Abstract
The immune system contains natural regulatory T cells that control the magnitude of the immune response during physiologic and pathologic conditions. Although this suppressive function was historically attributed to CD8 T cells, most recent reports have focused on natural regulatory CD4 T cells. In the present study, we describe a new subset of natural CD8 regulatory T cells in normal healthy animals. This subset expresses low levels of CD45RC at its surface (CD45RClow); produces mainly interleukin-4 (IL-4), IL-10, and IL-13 cytokines upon in vitro stimulation; expresses Foxp3 and cytotoxic T-lymphocyte-associated antigen 4 (CTLA-4); and is not cytotoxic against allogeneic targets. This subset suppresses the proliferation and differentiation of autologous CD4 T cells into type-1 cytokines producing T cells after stimulation with allogeneic accessory cells. We also provide evidence that this regulatory subset mediates its suppression by cell-to-cell contact and not through secretion of suppressive cytokines. Finally, the regulatory activity of CD8 CD45RClow cells is also demonstrated in vivo in a rat model of CD4-dependent graft-versus-host disease. Collectively, these data demonstrate for the first time that freshly isolated rat CD8 CD45RClow T cells contain T cells with regulatory properties, a result that enlarges the general picture of T-cell-mediated regulation. (Blood. 2004;104:3294-3301)
Introduction
The delicate balance between pathogen-induced effector immunologic functions and natural self-tolerance mechanisms is of vital importance for preserving the integrity of a host in the course of an immune response. In different species of rodents and in humans, there is compelling evidence that the regulation of the magnitude of protective immunity to foreign antigens as well as the control of autoaggressive immune reactions are ensured by regulatory CD4 and CD8 T lymphocytes that display anti-inflammatory and antiproliferative functions. Convergent evidence indicates that multiple subtypes of regulatory T cells exist and that their regulatory activities are mediated either by immunosuppressive cytokines or by contact-dependent mechanisms.1-8
CD45 is a transmembrane tyrosine phosphatase expressed as isoforms of different molecular weight, which result in the differential splicing of 3 exons (A, B, and C) encoding part of the N-terminal extracellular domain. In the rat, CD45RC expression levels define 2 subpopulations of CD4 T cells with different cytokine profiles and functions.3,9-12 Functional analyses of CD45RChigh and CD45RClow CD4 T cells have demonstrated that important regulatory interactions occur between these subsets in vivo.11,13,14 For example, the adoptive transfer of CD45RChigh CD4 T cells from congenic euthymic donors to nude rats induces a fatal wasting disease, while the transfer of both subpopulations, or of the CD45RClow cells alone, has no effect.11 Similar results were obtained using mouse CD4 T cells fractionated on the basis of CD45RB expression.15 It has also been demonstrated that a subpopulation of CD4 T cells from syngeneic healthy donors that constitutively express the CD25 (interleukin-2 receptor α [IL-2Rα]) molecule can prevent the development of autoimmunity upon transfer.1,2,16-19 These results demonstrate that the immune system from healthy animals contains natural regulatory CD4 T cells that actively suppress autoreactivity. In animal models, several studies have shown that regulatory CD4 T cells, besides their pivotal role in maintaining self-tolerance, also regulate homeostasis of the peripheral T-cell pool,20 contribute to tolerance induction after solid organ transplantation,21,22 and promote tolerance to allogeneic bone marrow grafts.23,24 These findings have opened new prospects for immunotherapeutic interventions. One phenotypic marker of natural CD4 regulatory T cells is the constitutive expression of CD25. However, regulatory CD4 T cells also reside in the CD25-CD45RB/RClow compartment.25
CD8 T cells play important roles, either as effector or regulatory T cells, in several immunopathologic manifestations such as autoimmune diseases,26-29 transplantation,30,31 and protection of the host against infectious diseases and cancer.31,32 Several studies have demonstrated that the manipulation of the immune system in vitro or in vivo can lead to the generation of CD8 regulatory T cells. These CD8 regulatory cells act through the production of suppressor cytokines (transforming growth factor β [TGFβ] or IL-10)28,33 or by inactivating dendritic cells.6 Despite the long-held notion that CD8 T cells could mediate suppression, most recent reports have focused on the regulatory properties of CD4 T-cell subsets and only few studies have addressed this point for CD8 T cells.34-36 This was mainly related to the absence of markers allowing the identification of natural regulatory CD8 T cells.
In the present study, we investigated if the level of CD45RC expression could represent a marker to identify a subset of natural regulatory CD8 T cells in normal animals. We demonstrate that freshly isolated rat CD8 CD45RClow T cells contain cells with regulatory properties. These cells are not cytotoxic, produce mainly type 2 cytokines, express selectively Foxp3 and cytotoxic T-lymphocyte-associated antigen 4 (CTLA-4), and suppress alloreactive immune responses both in vitro and in vivo. The regulatory functions and mechanisms of action of these regulatory CD8 T cells are related to an inhibition of the expansion of effector CD4 T helper 1 (Th1) cells. Finally, we provide evidence that this regulation is not cytokine mediated but instead requires cell-to-cell interaction.
Materials and methods
Animals
Lewis (LEW), Brown-Norway (BN), and (LEW × BN) F1 male rats (all 8 to 10 weeks old) were used in this study. These animals were obtained from Centre d'Elevage R. Janvier (Le Genest St Isle, France) and maintained in our animal house facility. All experimental procedures were carried out in accordance with European Union guidelines.
Antibodies
The monoclonal antibodies (mAbs) used for flow cytometry and for the purification of T-cell subpopulations were as follows: W3/25 (anti-rat CD4), OX6 (anti-rat major histocompatibility complex [MHC] class II), OX8 (anti-rat CD8), OX12 (anti-rat kappa light chain), OX18 (anti-rat MHC class I), OX21 (anti-human C3b inactivator), OX22 (anti-rat CD45RC), OX39 (anti-rat CD25), R73 (anti-rat T-cell receptor αβ [TCRαβ]), V65 (anti-rat TCRγδ), JJ319 (anti-rat CD28), and 3.2.3 (anti-rat natural killer cell receptor protein 1 [NKR-P1]). For cytokine neutralization we used mAbs OX81 (anti-rat IL-4) and 2G7 (which recognizes both active and latent forms of TGFβ1, TGFβ2, and TGFβ3), and rabbit anti-rat IL-10 (kindly provided by Dr J. Khalife, Lille, France). The hybridomas OX6, OX8, OX12, OX18, OX21, OX22, OX39, OX81, and W3/25 were kindly provided by Dr D. Mason (Oxford, United Kingdom); the hybridomas JJ319, V65, and R73, by Dr T. Hünig (Würzburg, Germany); and the hybridoma 2G7, by Dr C. Melief (Leiden, the Netherlands). The mouse IgG1 (OX21) and mouse IgG2b (Y3) were used as isotype control mAbs. The hybridoma Y3 (HB176) was obtained from the American Type Culture Collection (Manassas, VA).
