Abstract
In sickle cell disease, deoxygenation of intra-erythrocytic hemoglobin S leads to hemoglobin polymerization, erythrocyte rigidity, hemolysis, and microvascular occlusion. Ischemia-reperfusion injury, plasma hemoglobin-mediated nitric oxide consumption, and free radical generation activate systemic inflammatory responses. To characterize the role of circulating leukocytes in sickle cell pathogenesis we performed global transcriptional analysis of blood mononuclear cells from 27 patients in steady-state sickle cell disease (10 patients treated and 17 patients untreated with hydroxyurea) compared with 13 control subjects. We used gender-specific gene expression to validate human microarray experiments. Patients with sickle cell disease demonstrated differential gene expression of 112 genes involved in heme metabolism, cell-cycle regulation, antioxidant and stress responses, inflammation, and angiogenesis. Inducible heme oxygenase-1 and downstream proteins biliverdin reductase and p21, a cyclin-dependent kinase, were up-regulated, potentially contributing to phenotypic heterogeneity and absence of atherosclerosis in patients with sickle cell disease despite endothelial dysfunction and vascular inflammation. Hydroxyurea therapy did not significantly affect leukocyte gene expression, suggesting that such therapy has limited direct anti-inflammatory activity beyond leukoreduction. Global transcriptional analysis of circulating leukocytes highlights the intense oxidant and inflammatory nature of steady-state sickle cell disease and provides insight into the broad compensatory responses to vascular injury.
Introduction
Sickle cell disease arises from a point mutation in the β-globin gene, resulting in the expression of hemoglobin S (HbS). Deoxygenated HbS polymerizes, leading to erythrocyte rigidity, distortion, membrane damage, and hemolysis.1,2 Consequently, sickle cell patients suffer repeated vaso-occlusive events characterized by ischemia-reperfusion injury and inflammation.3,4 These chronic vascular insults lead to numerous end-organ complications such as avascular necrosis of bones, retinal infarction, stroke, acute chest syndrome, pulmonary hypertension, and skin ulceration.5 While the molecular and biophysical details of the processes influencing HbS polymerization are well characterized, the explanation for the broad phenotypic heterogeneity and clinical variability of sickle cell disease, where patients with an identical genetic mutation suffer pleiotropic complications, remains a mystery.
A seminal feature that sets sickle cell disease apart from other chronic hemolytic syndromes and that predicts disease severity is a chronic, intense inflammatory state. Inflammation, leukocyte adhesion to vascular endothelium, and subsequent endothelial injury appear to contribute to the pathogenesis of sickle cell disease, driven in part by repeated episodes of ischemia-reperfusion injury.3,4,6-8 Elevated white blood cell counts have been shown to predict morbid events in sickle cell disease. Leukocytosis is a risk factor for hemorrhagic stroke in children and adults,9,10 acute chest syndrome,11 and early death.12 Further, elevated blood levels of inflammatory and anti-inflammatory cytokines (ie, interleukin 1 β [IL-1β], IL-4, IL-6, tumor necrosis factor α [TNFα]), increased adhesion molecule expression (ie, intercellular adhesion molecule [ICAM], vascular cell adhesion molecule [VCAM], integrins, and P-selectin), and increased inflammatory biomarkers such as C-reactive protein and isoprostanes have been described and appear to contribute to the development of chronic organ injury.6,13-28
Gene expression signatures have been used successfully to characterize tumor phenotype and to predict disease recurrence and mortality in B-cell lymphoma and breast cancer.29,30 This comprehensive analysis of transcription profiles provides a novel means to enhance knowledge of the pathogenesis and treatment of different diseases. Given the pivotal role of inflammation in sickle cell disease pathogenesis, we hypothesized that blood mononuclear cells from patients would express unique functional genomic profiles and that these gene signatures may provide insight into the complex inflammatory and homeostatic responses to vascular injury.
Patients, materials, and methods
Subjects
The study was approved by the National Heart, Lung and Blood Institute's institutional review board and all participants gave written informed consents. Twenty-seven clinically stable volunteers with sickle cell disease (23 HbSβ-thalassemia phenotypes [1 S allele and 1 β-thalassemia allele]) and 13 healthy African-American volunteers participated in the study. All primary analyses were restricted to the comparison of the 14 HbSS patients not taking hydroxyurea with control subjects. All volunteers had hemoglobin high-performance liquid chromatographic separation to confirm hemoglobin S or A phenotype, as well as hemoglobin F levels. Patient characteristics are summarized in Table 1 and Table 2. Sickle cell patients were excluded if they were clinically unstable, defined by having vasoocclusive crisis or acute chest syndrome within 30 days of the study, used tobacco products, or received blood transfusions within the preceding 4 weeks (or HbA > 5%). Controls were excluded if they used tobacco products or used aspirin or nonsteroidal anti-inflammatory products within the preceding 7 days.
