After cardiac transplantation, graft damage occurs secondary to ischemia-reperfusion injury and acute rejection. This damage ultimately leads to the development of graft coronary artery disease (GCAD), which limits long-term graft survival. Apoptosis is directly involved in graft injury, contributing to the development of GCAD. To assess the role of the antiapoptotic factor Bcl-2 in the process of GCAD, we transplanted hearts from FVB transgenic mice overexpressing human Bcl-2 under the control of α-myosin heavy chain promoter into allogenic C57BL/6 mice. Bcl-2 overexpression led to reduced cytochrome c–mediated caspase-9–dependent cardiomyocyte apoptosis and local inflammation (neutrophil infiltration and proinflammatory cytokine production) in cardiac allografts during ischemia-reperfusion injury and also led to reduced immune responses (inflammatory cell infiltration, production of TH1 cytokines and chemokines, and expression of adhesion molecules) during acute and chronic rejection without affecting host CD4+ and CD8+ cell responses in the spleen. Thus, local Bcl-2 expression directly contributes to the modulation of local immune responses in allograft rejection, resulting in attenuated GCAD. In conclusion, our findings suggest that the modulation of Bcl-2 expression by pharmacologic up-regulation or gene transfer may be of clinical benefit in the short- and long-term function of cardiac allografts.
Introduction
Progress in the clinical treatment of acute rejection and of posttransplantation infectious complications has substantially decreased deaths after cardiac transplantation. However, the late development of graft coronary artery disease (GCAD) continues to limit long-term survival. For patients who survive more than 1 year after cardiac transplantation, GCAD is the leading cause of death.1
Although the molecular mechanisms underlying the development of GCAD remain unclear, alloantigen-independent and -dependent factors are known contributors.2 Ischemia-reperfusion injury is the most influential alloantigen-independent factor in the subsequent development of GCAD,3 but the precise mechanisms and mediators involved in ischemia-reperfusion injury remain poorly defined. Although necrosis represents the classic manifestation of hypoxia-induced cell damage, myocyte apoptosis has been identified as an early event during ischemia-reperfusion injury.4 Thus, targeting myocardial apoptosis may be a reasonable therapeutic strategy to reduce the risk for ischemia-reperfusion injury in heart transplant patients.
Many heart transplant recipients experience episodes of acute rejection, another critical risk factor for the subsequent development of GCAD that can lead to late graft loss. Several studies have shown apoptotic cell death to be important during the rejection of renal, hepatic, and small intestinal allografts.5 Apoptosis occurs during cardiac allograft rejection, and the presence of apoptotic myocytes in endomyocardial biopsy specimens correlates with the histologic grade of rejection.6
We investigated the role of Bcl-2 in ischemia-reperfusion injury, in the immune response during acute allograft rejection, and in the development of GCAD in transgenic mice bearing extra copies of cloned human bcl-2 cDNA under the transcriptional control of mouse α-myosin heavy chain (MHC). We hypothesized that the antiapoptotic properties of Bcl-2 may modulate these processes and ultimately may reduce the incidence and severity of GCAD.
Materials and methods
Animals
Inbred male mice of several strains, 6 to 10 weeks old, were used. FVB (H-2q) bcl-2 transgenic (Tg) mice and wild-type (WT) littermates were used as allograft donors. C57BL/6 (H-2b) WT mice, purchased from The Jackson Laboratory (Bar Harbor, ME), were used as recipients. α-MHC bcl-2–overexpressing mice were donated by Dr Michael D. Schneider (Baylor College of Medicine, Houston, TX). To generate these mice, the EcoRI fragment of human bcl-2 cDNA was subcloned to a pKS vector containing the SV40 polyadenylation signal. The XbaI/HindIII fragment, containing human bcl-2 cDNA and the SV40 3′ untranslated region, were subcloned 3′ to the 5.5-kb mouse α-MHC promoter. The expression cassette was released with BamHI and was purified for injection into the pronuclei of FVB/N zygotes, which were transfected to pseudopregnant ICR females. Mice were screened by Southern blot analysis with a human bcl-2 fragment, and copy number was estimated by comparison with known amounts of α-MHC bcl-2. FVB male heterozygotes served as breeders. Housed at the animal care facilities at Stanford University Medical Center (Stanford, CA), the mice were kept under standard temperature, humidity, and timed-lighting conditions, provided mouse chow and water ad libitum, and treated in a humane manner in compliance with the Guide for the Care and Use of Laboratory Animals prepared by the Institute of Laboratory Animal Resources, National Research Council (National Academy Press, 1996).
The genotypes of Bcl-2 transgenic mice were identified by polymerase chain reaction (PCR) with the use of tail genomic DNA. The PCR primers were MHC-1 5′-ATA AGC TTA TAC ATT GAT GAG TTT GGA C-3′ and MHC-2 5′-ATG ATA TCG TCC CAA GGC TCA TTT CAG G-3′. PCR was as follows: initial denaturation at 94°C for 5 minutes followed by 30 cycles of denaturing at 94°C for 1 minute, annealing at 50°C for 30 seconds, and extension at 72°C for 1 minute, then a single final extension at 72°C for 10 minutes. Bcl-2 transgenic mice were identified by amplification of a 286-bp band.
Heterotopic heart transplantation
Heterotopic heart transplantation was performed according to the method of Corry et al7 with some modifications. Anesthesia was induced with 2.5% inhaled isoflurane (Halocarbon Laboratories, River Edge, NJ). During surgery, the animals were maintained on 2.5% inhaled isoflurane. Donor animals were systemically heparinized (50 mg/kg) before heart procurement. The donor heart was rapidly excised after retrograde coronary perfusion with ice-cold saline, and the procured heart was kept in ice-cold saline for 20 minutes. Standard graft implantation averages approximately 30 minutes; thus, total ischemic time was 50 minutes. Cardiac allograft survival was evaluated daily by direct abdominal palpation.
Experimental groups
Our study consisted of 4 parts. In the first part, indicators of ischemia-reperfusion injury were analyzed at 2 hours of reperfusion (Bcl-2 Tg and WT groups; n = 5 each). Cellular fractions were obtained from separate animals (Bcl-2 Tg and WT groups; n = 5 each) to analyze cytosolic and mitochondrial proteins. In the second part, local immune response during acute rejection was assessed 8 days after transplantation (Bcl-2 Tg and WT groups; n = 6 each). The time point of 8 days was chosen because a previous pilot study showed that allografts in both groups are acutely rejected within 9 days of transplantation. In the third part, host spleen cell response to allografts was assessed at 1, 3, 5 (Bcl-2 Tg and WT groups; n = 3 each), and 7 days after transplantation (Bcl-2 Tg and WT groups; n = 5 each). In the fourth part, GCAD and the local immune response during chronic rejection were measured at 30 days after transplantation (Bcl-2 Tg and WT groups; n = 8 each). Recipients in this chronic study group received daily doses (20 mg/kg) of cyclosporin A (Ben Venue Labs, Bedford, OH) by intraperitoneal injection for 30 days after transplantation to limit acute rejection.
