera by ammonium sulfate fractionation. For simplicity, we will refer to this partially purified antibody preparation as “αTF.” Preliminary validation experiments demonstrated that αTF bound to immobilized TF peptide (comprising the extracellular domain of murine TF), to cultured murine fibroblasts (which constitutively express TF) and cultured murine endothelial cells stimulated with lipopolysaccharide, and to perivascular cells in murine lung tissue. The original immunogenic peptide blocked αTF-binding ability. The antibody exhibited very modest blocking activity in a functional clotting assay using murine brain as the TF source. Some of these validation data are illustrated in Figure 1.
αTF validation data. The αTF preparation bound to murine TF peptide (extracellular domain) in solid-phase binding assay (left) and to murine fibroblasts (middle top), in which case binding was blocked by the immunogenic peptide (middle bottom). The αTF weakly inhibits TF-induced coagulation (right). BSA indicates bovine serum albumin; and OD, optical density. Original magnification, × 600.
αTF validation data. The αTF preparation bound to murine TF peptide (extracellular domain) in solid-phase binding assay (left) and to murine fibroblasts (middle top), in which case binding was blocked by the immunogenic peptide (middle bottom). The αTF weakly inhibits TF-induced coagulation (right). BSA indicates bovine serum albumin; and OD, optical density. Original magnification, × 600.
Immunostaining
This study focuses on the lung because TF expression was almost exclusively limited to this organ. Lungs were inflated with phosphate-buffered saline (PBS) using gentle hydrostatic pressure and immediately placed into OCT Compound (Sakura Rinetechnical, Tokyo, Japan) and then snap-frozen in liquid nitrogen. Five-micrometer frozen sections were mounted, prefixed with acetone, and stored at -80°C. For staining, slides were defrosted and allowed to dry for 15 minutes. After additional fixation with 4% paraformaldehyde for 10 minutes, they were rinsed and blocked using 3% bovine serum albumin. The αTF diluted 1:1000 and a rat antimurine CD31 (BD Bioscience Pharmingen, Palo Alto, CA) diluted 1:100 were applied for one hour at room temperature. Sections were rinsed and treated for 40 minutes with fluorescein isothiocyanate (FITC)–labeled antirabbit and TRITC (tetramethylrhodamine-5(and 6)-isothiocyanate)–labeled antirat antibodies (Jackson Immunoresearch Labs, West Grove, PA) at 1:100 dilution. In all experiments, nuclei were counterstained with DAPI (4,6 diamidino-2-phenylindole) to aid tissue identification. Nonspecific staining of tissue sections was tested for using the preimmune rabbit serum on adjacent sections.
For some studies we used staining with monoclonal antibodies against murine vascular cell adhesion molecule 1 (VCAM1; BD Biosciences, Palo Alto, CA) or EphB4 (Santa Cruz Biotechnology, Santa Cruz, CA) or xanthine oxidase (NeoMarkers, Fremont, CA). In others, we stained for fibrin/fibrinogen (GAM/Fbg/7S; Nordic Immunology, Tilburg, the Netherlands), in which case animals were treated with heparin immediately prior to being killed to avoid artifactual deposition of fibrin. Sections were mounted using fluoromount-G mounting solution (Southern Biotechnology, Birmingham, AL).
Data acquisition
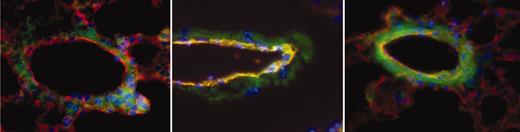
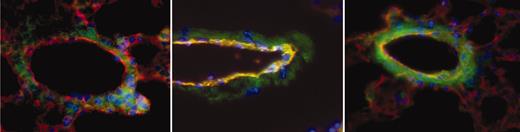
Sections were scored for TF positivity by a reader blinded to sample identity. Images were acquired using an Olympus IX70 inverted fluorescent microscope with a PlanApo 60×/1.4 oil objective lens (Tokyo, Japan). CD31 was used to identify endothelial cells in vessel walls. αTF, on the other hand, identified both endothelial cells and nonendothelial cells that express TF. The combined images of TF (green) and CD31 (red) yielded a yellow-orange merge image of TF-positive endothelial cells, allowing their identification. We examined a sufficient number of sections, each from separate portions of the lung, so that at least 50 veins per animal could be scored positive or negative for endothelial TF expression. Since TF was positive only in veins (and not arteries or capillaries), data are expressed as percent of veins that are TF positive. Figure 2 shows examples of a negative (Figure 2, left) and 2 positive (Figure 2, middle and right) pulmonary veins from a sickle mouse. In virtually all cases, if a vessel showed any endothelial TF positivity, the entire endothelial circumference was positive.