Eight controls participated in a study of intravenous endotoxin infusion; gene expression data from this study were queried to compare the inflammatory signature of sickle cell disease to the inflammatory response to intravenous endotoxin. This study was approved by the National Institute of Allergy, Immunology and Infectious Disease's institutional review board and all subjects gave written informed consent. Subjects received a single intravenous dose (4 ng/kg) of Clinical Center Reference Endotoxin (CCRE; Escherichia coli O:113; Clinical Center, National Institutes of Health [NIH], Bethesda, MD) as previously described.31 Peripheral blood mononuclear cells were collected before infusion and 6 hours after infusion for gene expression studies.
Peripheral blood mononuclear cell isolation
Peripheral blood from sickle cell patients, healthy African-American volunteers, and volunteers in the intravenous endotoxin study was collected into Vacutainer cell preparation tube (CPT) cell preparation tubes with sodium citrate and Ficoll (Becton Dickinson, Franklin Lakes, NJ) (supplemental data is available on the Blood website; see the Supplemental Materials link at the top of the online article). Purified peripheral blood mononuclear cell (PBMC) suspensions, containing predominantly lymphocytes and monocytes but also small amounts of neutrophils and platelets were resuspended in buffer RLT (700-1000 μL per 107 cells) and passed through Qiashredder columns (Qiagen, Valencia, CA) then stored at –70° C. Platelet-monocyte aggregates were not evaluated; however, platelet and neutrophil contamination were estimated and these methods and results can be found in the Supplemental Materials online.
RNA isolation, hybridization, and microarray analysis
Total RNA was extracted from peripheral blood mononuclear cells, neutrophils, and platelets using RNeasy Mini Kit (Qiagen). For PBMCs and neutrophils, 5 μg total RNA was used to synthesize cDNA using the SuperScript Double-Stranded cDNA synthesis kit (Invitrogen Life Technologies, Carlsbad, CA), which was reverse transcribed into fluorescently labeled cRNA using the Bioarray High Yield RNA transcript labeling kit (ENZO Diagnostics, Farmingdale, NY). Platelets had insufficient total RNA for further processing. Fluorescently labeled cRNA was fragmented by heating to 95 degrees for 30 minutes in fragmentation buffer consisting of 8 mL of 1 M tris acetate pH 8.1, 6.4 g magnesium acetate (MgOAc), 9.8 g potassium acetate (KOAc), and diethylprocarbonate (DEPC) water to a final volume of 200 mL. Microarrays were prepared according to manufacturer protocols using the HU95Av2 (Affymetrix, Eugene, OR) gene chip (for sickle cell and endotoxin PBMC arrays) and data were analyzed using Microarray Suite 4.0 software (Affymetrix, Santa Clara, CA). Data mining was performed using GeneSpring (Silicon Genetics, Redwood City, CA) and JMP Statistical Discovery Software (SAS Institute, Carrboro, NC; see “Statistical analysis”). In several cases, in order to control for batch effects (“Statistical analysis”), samples were hybridized to gene chips multiple times using additional aliquots from the original pool of total RNA for that sample. Neutrophil total RNAand PBMC total RNAfrom 3 additional sickle cell patients were hybridized to HU133A gene chips (Affymetrix) and analyzed with Micorarray Suite 5.1 software (Affymetrix) for the neutrophil-specific gene list validation experiments.
Validation of gene expression measurements using real-time polymerase chain reaction
Quantification of mRNA was performed using quantitative real-time polymerase chain reaction (qRTPCR; TaqMan system; Applied Biosystems, Rockville, MD) to confirm microarray data. Probes and primer sets were obtained as manufactured kits (p21/Waf1/Cip1) from Applied Biosystems Custom Oligo Synthesis Service (Foster City, CA) or custom designed (heme oxygenase-1) by using the software Primer Express (Applied Biosystems). Heme oxygenase-1 (HO-1) forward primer, reverse primer, and probe sequences are as follows: 5′-AGGCCAAGACTGCGTTCC-3′, 5′-GCAGAATCTTGCACTTTGTTGCT-3′, 5′-FAM-CTCAACATCCAGCTCTTTGAGGAG-TTGCAG-TAMRA-3′. Random hexamer primers were used to synthesize cDNA from sickle cell patients and healthy volunteers for qRTPCR. Real-time PCR was conducted using a High Capacity cDNA archive kit (Applied Biosystems) and quantified on a 7900HT Sequence Detection System (Applied Biosystems) according to the manufacturer's directions. The housekeeping gene RNase P1 was used as an internal standard.