ELISA, caspase activity, and MPO assays
Snap-frozen myocardial tissue specimens were homogenized in phosphate-buffered saline (PBS) and centrifuged at 12 000g for 20 minutes at 4°C. The protein concentration of the supernatant was determined by the bicinchoninic acid (BCA) method (Pierce Chemical, Rockford, IL), and aliquots were stored at -80°C. Tumor necrosis factor-α (TNF-α), MCP-1/CCL2, interferon-γ (IFN-γ), interleukin-4 (IL-4), and IL-10 enzyme-linked immunosorbent assay (ELISA) kits were purchased from BioSource International (Camarillo, CA). CXCL10 (IP-10), CXCL9 (MIG), Fas, Fas ligand, intercellular adhesion molecule-1 (ICAM-1), and vascular cell adhesion molecule-1 (VCAM-1) ELISA kits and caspase-8 and -9 activity assay kits were obtained from R&D Systems (Minneapolis, MN). A caspase-3 activity assay kit was purchased from BD Biosciences (Palo Alto, CA). Myeloperoxidase (MPO) activity was calculated as units per milligram total protein in lysates of reperfused cardiac grafts, as previously described.8
Immunohistochemistry
To confirm cardiomyocyte-specific human Bcl-2 overexpression, frozen sections of native hearts were costained with rabbit antihuman Bcl-2 (100) (Santa Cruz Biotechnology, Santa Cruz, CA) and mouse antimouse α-actinin (BM-75.2) (Sigma, St Louis, MO) and then were stained with fluorescein isothiocyanate (FITC)–conjugated antirabbit immunoglobulin G (IgG) and Texas red–conjugated antimouse IgG as secondary antibodies. Sections were counterstained with 4,6-diamidino-2-phenylindole (DAPI) (Molecular Probes, Eugene, OR). Stained tissues were examined with a Leica DMRB fluorescence microscope (Leica Microsystems, Frankfurt, Germany). To assess inflammatory cell infiltration to the cardiac allograft, frozen sections of transplanted hearts were stained with antimouse CD3 (145-2C11), CD4 (H129.19), CD8a (53-6.7), CD11c (HL3), Mac-1 (M1/70), and B220 (RA3-6B2) (BD PharMingen, San Diego, CA) and then were stained with biotinylated goat antirabbit, rabbit antirat, or antihamster IgG by the avidin-biotin complex method or were counter-stained with methyl green.
In situ oligoligation TUNEL
Apoptotic cell counts in allograft tissue were determined by in situ staining of DNA strand breaks in serial sections of each specimen using the ApopTag in situ oligoligation (ISOL) kit with oligo-A (Intergen, Purchase, NY) as directed, but with some modifications. Endogenous biotin was blocked with an avidin/biotin blocking kit (BioGenex, San Ramon, CA). TACS blue label and methyl green were used as a peroxidase substrate and a counterstain, respectively. Because conventional TdT-mediated dUTP nick-end labeling (TUNEL) assays can detect nonspecific DNA fragmentation caused by necrosis, this more specific in situ ligation assay to identify apoptotic nuclei was used with hairpin oligonucleotide probes.
Immunohistochemistry and ISOL TUNEL–positive cell counts
The number of positive cells in each cardiac allograft was manually counted by 2 investigators (G.K.M., R.D.T.) who were blinded to the experimental conditions. Cells were counted in 6 animals (4 fields each) at 200 × magnification. The percentage (ie, number of labeled cells divided by total number of cells) of positively stained cells was recorded.
Superoxide production
Superoxide levels were measured in excised tissue by the spin trap method after 2 hours of reperfusion. Superoxide accumulation was measured by using conditioned medium supplemented with the spin trapping agent 4-amino-2,2,6,6,-tetramethylpiperidine-1-oxyl (tempamine; Sigma), as previously described.9 Electron paramagnetic resonance (EPR) spectra were recorded at room temperature with a spectrometer (model 8400; Resonance Instruments). EPR signal intensity was quantified by comparing the double integration of the recorded first-derivative EPR peak of each sample with that of a standard tempamine spin solution. All measurements were normalized to the protein concentration of each sample.
Measurement of cytochrome c release from mitochondria to cytosol in cardiomyocytes
Two hours after reperfusion, cardiac grafts were minced and suspended in 10 mL digestion buffer (RPMI 1640 [Invitrogen, Carlsbad, CA], 10 mM HEPES [N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid] [Sigma], and 0.2% collagenase IV [Sigma]) and were incubated at 37°C for 1 hour. Then the single-cell suspension was filtered and washed. The pellet was resuspended in 3 mL red blood cell (RBC) lysing buffer (Sigma) to remove red blood cells and was washed. Isolated cardiomyocytes (20 million cells per sample) were fractioned into 4 subcellular protein fractions (cytosolic, nuclear, mitochondrial, and cytoskeletal fractions) using FractionPREP (BioVision, Mountain View, CA). The protein concentration of each fraction was determined using the BCA method. Cytochrome c levels in the cytosolic and mitochondrial fractions were determined using ELISA (R&D Systems). Percentage release of cytochrome c was calculated as the amount of cytosolic cytochrome c divided by the total amount of cytosolic and mitochondrial cytochrome c.
In vitro cardiomyocyte survival assay
Cardiomyocytes were isolated from Bcl-2 Tg and WT native hearts (n = 3 each), as described above. Cells (1 × 106 cells/well in a 12-well plate) were cultured in RPMI 1640 medium supplemented with or without 10% fetal calf serum at 37°C for 12, 24, 48, and 72 hours. Cell viability was determined with the trypan blue exclusion assay.10 Data are shown as the percentage of the initial number of plated cells that survive, derived from 2 independent experiments.
Morphometric analysis of GCAD
Thirty days after transplantation, the cardiac grafts were harvested and embedded in paraffin. Elastica von Gieson staining was performed for morphometric analysis of arterial intimal proliferation, as reported previously.11 Briefly, the neointima, media, and lumen were measured with the use of SPOT Advanced Version 3.4.2 software (Diagnostic Instruments, Sterling Heights, MI). The neointima was defined as the area bound by the internal elastic lamina and the lumen, the media as the region between the internal and external elastic membranes, and the lumen as the clear region in the vessel. Diseased vessels were defined as having more than 10% luminal narrowing. Multiple sections from the middle of the heart were used for analysis. Medium-sized coronary arteries were analyzed (more than 8 arteries for each graft).
Mixed lymphocyte reaction
Seven days after heart transplantation, CD4+ T cells from spleens of recipient mice were purified by CD4+ T-cell isolation kit (Miltenyi Biotec, Bergisch Gladbach, Germany) and were used as responder cells. As stimulator cells, T cell–depleted allogenic FVB or syngenic C57BL/6 splenocytes, which stained with anti-Thy 1.2 microbeads (Miltenyi Biotec) and were passed through a MACS column (Miltenyi Biotec), were treated with γ-irradiation (20 Gy) and were then used as stimulator cells. Cells were suspended in RPMI 1640 medium (Sigma) containing 5 μM 2-mercaptoethanol, 50 μg/mL streptomycin, 50 U/mL penicillin, and 10% heat-inactivated fetal calf serum. Responder cells (5 × 105) were cultured with 3 × 105 stimulator cells for 120 hours in a total volume of 200 μL in a 96-well flat-bottom plate. The proliferative response was assessed by pulsing with 0.25 μCi/mL (0.00925 MBq/mL) [3H]-thymidine (Amersham, Arlington, IL) for 6 hours. After 6 hours, cells were harvested (Harvester 96 Mach IIIM; TOMTEC, Hamden, CT), and incorporated [3H]-thymidine was measured with a MicroBeta System (Pharmacia Biotech, Piscataway, NJ).