Examples of pulmonary vessels from a sickle mouse. To obtain these images, TF (green), CD31 (red), and nuclei (blue) are overlaid to obtain a merged image in which TF-positive endothelial cells are yellow-orange in color. The vein in the left panel is negative for endothelial TF, whereas the ones in middle and right panels are positive. Note that in middle and, particularly, right images, nonendothelial TF staining has increased prominence as well. Original magnification, × 600.
Examples of pulmonary vessels from a sickle mouse. To obtain these images, TF (green), CD31 (red), and nuclei (blue) are overlaid to obtain a merged image in which TF-positive endothelial cells are yellow-orange in color. The vein in the left panel is negative for endothelial TF, whereas the ones in middle and right panels are positive. Note that in middle and, particularly, right images, nonendothelial TF staining has increased prominence as well. Original magnification, × 600.
To verify that our criteria for identification of veins were valid, in preliminary experiments we performed costaining of murine lungs for CD31/TF and for EphB4, a marker specific for venous endothelium.23 This indicated that veins were being correctly identified. In addition, a subset of the samples were read by a second blinded reader as a test of the veracity of scoring by the primary microscopist. For every group examined, the percentage of TF-positive veins identified by the second reader was within 2% of that identified by the primary reader.
Image acquisition and processing were performed using Spot camera model 1.4.0 and software SpotTM version 1.2-TWAIN (Diagnostic Instruments, Sterling Heights, MI), as well as Adobe Photoshop (Adobe Systems, San Jose, CA).
Hypoxia/reoxygenation
Some mice were subjected to H/R. This involved placement for 3 hours in a normobaric 8% O2 environment, after which animals were returned to ambient air for 18 hours. This duration of the hypoxia period is like that used in our prior studies of H/R in sickle mice,13,14 and the duration of the reoxygenation period was here determined empirically in preliminary studies as the time point being optimal.
Interventions
Lovastatin (Sigma Chemical, St Louis, MO) was dissolved at 25 mg in 400 μL ethanol, followed by addition of 3.6 mL mouse saline (330 mOsM). After vortexing well, it was given by intraperitoneal injection at either 2 or 20 μg/g/day. For short-term experiments, animals were given lovastatin once daily for 5 days prior to H/R stress (the last injection being given 4 hours before hypoxia). In longer-term experiments, animals were given lovastatin once daily for 2 weeks before being killed (the last injection being the day before being killed). Control animals received vehicle only.
In control experiments, Nor mice were raised under the same high-fat diet conditions (from onset of gestation until one week after weaning) as those used for BERK and hBERK animals.
Marrow transplantation
Animals underwent Nor-to-Nor, Nor-to-hBERK1, hBERK1-to-Nor, hBERK1-to-hBERK1, or NY1DD-to-Nor transplantation. Recipient animals were prepared by exposure to 137Cs (5.2 Gy [520 rad]), and this was repeated 3 hours later. Donor cells were freshly prepared between the 2 radiation doses. Donor animals were killed with CO2 gas, after which proximal and distal tips of both femurs were removed. Marrow cells were flushed out of bone with PBS. After washing, marrow cells were counted and diluted to 40 million cells per mL in PBS. Eight million cells were injected via tail vein. Confirmation of subsequent marrow conversion was performed using isoelectric focusing (IEF) to identify blood hemoglobin type.