Immunoblotting
Mononuclear cells were collected from 4 sickle cell patients in steady state and 4 control patients. PBMCs were isolated as described in “Peripheral blood mononuclear cell isolation,” cells were lysed, and total protein extracted. Twenty micrograms of crude cell lysate were used for Western blot. HO-1 protein expression was detected by using 1:1000 dilution of rabbit-anti–HO-1 polyclonal antibody (Calbiochem, La Jolla, CA). A 1:5000 dilution of horseradish peroxidase–conjugated goat antirabbit immunoglobulin G (IgG) was used as the secondary antibody (Jackson ImmunoResearch Laboratory, West Grove, PA).
Laboratory tests
Cell counts and differentials were performed using the Cell Dyn 3500 Analyzer (Abbott Diagnostics, Abbott Park, IL); hemoglobin high-performance liquid chromatography and serum chemistries were performed in the clinical pathology laboratory at the National Institutes of Health. Plasma heme was measured using benzidine assay.27
Statistical analysis
Data transformation. The average difference values (Affymetrix) for 102 chips were transformed and analyzed using special purpose scripts written in the JMP scripting language (SAS Instititute). Average difference values were standardized and transformed using the Symmetric Adaptive Transform, which yields quantile-normalized, homogeneous variance scale results. This transform has the practical advantage of eliminating the need to truncate or remove negative values prior to statistical analysis.
Principal components analysis. We performed principal components analysis32 on the transformed data matrix (chips by genes) to visualize the relative location of each chip in low-dimensional space, allowing for detection of outliers or other relevant patterns. Using the first 4 principal components, chips in a scatter plot matrix were labeled by various characteristics of the sample and laboratory procedures. The first 2 principal components clearly separated control and sickle cell patients. Separation of samples was also seen when points were labeled by the chip production lot number, suggesting a significant experimental batch/chip lot effect (data not shown).
Adjustment for sample, batch, and microarray lot effect. The data set represented 2 groups: sickle cell disease (14 patients of HbSS phenotype off hydroxyurea, 31 chips including replicates) and control group (13 controls, 25 chips including replicates), hybridized in 3 distinct lots of chips and reagents. With the observation of a clear experimental batch/chip lot effect, we corrected for this in our statistical analysis. Because samples were hybridized to multiple replicate chips, results for each sample were first averaged, keeping track of the occurrence frequency of each lot in the average. Average results were analyzed with 2-way analysis of variance (ANOVA), setting the first factor to the lot and the second to disease status, weighting each sample average according to the number of times that lot was used. Variability was first attributed to lot effect, and remaining variability (due to difference in expression between sickle cell disease and control) was checked for statistical significance (see “Filters for gene selection”). This is a conservative procedure in that the disease effect on gene expression might be underestimated relative to the experimental batch/chip lot effect.
Filters for gene selection. The false discovery rate33 computed for each gene list was required to be less than 5% and the relative change between groups (fold-change) was required to be at least 20%. The false discovery rate approach to controlling statistical error was utilized here as it is more appropriate for gene discovery, allows us to obtain a high quality list of genes, and is less restrictive than the family wise error rate approach which has a larger false-negative rate leading to disqualification of important genes. A 20% fold-change cut-off was chosen because fold changes less than 20% may be due to variability in background. An average difference of greater than or equal to 20 in either the sickle cell disease or control group was required to eliminate genes at or below accepted detection limits for this assay. Using these filters to compare the 13 controls (HbAA phenotype) and 14 sickle cell patients (HbSS phenotype only, not on hydroxyurea therapy), 112 genes showed significant expression changes (Figure 2). To define male versus female expression differences, the false discovery rate limit was relaxed to less than or equal to 10% and no fold-change requirement was made. In order to identify more possibly differentially regulated genes in sickle cell patients we further relaxed the false discovery rate requirement to less than or equal to 10% and eliminated the fold-change filter (Supplemental Table 1).