Flow cytometry analysis
At 1, 3, 5, and 7 days after heart transplantation, recipient splenocytes (4 × 106 cells) were cultured in the presence of 200 ng/mL ionomycin (Sigma), 1 ng/mL phorbol 12-myristate 13-acetate (PMA; Sigma), and 1 μM Monensin (Sigma) for 6 hours at a total volume of 1 mL in a 12-well plate and then were stained with monoclonal antibodies (mAbs) according to standard protocols. They were incubated with fluorescein isothiocyanate (FITC)–antimouse H-2Kb (AF6-88.5; BD PharMingen), peridin chlorophyll protein (PerCP)–antimouse CD4 (RM4-5; BD PharMingen), allophycocyanin (APC)–antimouse CD8 (53-6.7; BD PharMingen), phycoerythrin (PE)–antimouse TNF-α (MP6-XT22), PE antimouse IFN-γ (XMG1.2), and isotype-matched control mAbs obtained from BD PharMingen. TNF-α expression or IFN-γ expression on H-2Kb+CD4+ or H-2Kb+CD8+ cells was analyzed using a FACSCalibur system (BD Bioscience) and Cell Quest software (BD Bioscience).
Statistical analysis
All results are expressed as mean ± SD. Data were compared between the Bcl-2 Tg group and the WT group, and differences between groups were analyzed using the unpaired Student t test. Statistical analyses were performed with StatView 5.0 software (SAS Institute, Cary, NC). Statistical significance was accepted at P < .05.
Results
Bcl-2 overexpression prolongs cardiomyocyte survival under a culture condition of serum deprivation
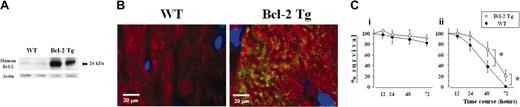
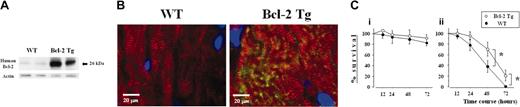
The overexpression of human Bcl-2 protein in cardiomyocytes of Bcl-2 Tg mice was confirmed by Western blotting and immunohistochemistry (Figure 1A-B). It is known that serum deprivation induces apoptosis in cultured cardiomyocytes.12 To determine the cytoprotective effect of Bcl-2, cardiomyocytes isolated from hearts of Bcl-2 Tg and WT mice were cultured in serum-rich and serum-free medium, and cell survival was assessed by trypan blue exclusion at 12, 24, 48, and 72 hours. In serum-rich medium, there was no significant difference in cardiomyocyte survival between the 2 groups (Figure 1Ci). In contrast, Bcl-2 Tg cardiomyocyte survival in serum-free medium was significantly higher at 48 and 72 hours compared with WT cardiomyocyte survival (Figure 1Cii). These observations suggest that Bcl-2 directly regulates cardiomyocyte survival.
Expression and effects of human Bcl-2 on cardiomyocytes from transgenic mice. (A) Western blot analysis of human Bcl-2 in heart lysates of naive Bcl-2 Tg (n = 2) and WT (n = 2) mice. Increased expression of human Bcl-2 was observed in the transgenic group. (B) Bcl-2 expression in cardiomyocytes. Heart sections were costained with antihuman Bcl-2 recognized by FITC-conjugated secondary antibody (green) and anti-α actinin recognized by Texas red–conjugated secondary antibody (red) antibodies. Sections were counterstained with 4,6-diamidino-2-phenylindole (DAPI, blue). Striations, within the cardiomyocyte, consistently displayed α-actinin staining throughout the entire cell. Human Bcl-2, located exclusively within the cytoplasm of the cardiomyocyte, was identified only in hearts from Bcl-2 Tg mice. A Leica DMRB fluorescent microscope (Leica Microsystems, Frankfurt, Germany) and SPOT Advanced Version 3.4.2 software (Diagnostic Instruments, Sterling Heights, MI) were used. Original magnification, × 400. (C) Effect of human Bcl-2 overexpression on cardiomyocyte survival. Cardiomyocytes from human Bcl-2 Tg (n = 3) and WT (n = 3) mice were cultured in the presence (i) or absence (ii) of 10% fetal calf serum. The percentage of surviving cells was assessed by the trypan blue exclusion assays at the indicated time points. Data are shown as the percentage of surviving cells against the number of initial plated cells and are representative of 2 independent experiments. Data are shown as mean ± SD. *P < .01.
Expression and effects of human Bcl-2 on cardiomyocytes from transgenic mice. (A) Western blot analysis of human Bcl-2 in heart lysates of naive Bcl-2 Tg (n = 2) and WT (n = 2) mice. Increased expression of human Bcl-2 was observed in the transgenic group. (B) Bcl-2 expression in cardiomyocytes. Heart sections were costained with antihuman Bcl-2 recognized by FITC-conjugated secondary antibody (green) and anti-α actinin recognized by Texas red–conjugated secondary antibody (red) antibodies. Sections were counterstained with 4,6-diamidino-2-phenylindole (DAPI, blue). Striations, within the cardiomyocyte, consistently displayed α-actinin staining throughout the entire cell. Human Bcl-2, located exclusively within the cytoplasm of the cardiomyocyte, was identified only in hearts from Bcl-2 Tg mice. A Leica DMRB fluorescent microscope (Leica Microsystems, Frankfurt, Germany) and SPOT Advanced Version 3.4.2 software (Diagnostic Instruments, Sterling Heights, MI) were used. Original magnification, × 400. (C) Effect of human Bcl-2 overexpression on cardiomyocyte survival. Cardiomyocytes from human Bcl-2 Tg (n = 3) and WT (n = 3) mice were cultured in the presence (i) or absence (ii) of 10% fetal calf serum. The percentage of surviving cells was assessed by the trypan blue exclusion assays at the indicated time points. Data are shown as the percentage of surviving cells against the number of initial plated cells and are representative of 2 independent experiments. Data are shown as mean ± SD. *P < .01.