As a positive graft-versus-host disease (GVHD) model, to provide an additional control for some of the above transplantations, we used transplantation of C57BL/6 marrow plus splenocytes into B10.BR recipients, as described previously.24 Within a week after transplantation, lungs from these animals scored highly positive for histologic changes of GVHD and were evaluated for endothelial TF expression.
FACS
Using a fluorescence-activated cell sorter (FACS; Becton Dickinson FACScalibur, San Jose CA), we studied degree of positivity of cells in the monocyte gate for both CD11b (Pharmingen, San Jose, CA) and TF. For this, we prepared lysed whole blood using the ammonium chloride method and examined data using Cellquestpro (Becton Dickinson).
Statistical analysis
The Student t test was used to determine statistical significance. Any multiple comparisons employed analysis of variance (ANOVA). All results are shown as mean ± SD.
Results
This study focuses upon the lung because our preliminary screening of multiple tissues in sickle mice indicated that endothelial TF expression is confined almost exclusively to this organ. Moreover, endothelial TF expression was limited to the veins in the lung, with no expression detected in arteries or capillaries. Therefore, present results are expressed as percent of veins positive for endothelial TF. Numbers of animals studied are indicated in the figure legends and Table 1.
Endothelial TF expression in lung
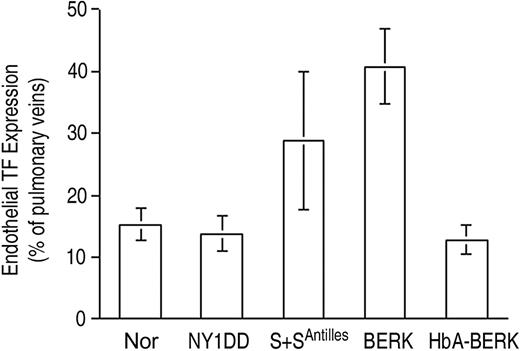
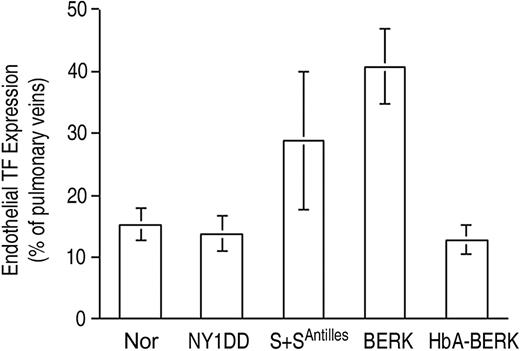
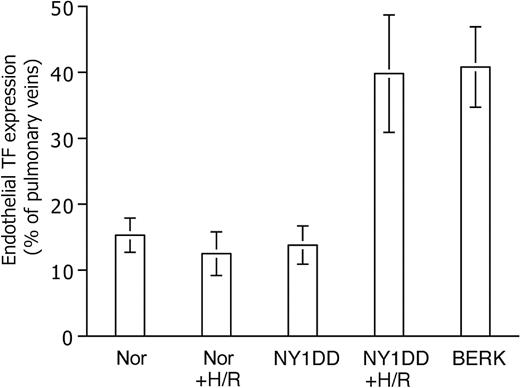
Endothelial TF expression (Figure 3) was low for both the normal C57BL/6 control mouse (Nor) and the HbA-expressing control transgenic mouse (HbA-BERK) at 15% ± 3% and 13% ± 2% positive, respectively. The mild sickle model,18 the NY1DD mouse, was indistinguishable from the Nor mouse (14% ± 3% positive). In contrast, the somewhat more severe S+S-Antilles mouse19 showed significantly increased TF expression (29% ± 11% positive; P = .004 vs Nor). The most severe mouse model, BERK,20 exhibited the highest TF expression (41% ± 6% positive; P < .001 vs Nor and P < .001 vs HbA-BERK). Interestingly, the 2 hBERK models, hBERK1 and hBERK2, were indistinguishable from BERK at 38% ± 10% and 39% ± 11% positive, respectively (for both, P < .001 vs HbA-BERK), indicating that they are more severe than the S+S-Antilles animals in regard to endothelial TF expression. However, it must be noted that the BERK and hBERK animals (and the HbA-BERK controls) are on a mixed genetic background,20 while the others are on a C57BL/6 background. We therefore performed additional corroborative studies using a marrow transplantation model (see “Controls”).