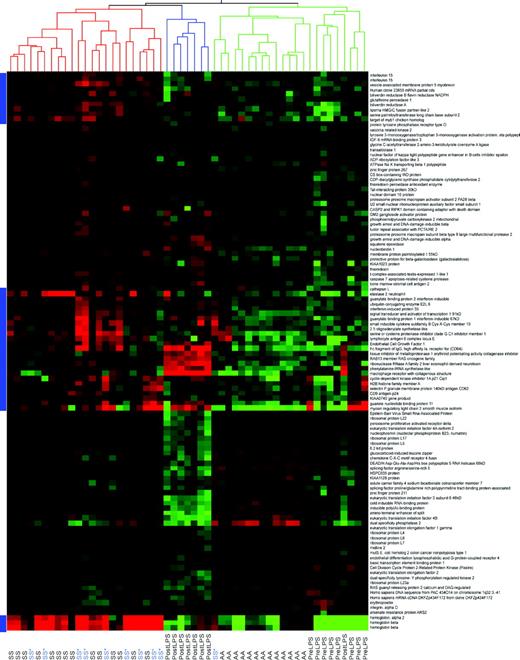
Hierarchical cluster analysis of 112 significantly differentially expressed genes successfully segregates sickle cell disease from control patients. These genes were derived using 2-way ANOVA, false discovery rate multiple comparisons correction of less than or equal to 5%, more than or equal to 20% fold-change cut-off, and a mean average difference more than 20 filter in either the sickle cell disease or control group. These 112 genes were obtained comparing mean gene expression levels in 14 sickle cell disease patients of HbSS phenotype (not on hydroxyurea therapy) to 13 African-American control subjects. Hierarchical clustering was performed across a larger set of all sickle cell disease patients (HbSS phenotype only) in the study, including patients on hydroxyurea treatment, using these 112 genes. The dendrogram at the top of the figure represents the relatedness of samples based on gene expression patterns. The white line separates the 2 main branches of the dendrogram. With the exception of one sickle cell patient on hydroxyurea, these 112 genes successfully segregate control (AA) from sickle cell disease (SS or SS*) patients. Sickle cell disease patients on hydroxyurea (SS*) therapy do not cluster separately from patients not on therapy. The single sickle cell patient on hydroxyurea who clustered with the control group may represent a misclassification or hydroxyurea altered the gene expression toward a normalized pattern. Gene names appear to the right of the figure and those of particular interest to our group are underlined and highlighted in yellow.
Hierarchical cluster analysis of 112 significantly differentially expressed genes successfully segregates sickle cell disease from control patients. These genes were derived using 2-way ANOVA, false discovery rate multiple comparisons correction of less than or equal to 5%, more than or equal to 20% fold-change cut-off, and a mean average difference more than 20 filter in either the sickle cell disease or control group. These 112 genes were obtained comparing mean gene expression levels in 14 sickle cell disease patients of HbSS phenotype (not on hydroxyurea therapy) to 13 African-American control subjects. Hierarchical clustering was performed across a larger set of all sickle cell disease patients (HbSS phenotype only) in the study, including patients on hydroxyurea treatment, using these 112 genes. The dendrogram at the top of the figure represents the relatedness of samples based on gene expression patterns. The white line separates the 2 main branches of the dendrogram. With the exception of one sickle cell patient on hydroxyurea, these 112 genes successfully segregate control (AA) from sickle cell disease (SS or SS*) patients. Sickle cell disease patients on hydroxyurea (SS*) therapy do not cluster separately from patients not on therapy. The single sickle cell patient on hydroxyurea who clustered with the control group may represent a misclassification or hydroxyurea altered the gene expression toward a normalized pattern. Gene names appear to the right of the figure and those of particular interest to our group are underlined and highlighted in yellow.
Validation of gene expression data. Expression levels of selected genes were compared with gene expression levels measured by quantitative real-time polymerase chain reaction and clinical laboratory values (total bilirubin, carboxy hemoglobin saturation, and plasma heme concentration) using linear regression. P values for the slope of the linear fit were calculated using a t test with a null hypothesis of slope equals 0.