Isolation of T cells, CD4 T cells, CD8 T cells, and CD45RC CD4 and CD8 T-cell subsets
Rat CD8 T cells were negatively selected from lymph nodes and spleen cells using a cocktail of the following mAbs: OX6, W3/25, OX12, 3.2.3, and V65. After washing and incubating with anti-mouse IgG magnetic microbeads (DYNAL, Oslo, Norway), CD8 T cells were purified by magnetic depletion. A similar technique was applied for the purification of CD4 T cells with the replacement of W3/25 mAb by OX8 mAb in the cocktail. The CD45RChigh and CD45RClow CD8 and CD4 T-cell subpopulations were obtained by positive and negative selection using magnetic beads. Purified CD8 or CD4 T cells were incubated with OX22-fluorescein isothiocyanate (FITC) and R73-phycoerythrin (PE) mAbs and selected using anti-FITC and anti-PE magnetic beads (AutoMacs; Miltenyi Biotec, Bergisch Gladbach, Germany). A similar procedure was applied for the purification of the CD25+ and CD25- CD4 T-cell subpopulations, using OX39-FITC and anti-FITC magnetic beads. Purity of the sorted populations was always higher than 95%.
Real-time quantitative reverse-transcription-polymerase chain reaction (RT-PCR)
Total RNA was extracted from freshly purified CD8 and CD4 T-cell subsets using Promega RNA isolation kit (Promega, Madison, WI). The cDNA was reverse transcribed as described.9 Transcript levels of Foxp3, cytotoxic T-lymphocyte-associated antigen 4 (CTLA-4), glucocorticoid-induced tumor necrosis factor receptor (GITR), and hypoxanthine phosphoribosyltransferase (HPRT) were quantified using real-time quantitative PCR and SYBR Green DNA dye (ABI Prism 7000; Perkin-Elmer Applied Biosystems, Foster City, CA). Primer sequences were as follows: Foxp3: forward, 5′-CCCAGGAAAGACAGCAACCTT-3′ and reverse, 5′-CTGCTTGGCAGTGCTTGAGAA-3′; CTLA-4: forward, 5′-GGACTGAGGGCTGCTGACAC-3′ and reverse, 5′-GGCATGGTTCTGGATCGATG-3′; GITR: forward, 5′-GCAGACTTTGGACCAACTGTTC-3′ and reverse, 5′-AGCGGCTGGGTATTGACCT-3′; and HPRT: forward, 5′-TGTTGGATACAGGCCAGACTTTGT-3′ and reverse, 5′-TCCACTTTCGCTGATGACACA-3′. Results were expressed as 2-ΔΔCT × 100 where the ΔΔCT is the difference between ΔCT of the sample and the ΔCT of the reference consisting of total CD8 or total CD4 T cells for the analysis of CD8 and CD4 T-cell subsets, respectively. ΔCT is the difference between the CT of the target gene and the HPRT gene.
T-cell stimulation and analysis of T-cell proliferation
The culture medium was RPMI 1640 (Gibco Life Technologies, Cergy Pontoise, France) containing 10% fetal calf serum (FCS), 1% sodium pyruvate, 1% nonessential amino acids, 1% l-glutamine, 1% penicillin-streptomycin, and 2 × 10-5 M 2-mercaptoethanol. Highly purified CD8 T cells and CD45RC CD8 T-cell subsets were polyclonally stimulated using anti-TCR and anti-CD28 mAbs, or specifically stimulated with alloantigens in a mixed lymphocyte reaction (MLR). For polyclonal activation, 105 T cells were incubated with plate-bound anti-TCR mAb and soluble anti-CD28 mAb as previously described.37 For MLR, stimulation was performed by incubating fractionated CD45RC CD8 T-cell subsets isolated from LEW rats (106 cells/well) with 5 × 105 T-cell-depleted and irradiated (25 grays) splenocytes from semiallogeneic ((LEW × BN) F1) or fully allogeneic (BN) rats in the presence of rat IL-2 (10 U/mL). For coculture experiments, CD4 CD45RChigh T cells isolated from LEW rats (106 cells/well) were stimulated with allogeneic, T-cell-depleted and irradiated BN spleen cells (5 × 105 cells/well) and variable numbers of LEW CD8 CD45RClow or CD8 CD45RChigh T cells. We decided to use purified CD45RChigh CD4 T cells rather than unfractionated CD4 T cells in order to avoid the intervention of regulatory CD4 T cells that are confined within the CD45RClow CD4 T-cell subset. To facilitate the reading, these CD4 CD45RChigh T cells will now be designated naive CD4 T cells. Transwell experiments were carried out in 24-well plates with naive CD4 T cells and allogeneic antigen-presenting cells (APCs) in the presence or absence of CD8 CD45RClow T cells and allogeneic APCs on the top of the Transwell. Proliferation was measured by the degree of (3H)-thymidine uptake during the last 18 hours of a 48-hour (for stimulation with anti-TCR and anti-CD28) or a 96-hour (for MLR) culture period. Results were expressed as mean counts/min of triplicate cultures. In some experiments, the proliferation of CD4 T cells was analyzed by measuring the cytoplasmic dilution of the carboxyfluorescein succinimidyl ester (CFSE) dye (Molecular Probes, Eugene, OR). Briefly, naive CD4 T cells were labeled with 4 μM CFSE in phosphate-buffered saline (PBS) containing 5% FCS for 10 minutes at room temperature. The unconjugated CFSE was eliminated by washing the cells with PBS-FCS 5%. The cells were stimulated with allogeneic APC and assessed for proliferation by flow cytometry at different time points. After gating for viable TCRαβ+ CD4+ cells, CFSE intensities were analyzed.
Cytokine analysis
At various times throughout the culture (36 and 60 hours for anti-TCR stimulation; 72, 96, and 120 hours for MLR), supernatants were removed and stored at -20°C for cytokine determination; cells were harvested and the RNA was purified for analysis of lymphokine gene expression by reverse transcription-PCR; interferon γ (IFNγ), IL-2, and IL-10 protein levels in the supernatant were measured by specific enzyme-linked immunosorbent assay (ELISA) as described.38 Transcript levels of IL-4, IL-13, and HPRT were quantified using real-time quantitative PCR and SYBR Green DNA dye as described.38 Results were expressed as the intrasample ratio of cytokine mRNA to HPRT mRNA copy numbers. Cytokine levels were also assessed by intracytoplasmic staining as described.39 Briefly, CD4 T cells were stimulated with allogeneic APCs in the presence or absence of autologous CD8 CD45RC cells for 5 days. Thereafter, T cells were labeled for membrane expression of TCR and CD4 and for intracytoplasmic production of either IFNγ, IL-4, or IL-10, using DB1-FITC, OX81-PE, and A5-6-PE mAbs, respectively (PharMingen, San Diego, CA).
In vitro cytotoxic responses
LEW CD8 T cells or fractionated CD45RChigh and CD45RClow CD8 T cells were differentiated into effector cells by in vitro stimulation with allogeneic APCs (BN rats) and exogenous rat IL-2 (10 U/mL) for a period of 4 days. In some experiments, the exogenous IL-2 was replaced by addition of autologous naive CD4 T cells. The target cells were fibroblasts derived from BN rats. Target cells were labeled with 0.1 mCi (3.7 × 106 Bq) 51Cr at 37°C for 2 hours, washed 3 times with medium, and incubated with effector cells at various effector-to-target (E/T) ratios, in a final volume of 200 μL/well. Some tests were performed in the presence of OX18, a blocking mAb directed against MHC class I determinant. After 6 hours of incubation, 50 μL of the supernatant was collected and radioactivity was counted in a gamma counter. The percent of specific 51Cr release was calculated according to the formula: 100 × (test release - spontaneous release)/(maximal release - spontaneous release), where test release, spontaneous release, and maximal release represent the release in the presence of effector cells, in the presence of medium alone, and in the presence of 10% bleach, respectively.