Endothelial TF expression in pulmonary veins. TF expression is scored as percent of pulmonary veins positive for TF (mean ± SD). Normal mice (Nor), HbA-expressing control mice (HbA-BERK), and mild sickle mice (NY1DD) have the same low-TF expression. TF expression by the moderately severe S+S-Antilles mouse is higher, and that of the severe BERK mouse is the highest. Statistical significance: P = ns (not significant) for NY1DD (n = 10) versus Nor (n = 9); P = .004 for S+S-Antilles (n = 4) versus Nor (n = 9); and P < .001 for BERK (n = 5) versus HbA-BERK (n = 5).
Endothelial TF expression in pulmonary veins. TF expression is scored as percent of pulmonary veins positive for TF (mean ± SD). Normal mice (Nor), HbA-expressing control mice (HbA-BERK), and mild sickle mice (NY1DD) have the same low-TF expression. TF expression by the moderately severe S+S-Antilles mouse is higher, and that of the severe BERK mouse is the highest. Statistical significance: P = ns (not significant) for NY1DD (n = 10) versus Nor (n = 9); P = .004 for S+S-Antilles (n = 4) versus Nor (n = 9); and P < .001 for BERK (n = 5) versus HbA-BERK (n = 5).
Effect of hypoxia/reoxygenation
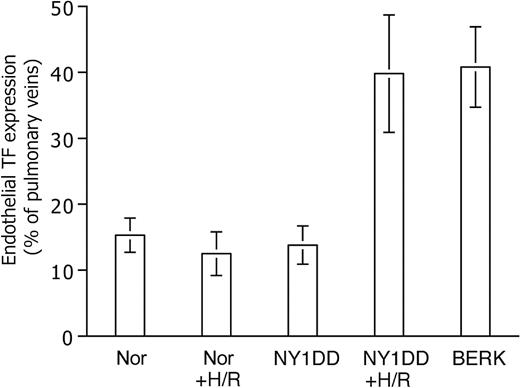
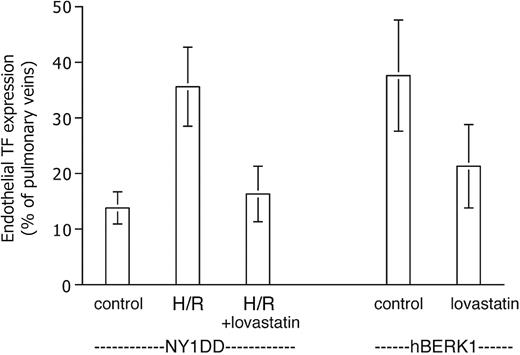
Exposure to H/R (Figure 4) did not increase endothelial TF expression in normal mice. In striking contrast, H/R did cause NY1DD mice to assume the TF phenotype (36% ± 7% positive; P < .001 vs prehypoxia NY1DD) exhibited by the most severe BERK model. We did not have IACUC permission to subject the more severe animal models to the hypoxia protocol.
Effect of hypoxia-reoxygenation (H/R) on TF expression. Data are expressed as for Figure 3. H/R does not increase TF expression in the normal (Nor) mouse, but it converts the mild sickle mouse (NY1DD) to the severe, BERK-like TF-positive phenotype. Statistical significance: P < .001 for posthypoxia NY1DD (n = 9) versus prehypoxia NY1DD (n = 10); and P = ns for posthypoxia NY1DD (n = 9) versus BERK (n = 5); and P = ns for posthypoxia Nor (n = 11) versus prehypoxia Nor (n = 9).
Effect of hypoxia-reoxygenation (H/R) on TF expression. Data are expressed as for Figure 3. H/R does not increase TF expression in the normal (Nor) mouse, but it converts the mild sickle mouse (NY1DD) to the severe, BERK-like TF-positive phenotype. Statistical significance: P < .001 for posthypoxia NY1DD (n = 9) versus prehypoxia NY1DD (n = 10); and P = ns for posthypoxia NY1DD (n = 9) versus BERK (n = 5); and P = ns for posthypoxia Nor (n = 11) versus prehypoxia Nor (n = 9).