Results
Validation of global transcriptional analysis
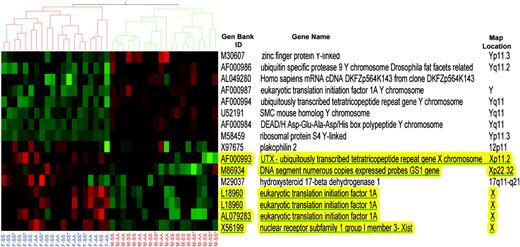
To validate the accuracy of our laboratory and statistical processes, we analyzed gene expression patterns from mononuclear cells of 8 male and 6 female sickle cell patients to determine if there were significant differences in gene expression based only on gender. After correcting for experimental batch and probe array lot effects and filtering for significant differentially expressed genes between male and female sickle cell patients, using 2-way ANOVA and less than or equal to 10% false discovery rate multiple comparisons correction (“Statistical analysis”), a list of 16 probe sets representing 14 genes was found to change significantly with gender. Notably, 14 of the 16 probe sets were located on either the X or Y chromosome. The Y chromosome genes from this list were elevated only in males; X chromosome genes were elevated only in females. The most likely mechanism for the increase in expression of X-linked genes is a double-gene-dose phenomenon with incomplete Lyonization of the second X chromosome,34 which has been previously reported for 4 of the X-linked genes on our list (GS1, eukaryotic translation and initiation factor 1A, UTX, and Xist [a presumed structural RNA that controls X chromosome inactivation from its center on the long arm of the X chromosome]; Figure 1).35-40 Hierarchical cluster analysis of the same 8 male and 6 female sickle cell subjects on these 16 gender-specific probe sets demonstrated clear discrimination of gender. We extended our hierarchical cluster analysis to include all 37 study patients, including 10 sickle cell patients on hydroxyurea therapy and 13 controls in addition to the original 14 sickle cell patients from which the 16 genes were derived. Again, the expression signature accurately segregated patients into male and female groups (Figure 1). Y-linked genes could, by themselves, segregate all patients. The most discriminatory X-linked gene (XIST or X56199) showed expression levels that overlapped between males and females on only 2 samples. However, a simple average of expression levels for 4 X-linked genes (L18960, M86934, X56199, AF000993) provided clear-cut discrimination between male and female. This need to average may reflect additional variability in the X-silencing process, compared with the all-or-none presence of the Y chromosome. Similar results have been obtained using HU95A microarrays in a study of gene expression in nonhuman primate pineal glands. Six of these human genes present in the primate samples were sufficient to predict primate gender (S.L. Coon, D.C. Klein, P.J.M., personal written communication, April 21, 2003). These data for X chromosomal genes demonstrate the fidelity of our expression array analysis as we replicate previous findings for several genes that escape X chromosome inactivation. The ability of our experimental and statistical algorithm to correctly identify gender and important sex-linked genes serves as an internal validation of our gene expression analysis. This comparison can be used as a novel validation technique in global transcriptional analysis studies.
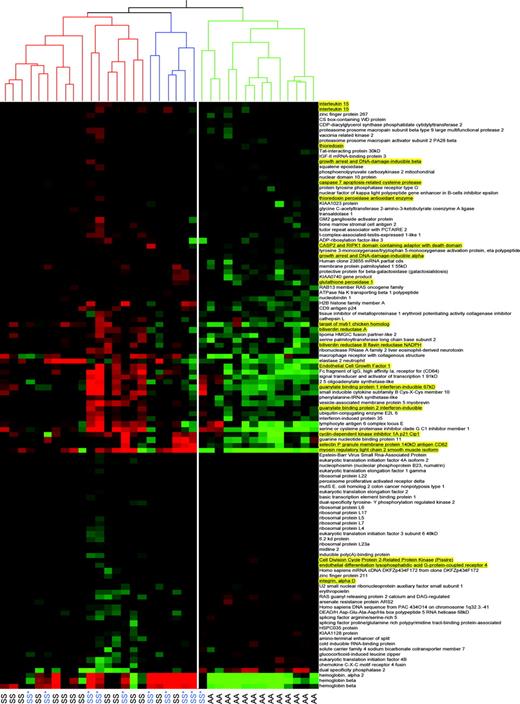
Heirarchical cluster analysis of 14 gender-specific genes as a novel validation of microarray expression data. Genes were chosen on the basis of comparison of mean gene expression levels between 8 male and 6 female sickle cell disease patients (HbSS phenotype only, designated as either M-SS for males or F-SS for females) with false discovery rate of less than or equal to 10%. Hierarchical cluster analysis of the expression pattern of these 16 probe sets (14 genes) in all sickle cell disease patients (HbSS phenotype), including the original 14 subjects as well as 10 additional sickle cell disease patients on hydroxyurea (M-SS* or F-SS*) and 13 controls (M-AA or F-AA) show that they discriminate gender with 100% accuracy. In this figure each column represents a sickle cell disease patient or a control subject and each row represents a probe set. Red signifies increased expression and green signifies decreased expression. Genes that are up-regulated in males over females are generally located on the Y chromosome and genes that are up-regulated in females over males are generally located on the X chromosome. One gene, plakophilin 2, maps to an autosomal location and has a low overall expression level. With the selected false discovery rate of less than or equal to 10% one would only expect 1 or 2 false positives. Eukaryotic translation initiation factor 1A is represented by 3 different probe sets. All 4 X-linked genes (underlined and highlighted in yellow) represent X chromosome genes known to escape X inactivation. This specificity is a remarkable additional validation of the experimental and analytical methodology.