Induction and clinical analysis of acute graft-versus-host disease (GvHD)
Recipient (LEW × BN) F1 rats were irradiated at a dose of 4.5 grays (Cesium 137 source) 24 hours prior to intravenous injection of parental CD8 CD45RC T-cell subsets, CD4 T cells, or combinations of both populations. Control rats were either injected intravenously with syngeneic F1 T cells or with PBS. Survival and body weight were monitored daily after T-cell transfer.
Statistical analysis
Results are expressed as the mean ± SD, and overall differences between variables were evaluated by Mann-Whitney U test. When indicated, analysis of variance (ANOVA) or χ2 test was also used.
Results
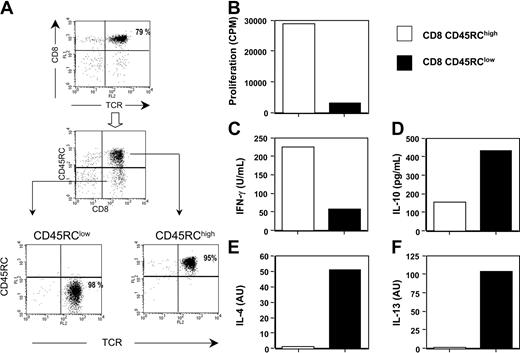
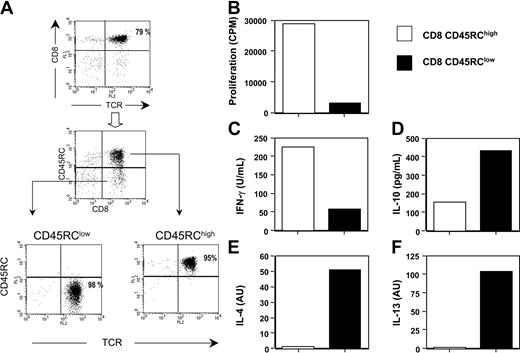
The level of CD45RC expression distinguishes 2 rat CD8 T-cell subsets with different cytokine profiles. Analysis of the spleen and lymph node cells of normal LEW rats by flow cytometry revealed that CD8 T cells could be divided into 2 subsets, according to their level of expression of the CD45RC molecule: CD45RChigh and CD45RClow. The CD45RClow CD8 population typically represented 20 ± 3% of CD8 T cells, while the CD45RChigh subset represented 80 ± 3%. For subsequent analyses, CD45RChigh and CD45RClow CD8 T cells were purified from LEW rat splenocytes in a 2-step procedure, combining positive and negative selection methods with immunomagnetic beads. Purified CD8 subpopulations routinely exhibited a purity of 95% to 98% (Figure 1A).
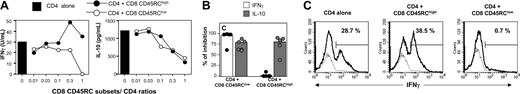
CD45RChigh and CD45RClow CD8 T cells produce different cytokine profiles. (A) CD8 T cells were purified from spleen and lymph nodes of naive LEW rats after depletion of CD4+, TCRγδ T cells, B cells, monocytes, and natural killer (NK) cells. The purified CD8 T cells (top panel) were further fractionated by anti-FITC and anti-PE magnetic beads on the basis of their expression of CD45RC using FITC-conjugated OX22 and of their expression of TCR using PE-conjugated R73. The values represent the purity of each cell population. (B-F) Highly purified CD45RChigh and CD45RClow CD8 T cells (5 × 105/well) from LEW rats were stimulated in vitro in mixed lymphocyte reaction using irradiated, T-cell-depleted, allogeneic BN spleen cells (2.5 × 105 cells/well) as stimulators in the presence of exogenous rat IL-2 (10 U/mL). Proliferation was assessed with an 18-hour (3H)-thymidine pulse added after 96 hours of culture. Tissue culture supernatants collected 96 hours after stimulation were assayed for IFNγ (C) and IL-10 (D) protein levels using capture ELISA. IL-4 (E) and IL-13 (F) mRNA expression were assayed by quantitative RT-PCR following stimulation for 72 hours. Results are expressed as arbitrary units (AU), representing the ratio between cytokine and HPRT mRNA × 100. The data shown represent the quantification made from a pool of triplicate cultures and are representative of 3 independent experiments.
CD45RChigh and CD45RClow CD8 T cells produce different cytokine profiles. (A) CD8 T cells were purified from spleen and lymph nodes of naive LEW rats after depletion of CD4+, TCRγδ T cells, B cells, monocytes, and natural killer (NK) cells. The purified CD8 T cells (top panel) were further fractionated by anti-FITC and anti-PE magnetic beads on the basis of their expression of CD45RC using FITC-conjugated OX22 and of their expression of TCR using PE-conjugated R73. The values represent the purity of each cell population. (B-F) Highly purified CD45RChigh and CD45RClow CD8 T cells (5 × 105/well) from LEW rats were stimulated in vitro in mixed lymphocyte reaction using irradiated, T-cell-depleted, allogeneic BN spleen cells (2.5 × 105 cells/well) as stimulators in the presence of exogenous rat IL-2 (10 U/mL). Proliferation was assessed with an 18-hour (3H)-thymidine pulse added after 96 hours of culture. Tissue culture supernatants collected 96 hours after stimulation were assayed for IFNγ (C) and IL-10 (D) protein levels using capture ELISA. IL-4 (E) and IL-13 (F) mRNA expression were assayed by quantitative RT-PCR following stimulation for 72 hours. Results are expressed as arbitrary units (AU), representing the ratio between cytokine and HPRT mRNA × 100. The data shown represent the quantification made from a pool of triplicate cultures and are representative of 3 independent experiments.
Highly purified LEW CD45RChigh and CD45RClow CD8 T cells were stimulated in vitro with bound anti-TCR and soluble anti-CD28 mAbs. Under these conditions, both T-cell subpopulations proliferated equally well but produced different cytokine profiles (Table 1). The anti-inflammatory cytokines IL-4, IL-10, and IL-13 were mainly produced by the CD45RClow CD8 T-cell subset. On the other hand, the inflammatory cytokines IL-2 and IFNγ were produced by both subsets, but the CD45RChigh CD8 T-cell population produced more IL-2 and more or similar levels of IFNγ depending on the experiment (Table 1), when compared with the CD8 CD45RClow subset. We also assessed the proliferative responses and cytokine profiles of these T-cell subsets after allogeneic stimulation, using irradiated T-cell-depleted BN spleen cells as a source of APCs and exogenous rat IL-2. As shown in Figure 1B, CD45RClow CD8 T cells did not proliferate in response to this allogeneic stimulation, whereas under the same experimental conditions, CD45RChigh CD8 T cells proliferated strongly. In addition, CD45RChigh CD8 T cells produced higher amounts of IFNγ (Figure 1C), but lower amounts of IL-10 (Figure 1D) and lower levels of IL-4 and IL-13 mRNA (Figure 1E-F) than CD45RClow CD8 T cells. Altogether, these data show that CD45RC expression divides CD8 T cells into 2 subsets with different cytokine profiles.