Bcl-2 overexpression results in reduction of cardiomyocyte apoptosis and inflammatory response during ischemia-reperfusion injury
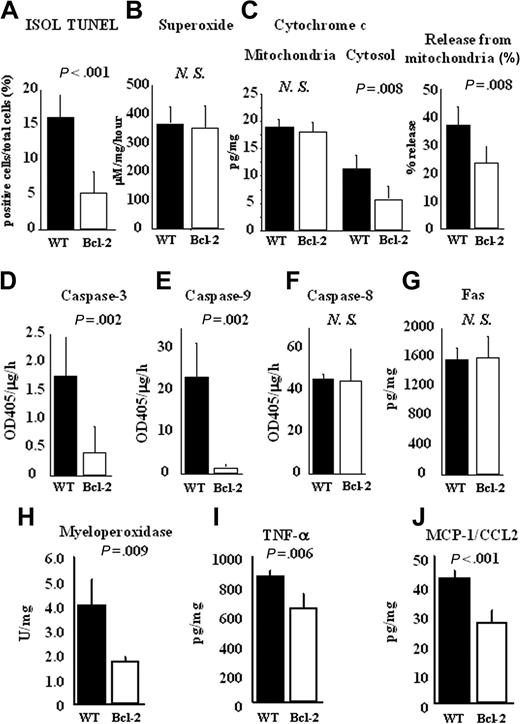
Cardiomyocyte apoptosis caused by ischemia-reperfusion injury is independent of alloantigen-specific immune responses. As shown in Figure 2A, ISOL TUNEL–positive apoptotic cardiomyocytes were significantly decreased in the Bcl-2 Tg cardiac allografts compared with WT allografts at 2 hours of reperfusion. During ischemia-reperfusion injury, superoxide is implicated in cardiomyocyte apoptosis.13 Because Bcl-2 is regarded as a strong antioxidant,14 we next examined superoxide production in the cardiac allografts. As shown in Figure 2B, superoxide production was comparable between the 2 groups. In contrast, the release of cytochrome c from mitochondria to cytosol, thought to be involved in apoptosis through activation of the superoxide pathway, was significantly decreased in the Bcl-2 Tg group compared with the WT group (Figure 2C). These results indicate that Bcl-2 overexpression in donor hearts inhibits cytochrome c–mediated apoptosis during ischemia-reperfusion injury; moreover, Bcl-2–mediated inhibition appears to occur downstream from superoxide production. In correlation with the reduction in cytochrome c release, caspase-3 and -9 activities, which are mediated by cytochrome c, were also reduced in lysates from the Bcl-2 Tg group compared with those from the WT group (Figure 2D- E). Caspase-8 activities in the lysates did not differ significantly between the 2 groups at 2 hours of reperfusion (Figure 2F). Consistent with that finding, the expression of Fas, which induces caspase-8–dependent apoptosis, was comparable in graft lysates between the 2 groups (Figure 2G). These results suggest that Bcl-2 overexpression in donor hearts inhibits cardiomyocyte apoptosis mediated by cytochrome c–dependent caspase-3 and -9 activation but not by superoxide and Fas-dependent pathways.
Reduction of cardiomyocyte apoptosis and inflammatory response by Bcl-2 overexpression during ischemia-reperfusion injury in cardiac allografts. Hearts of FVB WT or Bcl-2 Tg mice were transplanted into C57BL/6 mice. At 2 hours of reperfusion, the cardiac allografts were harvested, and histologic sections and lysates from the cardiac allografts were prepared as described in “Materials and methods.” (A) The number of apoptotic cells in cardiac allografts was counted by ISOL TUNEL staining. (B) Superoxide production was measured in excised tissue by the spin trap method. (C) Cytochrome c levels in mitochondria and cytosol were measured by ELISA, the percentage of cytochrome c release from mitochondria to cytosol was calculated, and the activities of (D) caspase-3, (E) caspase-9, and (F) caspase-8 and the levels of (G) Fas in heart lysates were measured by ELISA. (H) MPO activity was determined by MPO activity assay. (I) TNF-α, and (J) MCP-1/CCL2 production were measured by ELISA. Filled columns show the data from WT heart grafts (n = 5), and open columns show data from the Bcl-2 Tg heart grafts (n = 5). Mean ± SD values are shown. NS indicates not significant.
Reduction of cardiomyocyte apoptosis and inflammatory response by Bcl-2 overexpression during ischemia-reperfusion injury in cardiac allografts. Hearts of FVB WT or Bcl-2 Tg mice were transplanted into C57BL/6 mice. At 2 hours of reperfusion, the cardiac allografts were harvested, and histologic sections and lysates from the cardiac allografts were prepared as described in “Materials and methods.” (A) The number of apoptotic cells in cardiac allografts was counted by ISOL TUNEL staining. (B) Superoxide production was measured in excised tissue by the spin trap method. (C) Cytochrome c levels in mitochondria and cytosol were measured by ELISA, the percentage of cytochrome c release from mitochondria to cytosol was calculated, and the activities of (D) caspase-3, (E) caspase-9, and (F) caspase-8 and the levels of (G) Fas in heart lysates were measured by ELISA. (H) MPO activity was determined by MPO activity assay. (I) TNF-α, and (J) MCP-1/CCL2 production were measured by ELISA. Filled columns show the data from WT heart grafts (n = 5), and open columns show data from the Bcl-2 Tg heart grafts (n = 5). Mean ± SD values are shown. NS indicates not significant.
Apoptosis is known to occur through the induction of inflammation mediated by proinflammatory cytokines.15 We next investigated the effect of Bcl-2 overexpression in allografts on the inflammatory response during ischemia-reperfusion injury. We examined neutrophil-produced MPO because neutrophils are known to be predominant effector cells in the local inflammatory response.16 We also determined the levels of proinflammatory cytokines and chemokines, including TNF-α and MCP-1/CCL2. MPO activity and levels of tested proinflammatory cytokines were significantly lower in the cardiac allografts of the Bcl-2 Tg group compared with those of the WT group at 2 hours of reperfusion (Figure 2H-J). Taken together, these results suggest that local Bcl-2 overexpression in allografts inhibits cytochrome c release–mediated apoptosis and the inflammatory response in the early phase during ischemia-reperfusion injury.
Bcl-2 overexpression suppresses cardiomyocyte apoptosis and immune response during acute rejection
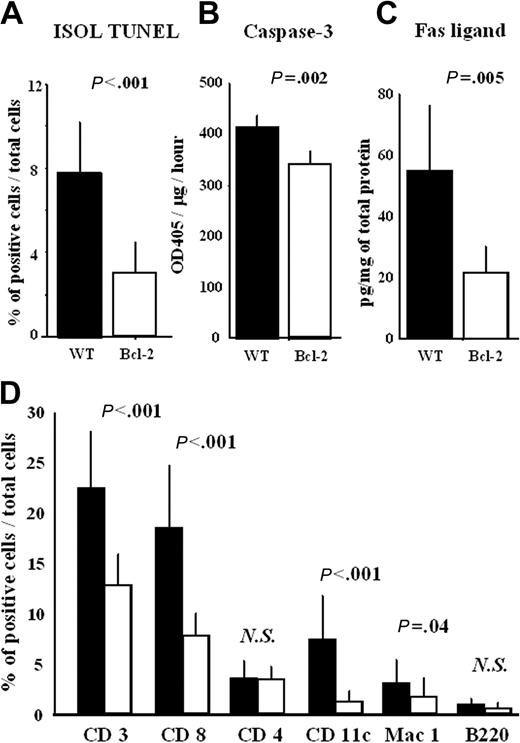
Excessive apoptosis is observed in acute rejection.6 We next investigated the role of Bcl-2 in cardiomyocyte apoptosis during acute rejection. Cardiomyocyte apoptosis quantified by ISOL TUNEL was significantly decreased in the cardiac allografts of the Bcl-2 Tg group compared with the WT group during acute rejection (Figure 3A). Consistent with this, caspase-3 activity was also significantly decreased in the cardiac allografts of the Bcl-2 Tg group (Figure 3B). Moreover, Fas ligand expression was significantly decreased in the cardiac allografts of the Bcl-2 Tg group, suggesting decreased infiltration of Fas ligand–expressing immune cells (especially CD8+ cells) (Figure 3C). Indeed, the infiltration of CD3+ and CD8+ T cells and of CD11c+ dendritic cells and Mac 1+ macrophages was reduced in the cardiac allografts of the Bcl-2 Tg group (Figure 3D). However, infiltration of CD4+ and B220+ cells did not differ significantly between the 2 groups (Figure 3D).