Heirarchical cluster analysis of 14 gender-specific genes as a novel validation of microarray expression data. Genes were chosen on the basis of comparison of mean gene expression levels between 8 male and 6 female sickle cell disease patients (HbSS phenotype only, designated as either M-SS for males or F-SS for females) with false discovery rate of less than or equal to 10%. Hierarchical cluster analysis of the expression pattern of these 16 probe sets (14 genes) in all sickle cell disease patients (HbSS phenotype), including the original 14 subjects as well as 10 additional sickle cell disease patients on hydroxyurea (M-SS* or F-SS*) and 13 controls (M-AA or F-AA) show that they discriminate gender with 100% accuracy. In this figure each column represents a sickle cell disease patient or a control subject and each row represents a probe set. Red signifies increased expression and green signifies decreased expression. Genes that are up-regulated in males over females are generally located on the Y chromosome and genes that are up-regulated in females over males are generally located on the X chromosome. One gene, plakophilin 2, maps to an autosomal location and has a low overall expression level. With the selected false discovery rate of less than or equal to 10% one would only expect 1 or 2 false positives. Eukaryotic translation initiation factor 1A is represented by 3 different probe sets. All 4 X-linked genes (underlined and highlighted in yellow) represent X chromosome genes known to escape X inactivation. This specificity is a remarkable additional validation of the experimental and analytical methodology.
Gene list generation using validated multiple comparisons correction: effect of sickle cell disease with and without hydroxyurea therapy
We chose to evaluate the expression profile of peripheral blood mononuclear cells because of their involvement in sickle cell disease pathogenesis7 and to assure a rapid isolation of cells without ex vivo gene activation. We analyzed mean mononuclear cell gene expression levels from 14 sickle cell patients (HbSS phenotype only) not taking hydroxyurea and compared them to 13 controls (HbAA phenotype). The characteristics of the subgroup of 14 HbSS patients were statistically similar to the entire group of 27 sickle cell patients. Patient characteristics of all 27 sickle cell patients and 13 controls are summarized in Table 1 and Table 2.
Using a 1.2 fold-change expression cut-off, a more than 20 average difference filter, and a less than or equal to 5% false discovery rate multiple comparisons correction, 112 genes were determined to have statistically significant differential levels of expression. Hierarchical cluster analysis was applied to those 112 genes in samples from the 13 controls and 14 sickle cell patients (HbSS phenotype) off hydroxyurea from the primary analysis and then prospectively applied to an additional 10 sickle cell patients (HbSS phenotype) on hydroxyurea therapy (Figure 2). Without prior knowledge of the functions of those 112 genes, their gene expression patterns predicted which patients had sickle cell disease, with the exception of a single patient on hydroxyurea therapy who was classified with the controls. Notably, sickle cell patients not on hydroxyurea therapy have very similar gene expression patterns to sickle cell patients on hydroxyurea therapy and are clearly distinguished from controls. A statistical comparison of sickle cell patients on and off hydroxyurea did not find any significant differentially expressed genes, even allowing a less than or equal to 20% false discovery rate in order to detect any differences that may have been missed using a more stringent false discovery rate, suggesting minimal direct anti-inflammatory effects of hydroxyurea therapy on the mononuclear cell population. The single patient on hydroxyurea treatment who was classified with controls had no substantial difference in clinical or laboratory parameters compared with the remaining hydroxyurea patients.