CD45RChigh and CD45RClow CD8 T cells exhibit different cytotoxic responses
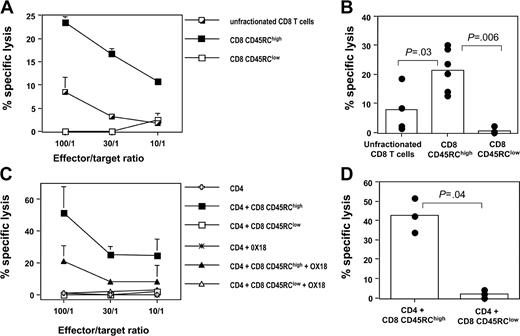
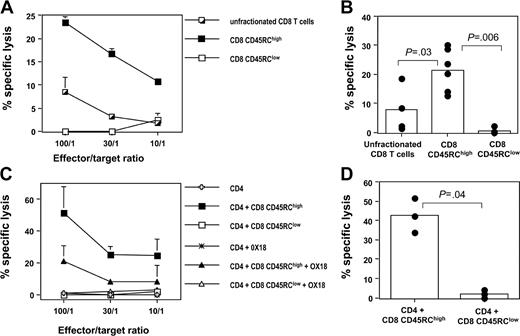
We next evaluated the cytotoxic capacity of these 2 CD8 T-cell subsets, using BN fibroblasts as targets. In a first set of experiments, CD8 CD45RC T-cell subsets isolated from LEW rats were tested after allogeneic stimulation, using irradiated T-cell-depleted BN or (LEW × BN) F1 spleen cells as a source of alloantigens and exogenous rat IL-2. As shown in Figure 2A-B, CD45RChigh CD8 T cells were cytotoxic against allogeneic targets, whereas CD45RClow CD8 T cells were not (in 6 different experiments at an E/T ratio of 100:1, the specific lysis was 21.5 ± 7.3% for CD8 CD45RChigh T cells versus 0.5 ± 1% for CD8 CD45RClow T cells, P = .0062). Similar results were obtained when CD8 T-cell subsets were stimulated in the presence of autologous CD4 T cells: in 3 different experiments at an E/T ratio of 100:1, the specific lysis was 42.5 ± 8.9% for CD8 CD45RChigh T cells versus 2 ± 2.1% for CD8 CD45RClow T cells (P = .049). These findings indicate that the inability of CD8 CD45RClow T cells to kill allogeneic targets is CD4-help independent (Figure 2C-D). We also demonstrated that this cytotoxic response was MHC class I dependent, since it was inhibited by the addition of anti-MHC class I mAb (Figure 2C and data not shown). Finally, we also showed that LEW CD4 T cells did not exhibit any cytotoxic response after allogeneic stimulation, using similar condition as for the CD8 T-cell subsets (Figure 2C). Altogether, these data show that cytotoxic responses mediated by alloreactive CD8 T cells are confined within the CD8 CD45RChigh subset.
The alloreactive cytotoxic response mediated by LEW CD8 T cells is mainly confined to the CD45RChigh CD8 T-cell subset. Purified CD8 CD45RC subsets (106 cells/mL) from LEW rats were differentiated into effector cells by in vitro stimulation with allogeneic BN or (LEW × BN) F1 splenocytes (5 × 105/mL). Differentiation was carried out for 4 days in the presence of either exogenous IL-2 (A-B) or autologous CD4 T cells (C-D). Thereafter, effector cells were tested for their ability to kill 51Cr-labeled BN fibroblasts as described in “Materials and methods.” The contribution of MHC class I molecules in this cytotoxic response was tested by adding OX18, an anti-rat MHC class I mAb. (A, C) Results were expressed as means ± SD of the percentage of specific lysis at graded effector-target ratios. (B, D) Results were represented as the percentage of specific lysis at an effector-target ratio of 100:1. Bars represent the mean values of 6 (B) or 3 (D) individual experiments, and filled circles represent the results of each individual experiment. The statistical results of group comparisons (Mann-Whitney U test) are indicated above with horizontal lines.
The alloreactive cytotoxic response mediated by LEW CD8 T cells is mainly confined to the CD45RChigh CD8 T-cell subset. Purified CD8 CD45RC subsets (106 cells/mL) from LEW rats were differentiated into effector cells by in vitro stimulation with allogeneic BN or (LEW × BN) F1 splenocytes (5 × 105/mL). Differentiation was carried out for 4 days in the presence of either exogenous IL-2 (A-B) or autologous CD4 T cells (C-D). Thereafter, effector cells were tested for their ability to kill 51Cr-labeled BN fibroblasts as described in “Materials and methods.” The contribution of MHC class I molecules in this cytotoxic response was tested by adding OX18, an anti-rat MHC class I mAb. (A, C) Results were expressed as means ± SD of the percentage of specific lysis at graded effector-target ratios. (B, D) Results were represented as the percentage of specific lysis at an effector-target ratio of 100:1. Bars represent the mean values of 6 (B) or 3 (D) individual experiments, and filled circles represent the results of each individual experiment. The statistical results of group comparisons (Mann-Whitney U test) are indicated above with horizontal lines.
Freshly isolated CD8 CD45RClow T cells suppress the proliferation of alloreactive CD4 T cells in vitro
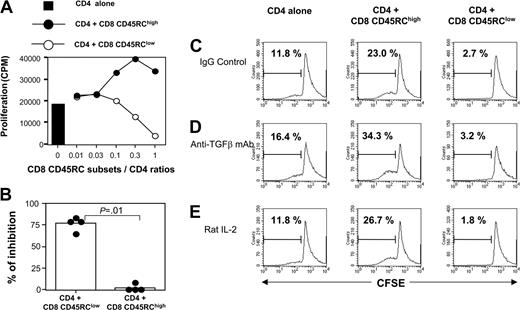
Using coculture experiments, we evaluated the suppressive effect of CD45RC CD8 T-cell subsets on the proliferative response of syngeneic CD4 T cells after allogeneic stimulation. As shown in Figure 3A, CD8 CD45RClow T cells from LEW rats inhibited in a dose-dependent fashion the proliferation of LEW CD4 T cells stimulated with semiallogeneic, T-cell-depleted spleen cells. In contrast, the CD8 CD45RChigh subset did not exhibit any suppressive effect. In 4 separate experiments using a suppressor/responder ratio of 1:1, we observed a significant suppression of the response to alloantigen in the presence of CD45RClow CD8 T cells, but not in the presence of CD45RChigh CD8 T cells (Figure 3B). Similar results were obtained when CD4 T-cell proliferation was specifically assessed by CFSE dilution (Figure 3C). In 3 different experiments of allogeneic stimulation, we showed that the proportion of LEW CD4 T cells with reduced CFSE intensity was significantly lower in the presence of CD45RClow CD8 T cells (5 ± 4%, P = .04) when compared with that obtained when CD4 T cells were stimulated alone (25 ± 17%), or in the presence of CD45RChigh CD8 T cells (28 ± 18%).