Therapeutic interventions
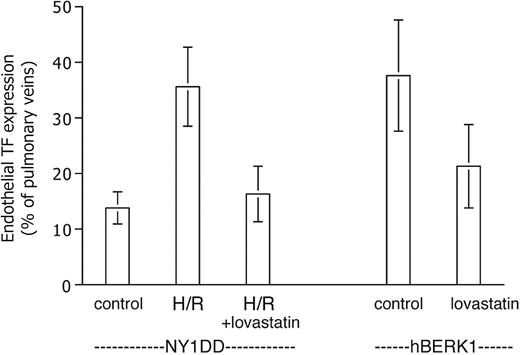
We tested the effect of lovastatin on endothelial TF expression in our mice because it has been reported to inhibit TF expression in vitro (see “Discussion”). We observed a significant therapeutic effect of lovastatin in both short-term and longer-term experiments (Figure 5). Pretreatment of NY1DD mice eliminated their response to H/R in terms of endothelial TF expression, which dropped to 16% ± 5% positive (P < .001) at the lowest lovastatin dose tested, 2 mg/kg/day. On the other hand, lovastatin did not decrease the basal degree of endothelial TF expression exhibited by unstressed NY1DD mice (not shown). For hBERK1 animals left at ambient air, the longer-term treatment (for 2 weeks, an arbitrarily selected time point) with 2 mg/kg/day lovastatin resulted in a similar decrease in TF expression (to 21% ± 8% positive; P = .002). A 10-fold higher lovastatin dose did not further increase therapeutic benefit in either the short-term or long-term models (data not shown).
Lovastatin inhibits TF expression. Data are expressed as for Figure 3. The elevated TF expression of the posthypoxic NY1DD mouse is inhibited by a 5-day pretreatment with lovastatin. The elevated TF expression of the severe hBERK1 mouse at ambient air is inhibited by a 2-week pretreatment with lovastatin (n = 8). Statistical significance: P < .001 for posthypoxic NY1DD animals with (n = 6) versus without (n = 9) lovastatin; P = .002 for hBERK1 animals with (n = 8) versus without (n = 9) lovastatin.
Lovastatin inhibits TF expression. Data are expressed as for Figure 3. The elevated TF expression of the posthypoxic NY1DD mouse is inhibited by a 5-day pretreatment with lovastatin. The elevated TF expression of the severe hBERK1 mouse at ambient air is inhibited by a 2-week pretreatment with lovastatin (n = 8). Statistical significance: P < .001 for posthypoxic NY1DD animals with (n = 6) versus without (n = 9) lovastatin; P = .002 for hBERK1 animals with (n = 8) versus without (n = 9) lovastatin.
Pulmonary VCAM1 expression
In notable contrast to the absence of TF response in Nor mice after H/R (Figure 4), lungs of normal mice did reveal significantly enhanced expression of endothelial VCAM after H/R, changing from 40% ± 17% to 61% ± 6% positive (n = 8 each; P = .006). This indicates that the degree of hypoxia was sufficient to induce a physiologic response by endothelium in normal mice. The NY1DD sickle animals, in contrast, were already highly positive for VCAM under ambient air (83% ± 3% positive, n = 10) and no further increase was notable after H/R (85% ± 4% positive, n = 9). Lovastatin decreased VCAM expression modestly in the posthypoxic NY1DD mouse (to 68% ± 9% positive; n = 6; P < .001)
TF expression by nonendothelial cells
Of course, TF expression was not limited to endothelial cells. In fact, we noted that when endothelial TF was enhanced (in the posthypoxic NY1DD and the ambient air BERK-type mice), so was TF for the surrounding nonendothelial cells that constitutively express it, such as fibroblasts or smooth muscle cells. This is evident in Figure 2 (green staining). Interestingly, lovastatin pretreatment did not appear to substantially alter the nonendothelial TF expression in either hBERK1 (n = 8) or posthypoxic NY1DD (n = 6) models. It should be noted, however, that this is a preliminary impression and is not based on formal quantitation of nonendothelial TF expression.