Specific annotated pathways of interest
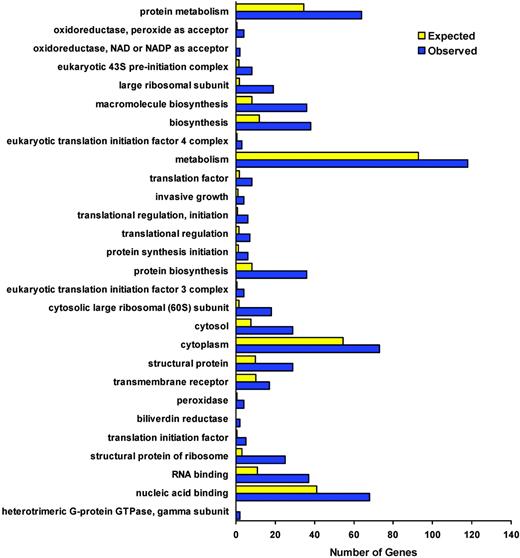
We applied less stringent statistical filters to the data in order to capture a larger group of differentially expressed genes in sickle cell disease and to identify more pathways for hypothesis generation. Using a less than or equal to 10% false discovery rate correction and no fold-change cut-offs, we detected 385 genes that were differentially expressed in sickle cell disease (Supplemental Table S1). Closer inspection of these 385 genes by hierarchical cluster analysis shows that the genes fall into functional clusters including oxido-reductase antioxidant pathways, growth factors, cell-cycle regulators, and heme metabolizing enzymes. We annotated this list of 385 genes using our own gene ontology (GO)–Scan software based on the Gene Ontology Consortium (http://www.geneontology.org). Using GO-Scan we determined whether any categories of genes were significantly overrepresented in our gene list, using a Fisher exact test. The oxido-reductase and cell signaling genes (which fall in the broader GO category of metabolism) represented a large majority of genes in our list (Figure 3). Examination of these pathways suggests a role of circulating cells in the response to oxidant and hemolytic stress, vascular injury, and participation in repair and homeostasis (Table 3). Of particular interest is the HO-1 pathway and downstream proteins affected by this system (Figure 4).
GO-Scan classifications of 385 significantly differentially expressed genes. The x axis reflects the number of genes in a particular category of annotations from our list of 385 genes (generated using a less stringent multiple comparisons correction: false discovery rate ≤ 10%). Blue: observed number; yellow: expected number, based on the total number of genes on the chip given each annotation term multiplied by the average differential expression rate (number of differentially expressed, annotated genes/number of annotated genes). Annotation terms were selected from GO-Scan when they were significantly overrepresented in the list of differentially expressed genes. Significance was determined with a Fisher exact test and P less than or equal to .01. Individual genes from selected categories are listed in Table 3.
GO-Scan classifications of 385 significantly differentially expressed genes. The x axis reflects the number of genes in a particular category of annotations from our list of 385 genes (generated using a less stringent multiple comparisons correction: false discovery rate ≤ 10%). Blue: observed number; yellow: expected number, based on the total number of genes on the chip given each annotation term multiplied by the average differential expression rate (number of differentially expressed, annotated genes/number of annotated genes). Annotation terms were selected from GO-Scan when they were significantly overrepresented in the list of differentially expressed genes. Significance was determined with a Fisher exact test and P less than or equal to .01. Individual genes from selected categories are listed in Table 3.
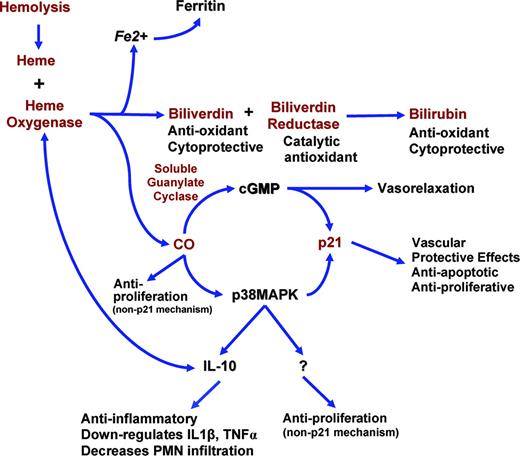
The potential role of the heme oxygenase-1 pathway and downstream effectors in the compensatory response to repeated ischemia-reperfusion injury and hemolytic stress in sickle cell disease. Red text represents up-regulated genes or molecules measured in this study in sickle cell patients. CO indicates carbon monoxide; cGMP, cyclic guanosine monophosphate; p38MAPK, p38 mitogen-activated protein kinase; p21, a cyclin-dependent kinase inhibitor.