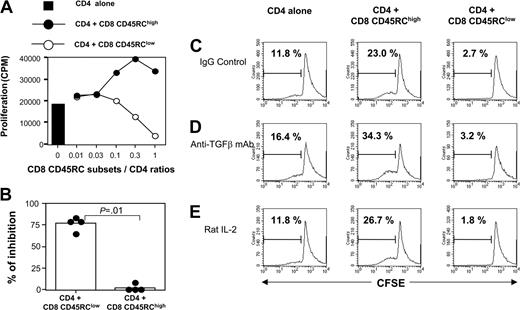
CD45RClow CD8 T cells suppress the proliferation of alloreactive CD4 T cells. (A) Purified naive CD4 T cells from LEW rats (5 × 105 cells/well) were stimulated for 5 days with irradiated, semiallogeneic, T-cell-depleted F1 splenocytes (2.5 × 105) either without (▪) or with variable numbers of LEW CD45RChigh (•) or CD45RClow (○) CD8 T cells. Proliferation was assessed with an 18-hour (3H)-thymidine pulse added after 4 days of culture. Similar results were obtained in 4 independent experiments. (B) Results were expressed as the percentage of inhibition of proliferation at a CD4/CD8CD45RC ratio of 1:1, considering that the proliferation of CD4 T cells without CD8 T cells is set as 100%. Bars represent the mean values of 4 individual experiments, and filled circles represent the results of each individual experiment. (C-E) CFSE-labeled naive CD4 T cells from LEW rats were stimulated for 5 days with irradiated semiallogeneic T-cell-depleted F1 splenocytes. Stimulation took place without (left panels) or with either LEW CD45RChigh (middle panels) or CD45RClow (right panels) CD8 T cells added at a 1:1 ratio. Isotype control (C) or anti-TGFβ (D) mAbs (50 μg/mL) or exogenous IL-2 (E) (10 U/mL) was added on day 0. The division of CD4 T cells was assessed by flow cytometry by gating on CD4 T cells. The percentage in each plot represents dividing CD4 T cells. These data are representative of 3 independent experiments.
CD45RClow CD8 T cells suppress the proliferation of alloreactive CD4 T cells. (A) Purified naive CD4 T cells from LEW rats (5 × 105 cells/well) were stimulated for 5 days with irradiated, semiallogeneic, T-cell-depleted F1 splenocytes (2.5 × 105) either without (▪) or with variable numbers of LEW CD45RChigh (•) or CD45RClow (○) CD8 T cells. Proliferation was assessed with an 18-hour (3H)-thymidine pulse added after 4 days of culture. Similar results were obtained in 4 independent experiments. (B) Results were expressed as the percentage of inhibition of proliferation at a CD4/CD8CD45RC ratio of 1:1, considering that the proliferation of CD4 T cells without CD8 T cells is set as 100%. Bars represent the mean values of 4 individual experiments, and filled circles represent the results of each individual experiment. (C-E) CFSE-labeled naive CD4 T cells from LEW rats were stimulated for 5 days with irradiated semiallogeneic T-cell-depleted F1 splenocytes. Stimulation took place without (left panels) or with either LEW CD45RChigh (middle panels) or CD45RClow (right panels) CD8 T cells added at a 1:1 ratio. Isotype control (C) or anti-TGFβ (D) mAbs (50 μg/mL) or exogenous IL-2 (E) (10 U/mL) was added on day 0. The division of CD4 T cells was assessed by flow cytometry by gating on CD4 T cells. The percentage in each plot represents dividing CD4 T cells. These data are representative of 3 independent experiments.
The suppressive effect of CD8 CD45RClow cells is cytokine independent and requires cell-to-cell contact
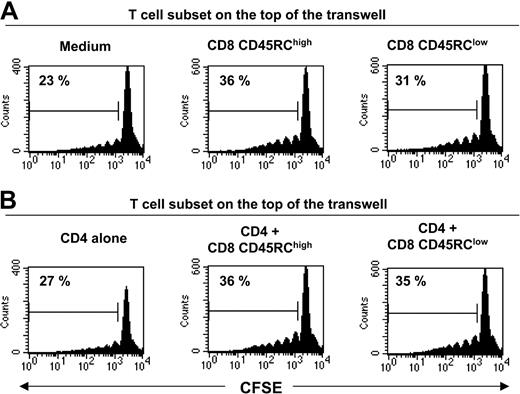
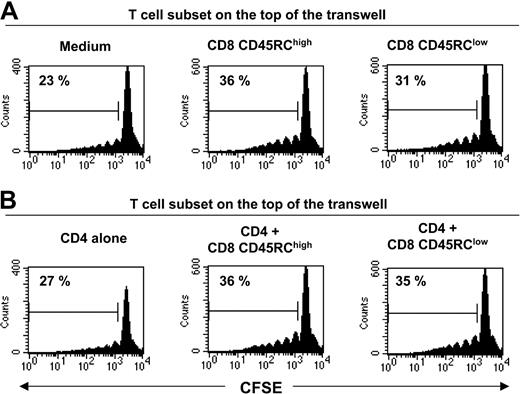
To investigate the molecular bases of this suppression, we first examined the possible role of immunosuppressive cytokines such as IL-4, IL-10, and TGFβ. Addition of a neutralizing antibody to TGFβ (Figure 3D) at appropriate concentrations was unable to abrogate the suppression (the inhibition in 2 different experiments was 77% and 83% in the absence of 2G7; 88% and 66% in the presence of 2G7). Similar results were obtained with antibodies neutralizing either rat IL-4 or rat IL-10 (data not shown). We also showed that the addition of 10 U/mL exogenous rat IL-2 did not overcome the suppressive effect mediated by CD8 CD45RClow (Figure 3E). To assess the potential contribution of unknown suppressive soluble factor(s), we performed Transwell experiments. As shown in Figure 4, CD45RClow CD8 T cells from LEW rats failed to suppress the proliferation of alloreactive CD4 T cells across a 0.45-μM pore size membrane, after allogeneic stimulation without (Figure 4A) or with (Figure 4B) LEW CD4 T cells. This contrasted with the marked suppression that we observed when the 2 populations were in the same side of the membrane. Collectively, these studies demonstrate that CD8 CD45RClow cells do not mediate their suppressive effect by secreting soluble suppressor factors or by sequestering IL-2, but that cell contact is required.
Soluble factors do not play a significant role in the suppression mediated by CD45RClow CD8 T cells. Transwell culture experiments. CFSE-labeled CD4 T cells from LEW rats (5 × 105 cells/well) were placed in the bottom well. They were separated by a 0.45-μM pore size membrane from an equal number of either LEW CD8 CD45RClow, CD8 CD45RChigh, and CD4 T cells or cocultures of CD4 T cells with either CD8 CD45RClow or CD8 CD45RChigh T cells (top well). Cells were stimulated with irradiated, T-cell depleted, BN splenocytes for 5 days. CD4 T-cell division was assessed by flow cytometry by gating on CD4 T cells. The percentage in each plot represents dividing T cells. Data are representative of 2 independent experiments.