Analysis of blood monocytes (group size n = 4 in each case) showed Nor and NY1DD animals to have a low baseline TF positivity (4.9% ± 1.2% and 5.2% ± 1.8%, respectively). Normal animals showed no increase in response to H/R (5.2% ± 1.3% positive), but NY1DD animals showed a TF increase to 12.9% ± 4.7% positive (P = .024). Thus, TF expression by murine monocytes paralleled that of pulmonary vein endothelium in the posthypoxic mouse. Monocyte TF was also increased in hBERK1 mice at ambient air (9.5% ± 2.6% positive; P = .02 vs Nor). Lovastatin pretreatment did not diminish TF expression by monocytes.
Colocalization
Given a report of xanthine oxidase localization in and on the thoracic aorta of sickle mice,12 we stained lung sections from NY1DD mice for this enzyme. There was only faint staining for xanthine oxidase and no tendency for it to colocalize with TF-positive endothelium (data not shown). Similarly, we also stained for vessel wall fibrin25 in posthypoxic NY1DD mice and again found very modest staining and no colocalization with endothelial TF expression (data not shown).
Controls
We subjected control (n = 6) and sickle animals (NY1DD and hBERK1 models, n = 7 each) to control intraperitoneal vehicle injections. In no case did this cause any detectable effect on TF expression compared with uninjected animals (data not shown).
There are 2 potentially important differences between Nor and BERK-type animals, above and beyond the latter's sickle state. First, BERK-type animals are generated using a high-fat diet (until 1 week after weaning), which might be hypothesized to alter expression of an endothelial surface molecule. To address this dietary issue, we raised some Nor animals (n = 6) under the same high-fat diet exposure used for BERK and hBERK animals. This resulted in no alteration of their TF expression (10% ± 4% positivity). Also, we measured serum cholesterol levels of BERK animals and found them to be the same as that of the Nor animals (data not shown).
Second, Nor, NY1DD, and S+S-Antilles mice have a C57BL/6 background, whereas the BERK-type animals have a mixed genetic background.20 To address the issue of genetic background, we studied HbA-expressing transgenic animals as controls, as noted above (Figure 3). In addition, we performed phenotype-changing marrow transplantations so that Nor mice acquired an hBERK1 marrow and so that hBERK1 mice acquired a Nor marrow. Animals were studied 3 to 4 months after transplantation, at which time IEF had confirmed greater than 95% marrow conversion. As summarized in Table 1, acquisition of an hBERK1 marrow caused Nor animals to assume the sickle phenotype of TF positivity, whereas acquisition of a Nor marrow caused BERK animals to lose their TF positivity. Similarly, acquisition of an NY1DD marrow by Nor animals caused them to become responsive to H/R in terms of TF expression. Control animals undergoing Nor-to-Nor or hBERK1-to-hBERK1 transplantations, which included the radiation exposure but no marrow conversion, exhibited no change in TF phenotype. Because the transplantations involving Nor and hBERK1 animals could entail some antigen mismatches, we examined a deliberate GVHD model and found the pulmonary venous endothelium to have normal, not elevated, TF expression (Table 1).
Discussion
Our results show that TF expression in the pulmonary veins of sickle transgenic mice parallels the accepted understanding of the overall disease severity of these models: BERK is more severe than S+S-Antilles, which is more severe than NY1DD.18-20 Notably, the mild NY1DD mouse is indistinguishable from normal mice in pulmonary endothelial TF expression, but its phenotype is converted to that of the severe BERK mouse after exposure to H/R. Interestingly, the hBERK1 and hBERK2 mice are identical to the BERK mouse in terms of TF expression, indicating that they also have the crucial characteristic(s) responsible for this phenotype. Control experiments demonstrated that the high-TF expression by BERK and hBERK animals is not just due to dietary fat or genetic background issues.