Bcl-2 overexpression results in reduced cardiomyocyte apoptosis and immune cell infiltration in cardiac allografts during acute rejection. At 8 days after transplantation, the cardiac allografts were harvested, and histologic sections and lysates from the cardiac allografts were prepared as described in “Materials and methods.” (A) The number of apoptotic cells in cardiac allografts was counted by ISOL TUNEL staining, (B) the activity of caspase-3, and (C) the level of Fas ligand in heart lysates were measured by ELISA. Histologic sections were also stained with anti-CD3, -CD4, -CD8a, -CD11c, -Mac-1, and -B220 antibodies, as described in “Materials and methods.” The number of positive cells in each cardiac allograft was manually counted by 2 investigators who were blinded to the experimental conditions. Cells were counted in 6 animals (4 fields each) at 200 × magnification. (D) The percentage of positively stained cells—that is, the number of labeled cells divided by total number of cells—was recorded. Filled columns show the data from WT heart grafts (n = 6), and open columns show data from the Bcl-2 Tg heart grafts (n = 6). Mean ± SD values are shown. NS indicates not significant.
Bcl-2 overexpression results in reduced cardiomyocyte apoptosis and immune cell infiltration in cardiac allografts during acute rejection. At 8 days after transplantation, the cardiac allografts were harvested, and histologic sections and lysates from the cardiac allografts were prepared as described in “Materials and methods.” (A) The number of apoptotic cells in cardiac allografts was counted by ISOL TUNEL staining, (B) the activity of caspase-3, and (C) the level of Fas ligand in heart lysates were measured by ELISA. Histologic sections were also stained with anti-CD3, -CD4, -CD8a, -CD11c, -Mac-1, and -B220 antibodies, as described in “Materials and methods.” The number of positive cells in each cardiac allograft was manually counted by 2 investigators who were blinded to the experimental conditions. Cells were counted in 6 animals (4 fields each) at 200 × magnification. (D) The percentage of positively stained cells—that is, the number of labeled cells divided by total number of cells—was recorded. Filled columns show the data from WT heart grafts (n = 6), and open columns show data from the Bcl-2 Tg heart grafts (n = 6). Mean ± SD values are shown. NS indicates not significant.
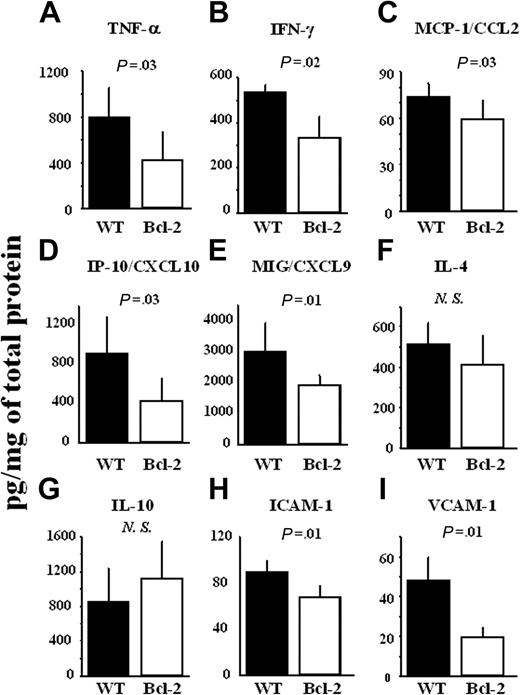
The levels of TH1 cytokines (TNF-α and IFN-γ) and chemokines (MCP-1/CCL2, IP-10/CXCL10, and Mig/CXCL9) were significantly decreased in the cardiac allografts of the Bcl-2 Tg group (Figure 4A-E), whereas the levels of TH2 cytokines (IL-4 and IL-10) did not differ (Figure 4F-G). The levels of adhesion molecules (ICAM-1 and VCAM-1) were also significantly lower in the Bcl-2 Tg group (Figure 4H-I).
Reduction of TH1 cytokines, but not TH2 cytokines, chemokines, and adhesion molecules by Bcl-2 overexpression during acute rejection in allografts. At 8 days after transplantation, the cardiac allografts were harvested, and lysates from the cardiac allografts were prepared as described in “Materials and methods.” (A) Production of TNF-α, (B) IFN-γ, (C) MCP-1/CCL2, (D) IP-10/CXCL10, (E) MIG/CXCL9, (F) IL-4, (G) IL-10, (H) ICAM-1, and (I) VCAM-1 in heart lysates was measured by ELISA. Filled columns show the data from WT heart grafts (n = 6), and open columns show data from the Bcl-2 Tg heart grafts (n = 6). Mean ± SD values are shown. NS indicates not significant.
Reduction of TH1 cytokines, but not TH2 cytokines, chemokines, and adhesion molecules by Bcl-2 overexpression during acute rejection in allografts. At 8 days after transplantation, the cardiac allografts were harvested, and lysates from the cardiac allografts were prepared as described in “Materials and methods.” (A) Production of TNF-α, (B) IFN-γ, (C) MCP-1/CCL2, (D) IP-10/CXCL10, (E) MIG/CXCL9, (F) IL-4, (G) IL-10, (H) ICAM-1, and (I) VCAM-1 in heart lysates was measured by ELISA. Filled columns show the data from WT heart grafts (n = 6), and open columns show data from the Bcl-2 Tg heart grafts (n = 6). Mean ± SD values are shown. NS indicates not significant.
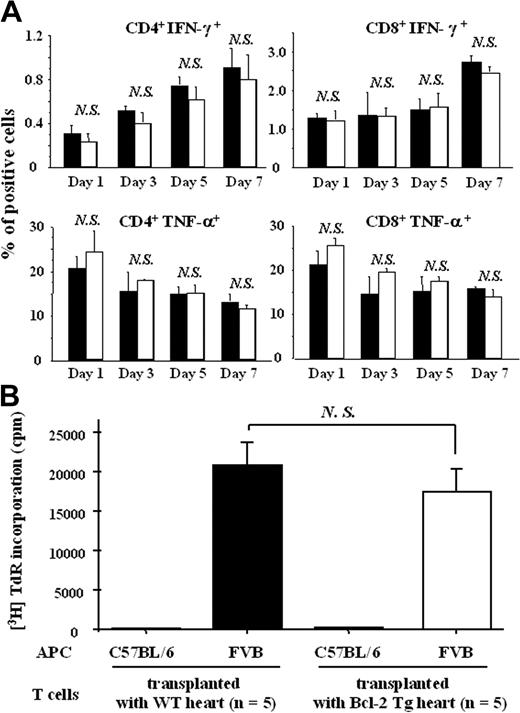
We next determined the effect of Bcl-2 overexpression in cardiac allografts on host immune cells. Expression levels of IFN-γ and TNF-α on splenic CD4+ or CD8+ T cells from recipients of Bcl-2 Tg allografts were comparable to those from recipients of WT allografts 1, 3, 5, and 7 days after transplantation (Figure 5A). Furthermore, when splenic CD4+ T cells from Bcl-2 Tg graft recipient or WT graft recipients were cocultured with allogenic FVB spleen cells 7 days after transplantation, the alloantigen-specific CD4+ T-cell proliferative response did not differ between the 2 groups (Figure 5B).