Soluble factors do not play a significant role in the suppression mediated by CD45RClow CD8 T cells. Transwell culture experiments. CFSE-labeled CD4 T cells from LEW rats (5 × 105 cells/well) were placed in the bottom well. They were separated by a 0.45-μM pore size membrane from an equal number of either LEW CD8 CD45RClow, CD8 CD45RChigh, and CD4 T cells or cocultures of CD4 T cells with either CD8 CD45RClow or CD8 CD45RChigh T cells (top well). Cells were stimulated with irradiated, T-cell depleted, BN splenocytes for 5 days. CD4 T-cell division was assessed by flow cytometry by gating on CD4 T cells. The percentage in each plot represents dividing T cells. Data are representative of 2 independent experiments.
CD8 CD45RClow cells affect the differentiation of alloreactive CD4 T cells
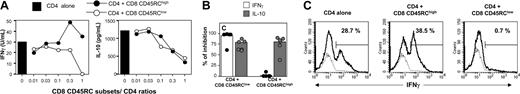
We next assessed whether CD45RC CD8 T-cell subsets could influence the differentiation of syngeneic CD4 T cells after allogeneic stimulation in vitro. When CD4 T cells from LEW rats were stimulated with allogeneic APCs in the presence of variable numbers of LEW CD45RClow CD8 T cells, the production of IFNγ and IL-10 was suppressed in a dose-dependent fashion (Figure 5A-B). In contrast, only IL-10 production was suppressed in the presence of LEW CD45RChigh CD8 T cells (Figure 5A-B). In these coculture experiments, the cytokines produced could originate from both CD4 T cells and CD8 T-cell subsets. To evaluate specifically the CD4 T-cell response, we assessed the proportion of CD4 T cells producing IFNγ, IL-4, and IL-10 by intracytoplasmic cytokine staining (Figure 5C). In 7 different experiments, a large proportion of LEW CD4 T cells stimulated with allogeneic (BN) or semiallogeneic (F1) APCs produced IFNγ (21.9 ± 7.9%). This proportion did not change significantly (P = .48) when CD4 T cells were stimulated in the presence of syngeneic CD8 CD45RChigh T cells (21.2 ± 14.3%), while IL-4 and IL-10 production were undetectable (data not shown). In contrast, when LEW CD4 T cells were stimulated in the presence of syngeneic CD8 CD45RClow T cells, the proportion of IFNγ-producing CD4 T cells was significantly reduced (3 ± 1.1%, P = .001), while IL-4- and IL-10-producing CD4 T cells were still undetectable (data not shown). Together, these studies demonstrate that the CD8 CD45RClow subset inhibits the differentiation of alloreactive CD4 T cells into IFNγ-secreting cells. This effect was not accompanied by an increase of IL-4- or IL-10-producing cells.
CD45RClow CD8 T cells inhibit the differentiation of alloreactive CD4 T cells into IFNγ-producing T cells. (A) Purified CD4 T cells from LEW rats (5 × 105 cells/well) were stimulated with irradiated allogeneic, T-cell-depleted, BN splenocytes (2.5 × 105) either without (▪) or with variable numbers of LEW CD45RChigh (•) or CD45RClow (○) CD8 T cells. Tissue culture supernatants were collected on day 5 after allogeneic stimulation and assayed for IFNγ (left panel) and IL-10 (right panel) titer using capture ELISA. Similar results were obtained in 8 different experiments. (B) Results were expressed as the percentage of inhibition of IFNγ (□) and IL-10 (▦) production at a CD4/CD8 CD45RC subsets ratio of 1:1. The cytokine production by CD4 T cells activated without CD8 T cells was set as 100% of the response. Bars represent the mean values of 7 individual experiments, and filled circles represent the results of each individual experiment. (C) Purified CD4 T cells from LEW rats were stimulated with irradiated allogeneic, T-cell-depleted BN splenocytes, either with or without LEW CD45RChigh or CD45RClow CD8 T cells added at a 1:1 ratio. T-cell blasts were collected after 96 hours of culture and were stimulated with phorbol myristate acetate (PMA) and ionomycin for 4 hours. Brefeldin A was added for the last 2 hours of incubation and staining was performed using anti-CD4 mAb and FITC anti-IFNγ, PE anti-IL-4, or PE anti-IL-10, as indicated in “Materials and methods.” The percentage of IFNγ-producing cells was enumerated after gating on CD4 T cells and is indicated in each plot. The dotted lines represent the isotype control Ab labeling. These data are representative of 3 independent experiments.
CD45RClow CD8 T cells inhibit the differentiation of alloreactive CD4 T cells into IFNγ-producing T cells. (A) Purified CD4 T cells from LEW rats (5 × 105 cells/well) were stimulated with irradiated allogeneic, T-cell-depleted, BN splenocytes (2.5 × 105) either without (▪) or with variable numbers of LEW CD45RChigh (•) or CD45RClow (○) CD8 T cells. Tissue culture supernatants were collected on day 5 after allogeneic stimulation and assayed for IFNγ (left panel) and IL-10 (right panel) titer using capture ELISA. Similar results were obtained in 8 different experiments. (B) Results were expressed as the percentage of inhibition of IFNγ (□) and IL-10 (▦) production at a CD4/CD8 CD45RC subsets ratio of 1:1. The cytokine production by CD4 T cells activated without CD8 T cells was set as 100% of the response. Bars represent the mean values of 7 individual experiments, and filled circles represent the results of each individual experiment. (C) Purified CD4 T cells from LEW rats were stimulated with irradiated allogeneic, T-cell-depleted BN splenocytes, either with or without LEW CD45RChigh or CD45RClow CD8 T cells added at a 1:1 ratio. T-cell blasts were collected after 96 hours of culture and were stimulated with phorbol myristate acetate (PMA) and ionomycin for 4 hours. Brefeldin A was added for the last 2 hours of incubation and staining was performed using anti-CD4 mAb and FITC anti-IFNγ, PE anti-IL-4, or PE anti-IL-10, as indicated in “Materials and methods.” The percentage of IFNγ-producing cells was enumerated after gating on CD4 T cells and is indicated in each plot. The dotted lines represent the isotype control Ab labeling. These data are representative of 3 independent experiments.