In general, TF is not believed to be expressed by endothelial cells under normal circumstances in humans. On the other hand, it can be in disease states such as atherosclerosis,26 and we are aware of no published studies on endothelial TF expression in highly relevant human clinical situations such as disseminated intravascular coagulation or systemic inflammatory states. Thus, the degree to which endothelial TF expression plays a role in biology is unknown. However, it is notable that there are approximately 100 endothelial cells for every blood monocyte in the human, so even a small percentage of positive endothelial cells could, potentially, have the impact of very high degrees of monocyte positivity. Whether this mathematical inference has any basis in biology remains to be proven. Another caveat is that 2 in vitro culture studies have indicated that endothelial expression of TF is largely, if not entirely, on the basolateral rather than the luminal surface.27,28 On the other hand, sickle disease is believed to be a state of endothelial injury,10 and certainly thrombin (for example) causes retraction of endothelial margins29 and would be a potential cause of ablumenal TF exposure to the blood.
It is reported that administration of endotoxin to rats30 or rabbits31 did not result in endothelial TF expression in the lung. This discrepancy with our results may be because of species differences (our study used mice), the different reagents having different avidities for TF, technical differences, or because endotoxin is simply an insufficient stimulus compared with reperfusion injury physiology.
Specificity for the lung?
Given our previous studies suggesting the relevance of reperfusion injury physiology to sickle disease,13,14 we are not surprised that a limited-duration exposure to hypoxia followed by reoxygenation (H/R) has a different effect on sickle mice than normal mice. This experimental maneuver results in exposure of the normal animal only to temporary hypoxia, while it exposes the sickle animal to actual ischemia-reoxygenation, caused by vascular occlusion resulting from the unique presence of sickling red blood cells. Notably, hypoxia, per se, is reported to induce TF in lung smooth muscle cells and monocytes, but not in endothelial cells, in normal mice (of a different genetic background).25 Consistent with this, we found here that normal mice did not develop endothelial TF expression after H/R, even though they did exhibit up-regulation of VCAM1 expression (indicating that the degree of hypoxia was sufficient to exert a physiologic effect). Thus, we have again observed that experimental H/R has a markedly different effect in the sickle context.
However, we find it very interesting that vigorous endothelial TF expression is largely limited to the lung. This could reflect an instance of tissue-specific endothelial heterogeneity. Arguing against this, however, is the fact that TF expression by pulmonary vein endothelial cells was paralleled by TF expression by nonendothelial cells. Therefore, the more likely explanation for this apparent tissue specificity is that the biologic impact of H/R in the sickle mouse is greater in lung than elsewhere. This could be because H/R simply induces a stronger signal of the same type in the sickle animal (ie, greater degree of hypoxia). Or it could be because H/R in the sickle mouse involves aspects that comprise a second, dissimilar stress on the endothelium found in sickle but not normal animals. Thus, it is interesting to note the large number of differences in the sickle mouse upon which hypoxia would be superimposed.
First and foremost, the lung, unlike other organs, will be flooded with high numbers of presickled red cells.10 Yet, sickle disease is accompanied by additional, independent stressors: abnormally adhesive and phosphatidylserine-expressing red cells,10 elevated levels of vascular endothelial growth factor (VEGF),32 increased inflammatory mediators,10 increased generation of thrombin,3 abnormally oxidized lipids,33 elevated levels of erythropoietin, elevated vessel wall shear rate,14 and abnormally adherent white cells.14 Each of these can induce endothelial TF expression,34-41 so any of these additional facets that make the sickle context different from normal could comprise a second, distinct stress that is particularly promotive of TF expression.
Signaling mechanisms
Regardless of the identity of the proximate stress, the mechanism of signaling leading to TF expression by pulmonary venous endothelium probably involves several transcription factors. The promoter region of the TF gene includes sites for nuclear factor κB (NFκB), early growth response-1 (Egr-1), activator protein-1 (AP-1), and specificity protein 1 (Sp1).42 H/R activates NFκB.43 Hypoxia itself induces Egr-1 and VEGF, which itself also augments Egr-1.44 Furthermore, the mechanism of TF activation by a number of stresses characteristic of sickle disease noted in “Specificity for the lung?” involves extracellular signal-related kinase 1/2 (ERK1/2) and transcription factor Egr-1. This seems to be true for erythropoietin,39 VEGF,45 tumor necrosis factor (TNF),46 oxidized lipids,38 and thrombin,47 for example. Thus, we hypothesize that the difference of H/R exposure in normal versus sickle mice lies in the latter's activation of Egr-1.