Bcl-2 overexpression in cardiac allografts does not modulate the allospecific host immune cell response in the spleen. Splenocytes from recipient mice were prepared at 1, 3, 5, and 7 days after heart transplantation (n = 3 each), as described in “Materials and methods.” (A) Expression levels of IFN-γ and TNF-α on splenic CD4+ or CD8+ T cells from host C57BL/6 mice that received transplanted Bcl-2 Tg hearts and WT hearts were assessed by intracellular cytokine staining analysis using flow cytometry. Splenocytes from recipient mice were prepared at 7 days (n = 5 each) after heart transplantation, as described in “Materials and methods.” (B) Anti-FVB (stimulator) activity of C57BL/6 splenocytes from WT naive (control) mice, mice that received transplanted WT hearts, and mice that received transplanted Bcl-2 Tg hearts was assessed by a mixed lymphocyte reaction. Filled columns show the data from WT heart grafts, and open columns show data from the Bcl-2 Tg heart grafts. Data are representative of 3 separate experiments. Mean ± SD values are shown. NS indicates not significant.
Bcl-2 overexpression in cardiac allografts does not modulate the allospecific host immune cell response in the spleen. Splenocytes from recipient mice were prepared at 1, 3, 5, and 7 days after heart transplantation (n = 3 each), as described in “Materials and methods.” (A) Expression levels of IFN-γ and TNF-α on splenic CD4+ or CD8+ T cells from host C57BL/6 mice that received transplanted Bcl-2 Tg hearts and WT hearts were assessed by intracellular cytokine staining analysis using flow cytometry. Splenocytes from recipient mice were prepared at 7 days (n = 5 each) after heart transplantation, as described in “Materials and methods.” (B) Anti-FVB (stimulator) activity of C57BL/6 splenocytes from WT naive (control) mice, mice that received transplanted WT hearts, and mice that received transplanted Bcl-2 Tg hearts was assessed by a mixed lymphocyte reaction. Filled columns show the data from WT heart grafts, and open columns show data from the Bcl-2 Tg heart grafts. Data are representative of 3 separate experiments. Mean ± SD values are shown. NS indicates not significant.
These results suggest that Bcl-2 overexpression in cardiac allografts does not modulate the induction of allospecific host immune cells in the host spleen. Thus, Bcl-2 overexpression in allografts led to decreased inflammatory cell infiltration and expression of TH1 cytokines, chemokines, and adhesion molecules in cardiac allografts without modulating the induction of allospecific host immune cells in the spleen, contributing to the reduced cardiomyocyte apoptosis during acute rejection.
Bcl-2 overexpression suppresses cardiomyocyte apoptosis, inflammatory response, and GCAD 30 days after transplantation
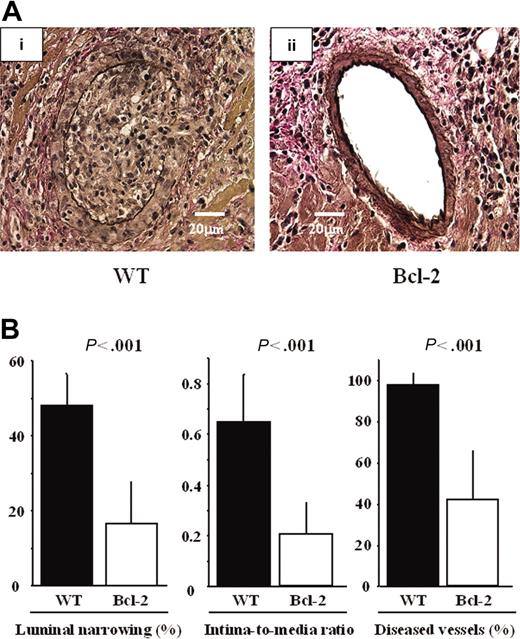
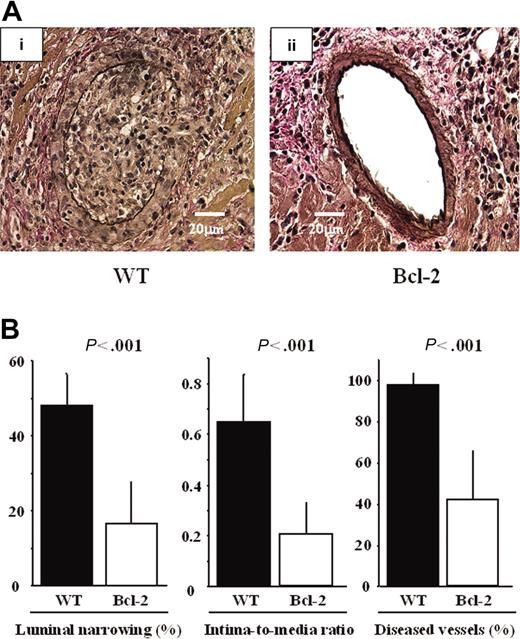
Overexpression of Bcl-2 in cardiac allografts led to reduced ischemia-reperfusion injury and inflammatory response during acute rejection. Because ischemia-reperfusion injury and acute rejection are known to be risk factors for the development of GCAD,2 we assessed GCAD indices and inflammatory responses in the chronic phase. Marked fibrointimal thickening and luminal narrowing, morphologically resembling typical human GCAD, were observed in the WT group, whereas decreased intimal thickening and vessel lumen preservation were observed in the Bcl-2 Tg group (Figure 6A). GCAD level, quantitatively determined by the mean percentage of luminal narrowing of the coronary arteries, the intima-to-media ratio, and the percentage of diseased vessels, was also significantly lower in the Bcl-2 Tg group than in the WT group (Figure 6B).
Reduction of GCAD by Bcl-2 overexpression 30 days after transplantation. Representative sections of cardiac allografts stained with elastic van Gieson for evaluation of GCAD in the (Ai) WT donor heart group and (Aii) the Bcl-2 Tg donor heart group. (Ai) Note that marked fibrointimal thickening and luminal narrowing, morphologically resembling typical human GCAD, are observed in the WT donor heart group. (Aii) In contrast, decreased intimal thickening and preserved vessel lumen are observed in the Bcl-2 Tg donor heart group. A Leica DMRB fluorescent microscope (Leica Microsystems, Frankfurt, Germany) and SPOT Advanced Version 3.4.2 software (Diagnostic Instruments, Sterling Heights, MI) were used. Original magnification, × 400. (B) Morphometric assessment of cardiac grafts. Note that compared with parameters in the WT donor heart group, all 3 GCAD parameters are significantly lower in the Bcl-2 Tg donor heart group 30 days after transplantation. ▪ indicates the data from WT heart grafts (n = 8); □, those from the Bcl-2 Tg heart grafts (n = 8). Mean ± SD values are shown.