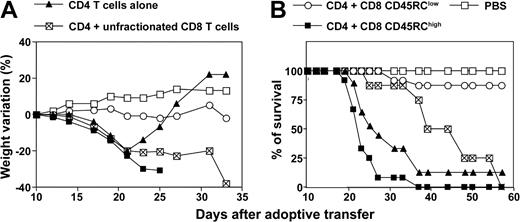
CD8 CD45RClow T cells suppress while CD8 CD45RChigh T cells exacerbate lethal acute graft-versus-host disease induced after allogeneic transplantation of CD4 T cells
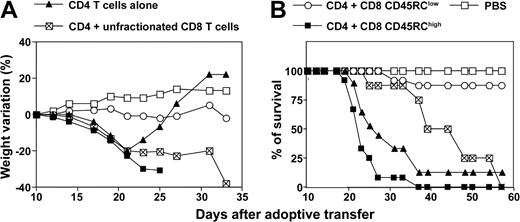
In order to evaluate the effect of CD45RC CD8 T-cell subsets on the development of alloreactive immune response in vivo, we used a CD4-dependent MHC-incompatible model of acute graft-versus-host disease (GvHD) in the rat.40 In preliminary experiments, we determined the dose of CD4 T cells required to induce a lethal GvHD in about 75% of the recipients. Irradiated (LEW × BN) F1 rats injected with 6 × 106 purified CD4 T cells from LEW rats developed severe signs of GvHD (diarrhea, hunched posture, ruffled fur, and significant weight loss), and 7 rats among 10 died between days 19 and 35 after cell transfer (Figure 6). The 3 surviving recipients regulated their disease and gained weight. Irradiated F1 recipients transferred with 6 × 106 LEW CD4 T cells in combination with 3 × 106 LEW unfractionated CD8 T cells also exhibited classical signs of GvHD that killed all 7 recipients (Figure 6). However, the mortality occurred later when compared with recipients injected with LEW CD4 T cells only. Irradiated F1 rats injected with LEW CD4 T cells in combination with 3 × 106 LEW CD8 CD45RChigh cells developed a very severe GvHD that killed all 10 recipients (Figure 6). Interestingly, irradiated F1 rats injected with LEW CD4 T cells associated with 3 × 106 LEW CD8 CD45RClow cells were clearly protected from lethal GvHD, and 10 recipients among 11 survived for more than 2 months. Occasionally, recipients of CD45RClow CD8 T cells in combination with CD4 T cells showed mild and transient signs of GvHD at 3 to 4 weeks after cell transfer. Irradiated F1 rats injected with PBS (Figure 6) or with fractionated LEW CD8 CD45RC subsets alone, although at high cell numbers (107/rat), appeared healthy and survived for more than 3 months (data not shown). Together, these studies demonstrate that CD45RChigh CD8 T cells exacerbate the GvHD induced by alloreactive CD4 T cells, while CD45RClow CD8 T cells prevent this disease.
CD45RClow CD8 T cells prevent graft-versus-host disease (GvHD) induced by allogeneic CD4 T cells. Purified CD4 T cells from LEW rats (6 × 106 cells/rat) were transferred into (LEW × BN) F1 rats 24 hours after total body irradiation (4.5 grays) either alone (group 1, ▴; n = 10), or in combination with 3 × 106 LEW CD45RChigh CD8 T cells (group 2, ▪, n = 10), CD45RClow CD8 T cells (group 3, ○, n = 11), or unfractionated CD8 T cells (group 4, ⊠, n = 7). Control rats were irradiated and injected with PBS (□, n = 4). Recipients were examined for induction of acute GvHD as revealed by weight loss and mortality. The comparison of maximal weight loss by ANOVA revealed statistical differences between the following groups: group 1/group 3: P < .01; group 1/group 4: P < .05; group 2/group 3: P < .01; group 2/group 4: P < .05; and group 3/group 4: P < .01. The statistical analysis of survival using χ2 test showed statistical differences between the following groups: group 1/group 3: P < .01; group 2/group 3: P < .01; and group 3/group 4: P < .01.
CD45RClow CD8 T cells prevent graft-versus-host disease (GvHD) induced by allogeneic CD4 T cells. Purified CD4 T cells from LEW rats (6 × 106 cells/rat) were transferred into (LEW × BN) F1 rats 24 hours after total body irradiation (4.5 grays) either alone (group 1, ▴; n = 10), or in combination with 3 × 106 LEW CD45RChigh CD8 T cells (group 2, ▪, n = 10), CD45RClow CD8 T cells (group 3, ○, n = 11), or unfractionated CD8 T cells (group 4, ⊠, n = 7). Control rats were irradiated and injected with PBS (□, n = 4). Recipients were examined for induction of acute GvHD as revealed by weight loss and mortality. The comparison of maximal weight loss by ANOVA revealed statistical differences between the following groups: group 1/group 3: P < .01; group 1/group 4: P < .05; group 2/group 3: P < .01; group 2/group 4: P < .05; and group 3/group 4: P < .01. The statistical analysis of survival using χ2 test showed statistical differences between the following groups: group 1/group 3: P < .01; group 2/group 3: P < .01; and group 3/group 4: P < .01.
CD45RChigh and CD45RClow CD8 T cells express different levels of Foxp3 and CTLA-4 mRNA
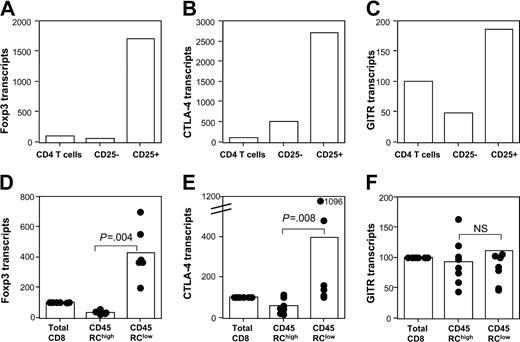
Since Foxp3,17 CTLA-4,41 and GITR42 have been shown as markers of natural regulatory CD4 T cells, it was important to examine whether the natural regulatory CD8 CD4R5RClow T cells also expressed these molecules. For this purpose, we assessed, using quantitative RT-PCR, the mRNA expression of these molecules in purified CD45RChigh versus CD45RClow CD8 T cells, as well as in purified CD25+ versus CD25- CD4 T cells. In agreement with previous data obtained for humans and mice, we showed that in rats, Foxp3 (Figure 7A), CTLA-4 (Figure 7B), and GITR (Figure 7C) were also mainly expressed by CD4 CD25+ regulatory T cells. For the CD8 CD45RC T-cell subsets, Foxp3 (Figure 7D) and CTLA-A (Figure 7E) mRNA were mainly expressed by CD45RClow CD8 T cells, although at levels significantly lower than CD4 CD25+ T cells. GITR mRNA expression (Figure 7F) did not differ between CD45RChigh and CD45RClow CD8 T cells (P = .83). Together, these data demonstrate that Foxp3, the key transcription factor involved in the development of regulatory CD4 T cells, and CTLA-4 are also expressed by the natural regulatory CD8 CD45RClow T cells.
Foxp3 and CTLA-4 are selectively expressed by CD45RClow CD8 T cells. Purified CD4 CD25 T-cell subsets from LEW rats (upper panels) and CD8 CD45RC T-cell subsets (lower panels) were tested for their expression of Foxp3 (A, D), CTLA-4 (B, E), and GITR (C, F) by quantitative RT-PCR as described in “Materials and methods.” The results shown for CD4 T-cell subsets are from 1 representative experiment of 2. The results shown for CD8 T-cell subsets are from 6 to 8 independent experiments. Bars represent the mean values, and filled circles represent the results of each individual experiment. The statistical results of group comparisons (Mann-Whitney U test) are indicated above by horizontal lines.