Although our studies were designed to collect detailed quantitative data only on endothelial cells, they did reveal increased TF expression by nonendothelial cells in both posthypoxic NY1DD and ambient air hBERK1 mice. Interestingly, nonendothelial TF expression in neither was substantially inhibited by lovastatin. Thus, in aggregate, our data suggest that there are some signaling differences in the stress-response axis for endothelial and nonendothelial cells. This will clearly require additional studies to completely define.
Physiologic consequences
The present focused study was intended to determine if endothelial TF is expressed in the sickle mouse and was not designed to discern if this leads to hemostatic consequences. However, in our short-term H/R study (3 hours hypoxia followed by 18 hours reoxygenation), we did not observe development of any thrombi. In contrast, de Franceschi et al48 recently observed a tendency for these to form in the lung of the SAD sickle mouse after 46 hours of hypoxia, which was seen to induce inflammation. That study, however, used long-term hypoxia with only 2 hours of reoxygenation, so it is unclear if the effects they describe are due to hypoxia per se or reperfusion injury physiology. The specific microarray used by those authors to interrogate their posthypoxia sickle lungs does not, in fact, include a probe for TF.
Inhibition by lovastatin
Lovastatin largely eliminated TF expression by hBERK1 mice at ambient air and by the posthypoxic NY1DD mice. We tested this drug because of the multiple anti-inflammatory and other salubrious effects of statins.49,50 One of these is induction of nitric oxide synthase,49 the product of which, NO, is an inhibitor of TF expression.51 In fact, statins are reported to inhibit TF expression induced in vitro in monocytes52,53 and endothelial cells.47 Additionally, statins protect rodents from experimental ischemic stroke54 and from renal H/R injury.55 It is believed that such therapeutic effects of statins are mediated by their ability to impair Rho activity and to activate Akt.50 Simvastatin is reported to inhibit ERK phosphorylation (which tends to parallel Egr-1 activation) in neutrophils56 and to inhibit Egr-1 itself in murine macrophages.57
We do not know the reason why lovastatin failed to inhibit monocyte TF expression in this study. Potential reasons include the theoretical possibilities that dose response to the statins differs for monocytes/macrophages versus endothelial cells, that concentrations achieved in vivo (unmeasured) did not correspond to those used in in vitro studies, and that details of transcriptional regulation may be different for monocytes/macrophages versus endothelial cells. In addition, not all statins have efficacy in any given model (eg, Colli et al53 ). Our study used lovastatin in vivo, whereas in vitro studies of TF47,52,53 or Egr-157 inhibition have employed other statins.
Conclusion
We previously observed that sulfasalazine did not diminish TF expression by circulating endothelial cells in sickle patients, even though it did diminish adhesion molecule expression.58 We believe that those data and our present results, in aggregate, suggest that a combination of sulfasalazine (or other strong NFκB inhibitor) plus a statin may be a rational therapeutic approach for vascular prophylaxis in sickle patients. We predict that this would be beneficial by inhibiting activation of the coagulation system, impairing adhesion of white blood cells and red blood cells to vessel wall, and preventing development of the chronic vasculopathy of sickle disease.10 This notion, of course, should be carefully investigated before clinical application.
Prepublished online as Blood First Edition Paper, April 8, 2004; DOI 10.1182/blood-2003-10-3719.
Supported by grants from the National Institutes of Health (HL55552, R.P.H.; HL54281, R.J.K.).
An Inside Blood analysis of this article appears in the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Darrell Johnson and Fuad Abdulla for technical assistance and Carol Taubert for manuscript preparation. We are grateful to Dr Frans Kuypers for providing us with HbA-BERK mice.