Reduction of GCAD by Bcl-2 overexpression 30 days after transplantation. Representative sections of cardiac allografts stained with elastic van Gieson for evaluation of GCAD in the (Ai) WT donor heart group and (Aii) the Bcl-2 Tg donor heart group. (Ai) Note that marked fibrointimal thickening and luminal narrowing, morphologically resembling typical human GCAD, are observed in the WT donor heart group. (Aii) In contrast, decreased intimal thickening and preserved vessel lumen are observed in the Bcl-2 Tg donor heart group. A Leica DMRB fluorescent microscope (Leica Microsystems, Frankfurt, Germany) and SPOT Advanced Version 3.4.2 software (Diagnostic Instruments, Sterling Heights, MI) were used. Original magnification, × 400. (B) Morphometric assessment of cardiac grafts. Note that compared with parameters in the WT donor heart group, all 3 GCAD parameters are significantly lower in the Bcl-2 Tg donor heart group 30 days after transplantation. ▪ indicates the data from WT heart grafts (n = 8); □, those from the Bcl-2 Tg heart grafts (n = 8). Mean ± SD values are shown.
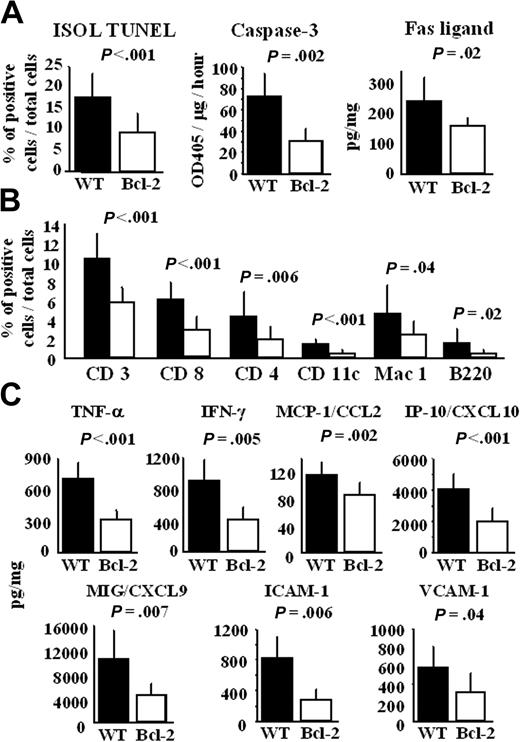
Along with the decrease in GCAD severity, the number of ISOL TUNEL–positive apoptotic cardiomyocytes, the level of caspase-3 activity, and the Fas ligand level were lower in the chronic phase in the cardiac allografts of the Bcl-2 Tg group (Figure 7A). Consistent with the Fas ligand level, infiltration of CD3+ and CD8+ cells was reduced in the cardiac allografts of the Bcl-2 Tg group (Figure 7B). In contradistinction to the cellular infiltrate profile seen in acute rejection, the chronic phase showed profoundly lower levels not only of CD11c+ and Mac-1+ cells but also of CD4+ and B220+ cells in the cardiac allografts of the Bcl-2 Tg group (Figure 7B). Furthermore, the levels of TNF-α, IFN-γ, chemokines (MCP-1/CCL2, IP-10/CXCL10, Mig/CXCL9), and adhesion molecules (ICAM-1 and VCAM-1) were significantly lower in the cardiac allografts of the Bcl-2 Tg group than in the WT group 30 days after transplantation (Figure 7C). These results indicate that local overexpression of Bcl-2 in cardiac allografts leads to reduced cardiomyocyte apoptosis and TH1 immune response in the chronic phase, contributing to the decreased development of GCAD.
Bcl-2 overexpression in cardiac allografts results in less cardiomyocyte apoptosis, immune cell infiltration, and production of TH1 cytokines, chemokines, and adhesion molecule 30 days after transplantation. Thirty days after transplantation, cardiac allografts were harvested, and histologic sections and lysates from the cardiac allografts were prepared, as described in “Materials and methods.” (A) The number of apoptotic cells in cardiac allografts was counted by ISOL TUNEL staining, and the activity of caspase-3 and the level of Fas ligand in heart lysates were measured using ELISA. (B) Histologic sections were also stained with anti-CD3, -CD4, -CD8a, -CD11c, -Mac-1, and -B220 antibodies, as described in “Materials and methods.” The number of positive cells in each cardiac allograft was manually counted by 2 investigators blinded to the experimental conditions. Cells were counted in 8 animals (4 fields each) at 200 × magnification. The percentage of positively stained cells—that is, the number of labeled cells divided by total number of cells—was recorded. (C) Production of TNF-α, IFN-γ, MCP-1/CCL2, IP-10/CXCL10, MIG/CXCL9, ICAM-1, and VCAM-1 in lysates from the cardiac allografts was measured by ELISA. Filled columns show the data from WT heart grafts (n = 8), and open columns show data from the Bcl-2 Tg heart grafts (n = 8). Mean ± SD values are shown.
Bcl-2 overexpression in cardiac allografts results in less cardiomyocyte apoptosis, immune cell infiltration, and production of TH1 cytokines, chemokines, and adhesion molecule 30 days after transplantation. Thirty days after transplantation, cardiac allografts were harvested, and histologic sections and lysates from the cardiac allografts were prepared, as described in “Materials and methods.” (A) The number of apoptotic cells in cardiac allografts was counted by ISOL TUNEL staining, and the activity of caspase-3 and the level of Fas ligand in heart lysates were measured using ELISA. (B) Histologic sections were also stained with anti-CD3, -CD4, -CD8a, -CD11c, -Mac-1, and -B220 antibodies, as described in “Materials and methods.” The number of positive cells in each cardiac allograft was manually counted by 2 investigators blinded to the experimental conditions. Cells were counted in 8 animals (4 fields each) at 200 × magnification. The percentage of positively stained cells—that is, the number of labeled cells divided by total number of cells—was recorded. (C) Production of TNF-α, IFN-γ, MCP-1/CCL2, IP-10/CXCL10, MIG/CXCL9, ICAM-1, and VCAM-1 in lysates from the cardiac allografts was measured by ELISA. Filled columns show the data from WT heart grafts (n = 8), and open columns show data from the Bcl-2 Tg heart grafts (n = 8). Mean ± SD values are shown.
Discussion
Tissue damage mediated by apoptosis is a key process in acute and chronic rejection caused by ischemia-reperfusion injury and alloantigen-specific immune responses.4-6,17 The Bcl-2 gene is implicated in prolonged cell survival by blocking the apoptotic process.18 Indeed, we observed that the cardiomyocyte survival of Bcl-2 Tg mice was longer than that of WT mice in the culture without fetal calf serum. Moreover, we showed not only inhibited cardiomyocyte apoptosis at different time points by local overexpression of Bcl-2 in allografts but also an anti-inflammatory effect of Bcl-2 during ischemia-reperfusion injury. This effect was caused by decreased cytochrome c release from mitochondria, caspase-3 and -9 activities, neutrophil infiltration, and proinflammatory cytokine production after ischemia-reperfusion injury and by reduced production of TH1 cytokines, chemokines, and adhesion molecules and reduced infiltration of CD8+ cells, dendritic cells, and macrophages during acute cardiac allograft rejection, ultimately leading to less development of GCAD.