Foxp3 and CTLA-4 are selectively expressed by CD45RClow CD8 T cells. Purified CD4 CD25 T-cell subsets from LEW rats (upper panels) and CD8 CD45RC T-cell subsets (lower panels) were tested for their expression of Foxp3 (A, D), CTLA-4 (B, E), and GITR (C, F) by quantitative RT-PCR as described in “Materials and methods.” The results shown for CD4 T-cell subsets are from 1 representative experiment of 2. The results shown for CD8 T-cell subsets are from 6 to 8 independent experiments. Bars represent the mean values, and filled circles represent the results of each individual experiment. The statistical results of group comparisons (Mann-Whitney U test) are indicated above by horizontal lines.
Discussion
In the present study, we show that CD45RC expression levels define functionally distinct CD8 T-cell subsets in normal rats, using in vitro and in vivo alloreactive responses as a readout. CD45RChigh CD8 T cells are cytotoxic against allogeneic targets and contain alloreactive lymphocytes that exacerbate the acute GvHD induced by CD4 T cells. In contrast, cells contained within the CD45RClow CD8 population fail to mount proliferative or cytotoxic responses against allogeneic cells. In addition, they inhibit the proliferation and the production of IFNγ by naive CD4 T cells in response to allogeneic stimulation. Finally, their transfer prevents GvHD induction by allogeneic CD4 T cells. In line with our results, it has been shown in mice that functionally defined CD8 T suppressor clones could be distinguished from cytotoxic clones by different patterns of cytokine production and by differential expression of the CD45RA isoform.43 All suppressors clones produced IL-10 and were negative for CD45RA expression, whereas cytotoxic clones did not produce IL-10 and expressed high levels of CD45RA antigen.43 However, although these in vitro data suggest that the expression of CD45RA is stable and characterizes 2 functionally distinct CD8 T-cell subsets, there is no evidence that this might be the case in vivo.
The existence of functionally distinct subsets within the CD8 T-cell pool has long been recognized. Initially, CD8 T lymphocytes had been defined as being either cytotoxic or suppressor cells. This distinction was based essentially on functional assays. Recently, it became clear that CD8 T cells may also be differentiated into several subsets depending on their pattern of cytokine production.44-48 To date, the ability to produce a polarized pattern of cytokines has been demonstrated only after in vitro primary stimulation of naive CD8 T cells in the presence of polarizing cytokines47-49 and for CD8 T-cell clones.50 Our results demonstrate for the first time that CD8 T cells from naive and healthy individuals can be separated, according to their levels of CD45RC expression, into different subtypes having different cytokine profiles. Upon in vitro stimulation with anti-TCR and CD28 mAbs or with allogeneic APCs, the CD8 CD45RChigh subpopulation secretes mainly IL-2 and IFNγ, while CD45RClow CD8 T cells produce mainly IL-4, IL-10, and IL-13. This difference in cytokine production indicates that CD45RC expression is not merely a marker of activated effector CD8 T cells. This was further confirmed by a phenotypic analysis of these CD8 T-cell subsets, using several activation markers (CD28, L-selectin, OX40, MHC class II, and CD25). Both subsets were not activated and had similar levels of these molecules (data not shown).
Concerning the effect on CD4 T-cell differentiation, it has been shown in vitro that CD8 T-cell clones producing type 1 cytokines (Tc1) favored the development of Th1-biased CD4 effectors, whereas Tc2 clones not only could promote Th2 effectors but also could efficiently suppress the development of Th1 cells.51 In vivo, CD8 T cells have been shown to alter the balance of Th1/Th2 responses by influencing the development of CD4 T cells secreting IL-4 or IFNγ.52-54 In the present study, we focused on the capacity of CD8 CD45RC subsets to influence the development of effector alloreactive CD4 T cells both in vitro and in vivo. We showed that CD45RClow CD8 T cells inhibited in vitro the proliferation and differentiation of alloreactive CD4 T cells into IFNγ-producing cells. This CD8 subset also inhibited in vivo a CD4-mediated acute GvHD, a Th1-mediated disorder. However, and in contrast to Tc2 cells, this inhibition was not accompanied by an up-regulation of Th2 cells.51 Similarly to Tc1 cells, CD45RChigh CD8 T cells favored the differentiation of alloreactive CD4 T cells into Th1 type and exacerbated the CD4-mediated GvHD.
Down-regulation of immune responses by regulatory T cells in vitro and in vivo has been amply documented.15,34,55,56 However, the mechanism whereby regulatory T cells mediate suppression remains unclear. Various mechanisms have been proposed, such as competition with antigen-specific T cells for APCs or growth factors and the production of suppressive cytokines.6,15,28,55,56 The mechanism of suppression by the CD8 CD45RClow subset appears to be cytokine independent and to be mediated by cell-to-cell contact. Indeed, no suppression was observed when the suppressors were separated from the responders by a semipermeable membrane, and supernatants from stimulated CD8 CD45RClow could not mediate suppression (data not shown). In addition, neutralizing Abs against IL-4, IL-10, or TGFβ failed to abrogate suppression. The requirement of T-cell contact to mediate suppression raises the possibility that CD45RClow CD8 T cells might mediate suppression by killing responder cells or allogeneic APCs. However, CD45RClow CD8 T cells are not cytotoxic and we did not observe evidence of increased cell death in the cocultures. Furthermore, the addition of exogenous IL-2 did not abrogate the suppression, excluding the possibility that CD45RClow CD8 T cells mediate suppression consumption of IL-2, a growth factor crucial for CD4 T-cell proliferation. We favor the hypothesis that CD45RClow CD8 T cells could mediate suppression by rendering APCs tolerogenic. Indeed, it has been shown that regulatory CD8 CD28- T cells induced CD4 T-cell unresponsiveness by decreasing the expression of costimulatory molecules and by increasing the expression of immunoglobulin-like transcripts 3 and 4 on dendritic cells.6 This hypothesis is currently under investigation.
In conclusion, our results demonstrate that CD8 T cells, separated according to the level of CD45RC expression, fall into subtypes having different cytokine profiles analogous to the 2 types of CD4 CD45RC T cells. The definition of CD4 CD45RC T-cell subsets was instrumental for the understanding of the cross-talk between CD4 T cells in the regulation of immune responses. It is now possible to consider the interaction between other T-cell subtypes, including CD8 CD45RC subsets, which enlarges the general figure of T-cell-mediated regulation. We also demonstrated that CD8 CD45RClow cells are very efficient at preventing GvHD. These data suggest that CD8 CD45RClow T cells may be used in cell therapy to prevent GvHD, a major complication of allogeneic bone marrow transplantation, an important therapy for a number of hematologic diseases.
Prepublished online as Blood First Edition Paper, July 22, 2004; DOI 10.1182/blood-2004-03-1214.
Supported by the Institut National de la Santé et de la Recherche Médicale (INSERM), Association de la Recherche contre le Cancer, Ligue Régionale de Recherche sur le Cancer, la Région Midi-Pyrénées and European Community (QLG1-CT2001-01918). A.S. and D.G.-D. are supported by the Centre National de la Recherche Scientifique; A.S.D. is supported by a grant from the Ministère de l'Education Nationale, de la Recherche et de la Technologie; and E.X. is supported by grants from INSERM and Fondation pour la Recherche Médicale.
E.X. and A.S.D. contributed equally to this work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
The authors wish to thank Maryline Calise, Audrey Boyer, Magali Toulouse, and Patrick Aregui (IFR 30, Toulouse, France) for taking care of the animal house.