Cardiomyocyte apoptosis, which is correlated with the severity of tissue rejection, is observed as an early event during cardiac ischemia-reperfusion injury.4,19,20 Oxygen-free radicals are implicated in the initiation of apoptosis by promoting cytochrome c release from mitochondria,21 whereas Bcl-2 has been implicated as a strong antioxidant.14 The precise mechanism by which Bcl-2 acts as an antioxidant is unknown. Human cardiomyopathy is known to be associated with the release of cytochrome c and the activation of caspase-3,22 which is regulated by at least 2 pathways: one is the mitochondria disruption-mediated stress pathway associated with the release of cytochrome c from the mitochondria into the cytosol, inducing activation of caspase-9 and caspase-3, and the other is the death receptor–mediated pathway, such as the Fas/Fas ligand system, which leads to caspase-8 and then caspase-3 activation.23 A certain substrate, such as poly-(ADP-ribose) polymerase, is cleaved by activated caspase-3, resulting in the induction of apoptosis associated with DNA fragmentation. However, we demonstrate that local overexpression of Bcl-2 did not affect superoxide production during ischemia-reperfusion injury. On the other hand, local overexpression of Bcl-2 in cardiac allografts led to significant reductions of cytochrome c release from mitochondria and diminished caspase-3, and -9 activities in cardiac allografts. These observations suggest that antiapoptotic effects of Bcl-2 in local grafts occur downstream of superoxide, suppressing cytochrome c–dependent caspase-9 apoptosis during ischemia-reperfusion injury. Another apoptotic pathway involving caspase-8 was not affected by the local expression of Bcl-2, and Fas expression, which is upstream of caspase-8, did not differ in heart allografts. Because ischemia-reperfusion injury is mediated by alloantigen-independent effectors such as neutrophils and macrophages, local overexpression of Bcl-2 in cardiac allografts contributes to the protection of the cardiomyocyte from apoptosis through inhibition of the mitochondria disruption–mediated, stress-dependent cell death pathway rather than the Fas-mediated cell death pathway. In support of this notion, other investigators have also reported that Bcl-2 inhibits the mitochondria disruption–mediated stress-dependent pathway.24,25 Furthermore, we showed that local overexpression of Bcl-2 leads to decreased production of TNF-α and MCP-1/CCL2 and to decreased MPO activity, suggesting that the infiltration of neutrophils and macrophages into allografts is also reduced during ischemia-reperfusion injury. Moreover, Fas ligand expression was very low in allografts (data not shown), suggesting that allospecific Fas ligand–expressing CD8+ cells were not involved in ischemia-reperfusion injury. These results suggest that the antiapoptotic and anti-inflammatory effects of Bcl-2 are beneficial blockades to the development of ischemia-reperfusion injury in allografts.
In the acute phase of rejection, we observed that the overexpression of Bcl-2 in cardiomyocytes did not prolong allograft survival compared with the WT group. However, in the acute and chronic phases of rejection, which are considered to be mediated by an alloantigen-specific immune response, we observed that Bcl-2 overexpression in cardiomyocytes resulted in reduced cardiomyocyte apoptosis. Classically, apoptosis is regarded as a type of cell death that does not induce inflammation and immune response; however, apoptotic cells have been shown to induce inflammation by the release of inflammatory cytokines15 and to induce immune response by the direct activation of dendritic cells.26 During ischemia-reperfusion injury, overexpression of Bcl-2 in cardiomyocytes protects cardiomyocytes from apoptotic death and inhibits local inflammation. Subsequently, these effects may also result in the potential decrease of graft immunogenicity, leading to reduced alloantigen-specific acquired immune responses and reduced development of GCAD.
Overexpression of Bcl-2 in cardiac allografts did not affect alloantigen-specific splenic T-cell responses, such as mixed lymphocyte reaction and cytokine production, suggesting that local overexpression of Bcl-2 modulates the local immune response in heart allografts rather than inducing alloantigen-specific effector/memory cells in spleen. Indeed, cells expressing Bcl-2 have been shown to block cytotoxicity mediated by allospecific cytotoxic T lymphocytes, thereby becoming resistant to cytotoxic effects mediated by perforin and granzymes.27 In support of this notion, we showed that the overexpression of Bcl-2 in cardiomyocytes in allografts led to decreased infiltration of CD8+ cells, which are major sources of IFN-γ, perforin, and granzymes, during acute and chronic rejection.28 IFN-γ directly affects vascular remodeling and intimal proliferation in the absence of immunocytes and is crucial for allograft rejection by activating macrophages after the production of IP-10/CXCL10 and MIG/CXCL9.28-31 IP-10/CXCL10 and MIG/CXCL9, which were ly identified as IFN-γ–inducible chemokines, contribute to acute rejection by inducing the chemoattraction of CXCR3+ cells, including CD8+ T cells.32-34 Thus, CD8+ T cell–derived IFN-γ induces the production of IP-10/CXCL10 and MIG/CXCL9 by macrophages. Subsequently, macrophage-derived IP-10/CXCL10 and MIG/CXCL9 induce additional CD8+ T-cell infiltration in cardiac allografts. Consistent with the decrease of CD8+ T-cell infiltration, Bcl-2 overexpression in cardiomyocytes also led to reduced IFN-γ, IP-10/CXCL10, and MIG/CXCL9 production in cardiac allografts.
ICAM-1 and VCAM-1, which are induced by TNF-α and IFN-γ,35 are also important for cell infiltration and adhesion to local inflammatory sites, and treatment with anti–ICAM-1 or anti–VCAM-1 antibody has an inhibitory effect on acute and chronic rejection.36-38 Consistent with the decrease of TNF-α and IFN-γ production in cardiac allografts, local overexpression of Bcl-2 resulted in the reduced expression of ICAM-1 and VCAM-1. In addition, Huber et al39 recently reported that increased Bcl-2 expression on TH2 cells led to the selective survival of TH2 cells in the mouse viral myocarditis model. In contrast, we show that the local overexpression of Bcl-2 in cardiac allografts has a significant influence on TH1-type immune responses but not on TH2-type immune responses.
In conclusion, we demonstrate that Bcl-2 overexpression in cardiomyocytes of transplanted allografts suppresses ischemia-reperfusion injury and limits local immune responses such as inflammatory cell infiltration and proinflammatory mediator production without affecting the host immune response during acute and chronic rejection and results in less GCAD in murine cardiac allografts. These findings may contribute to the development of a novel therapy aimed at improving the survival of cardiac allografts by modulating Bcl-2 expression through pharmacologic up-regulation or gene transfer.
Prepublished online as Blood First Edition Paper, July 27, 2004; DOI 10.1182/blood-2004-02-0666.
Supported by National Institutes of Health grant HL65669 (R.C.R.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.