The potential role of the heme oxygenase-1 pathway and downstream effectors in the compensatory response to repeated ischemia-reperfusion injury and hemolytic stress in sickle cell disease. Red text represents up-regulated genes or molecules measured in this study in sickle cell patients. CO indicates carbon monoxide; cGMP, cyclic guanosine monophosphate; p38MAPK, p38 mitogen-activated protein kinase; p21, a cyclin-dependent kinase inhibitor.
Heme oxygenase-1 pathway and p21 are up-regulated in sickle cell disease
We analyzed the expression levels of enzymes in the heme catabolism pathway and found that average gene expression of HO-1 and biliverdin reductase (α and β) was increased 2-fold and more than 1.5-fold respectively in patients with steady-state sickle cell disease as compared with controls (Figure 5A, left y-axis). Total bilirubin, the final product of the HO-1 pathway, is increased 3-fold in sickle cell patients (Figure 5A, right y-axis). Carbon monoxide production, reflected by carboxy hemoglobin saturation measured by co-oximetry, correlated with plasma heme levels in sickle cell patients (Figure 5B), suggesting that carboxy hemoglobin can be used as a marker of HO-1 activity and carbon monoxide production. HO-1 gene expression levels correlated significantly with this marker of carbon monoxide production, (r = 0.51, P = .01, Figure 5C) and with total bilirubin levels (r = 0.66; P < .001; data not shown), both end-products of HO-1–mediated heme catabolism, suggestive of increased HO-1 activity. Expression levels of biliverdin reductase-α measured by microarray also correlated with total bilirubin levels (Figure 5D). Additionally, HO-1 and biliverdin reductase gene expression correlated with another marker of hemolysis, lactate dehydrogenase (r = 0.66, P < .0001 and r = 0.58, P < .0001).
Validation of gene expression data for the HO-1 pathway and p21. Gene expression levels are reflected as arbitrary units of fold-change of expression relative to mean control levels. Black lines in B, C, and D are regression lines. (A) Sickle cell disease patients (red bars) have increased mean gene expression (left y-axis) of all enzymes in the heme catabolism pathway and have higher mean serum total bilirubin (right y-axis), the end product of heme breakdown, compared with healthy volunteers (gray bars). Error bars reflect SEM. (B) Carbon monoxide production (determined by carboxy hemoglobin levels measured by co-oximetry) was measured in 24 patients with sickle cell disease (13 HbSS not on hydroxyurea therapy [▴], 8 HbSS on hydroxyurea [▪], 2 HbSC and 1 HbSβ-thalassemia phenotype [○]). Carbon monoxide production correlates with plasma heme levels measured by benzidine assay. (C) Carbon monoxide production correlates with HO-1 gene expression measured by microarray in these same 24 sickle cell disease patients. (D) Serum total bilirubin levels were measured in 27 sickle cell disease patients (14 HbSS not on hydroxyurea therapy [▴], 10 HbSS on hydroxyurea [▪], 2 HbSC and 1 HbSβ-thalassemia phenotype [○], and 13 controls [•]). Serum total bilirubin correlates with biliverdin reductase gene expression measured by microarray. Gene expression levels for biliverdin reductase also correlate with CO production (r = 0.55, P < .005, data not shown). (E) HO-1 and p21 gene expression measured by microarray and real-time PCR show increased expression in 27 sickle cell disease patients. Error bars reflect SEM. (F) Patients with sickle cell disease (n = 4) have increased cellular HO-1 protein levels in their peripheral blood mononuclear cells compared with healthy volunteers (n = 4) as measured by Western blot. Lane 1 represents an HO-1–positive control.
Validation of gene expression data for the HO-1 pathway and p21. Gene expression levels are reflected as arbitrary units of fold-change of expression relative to mean control levels. Black lines in B, C, and D are regression lines. (A) Sickle cell disease patients (red bars) have increased mean gene expression (left y-axis) of all enzymes in the heme catabolism pathway and have higher mean serum total bilirubin (right y-axis), the end product of heme breakdown, compared with healthy volunteers (gray bars). Error bars reflect SEM. (B) Carbon monoxide production (determined by carboxy hemoglobin levels measured by co-oximetry) was measured in 24 patients with sickle cell disease (13 HbSS not on hydroxyurea therapy [▴], 8 HbSS on hydroxyurea [▪], 2 HbSC and 1 HbSβ-thalassemia phenotype [○]). Carbon monoxide production correlates with plasma heme levels measured by benzidine assay. (C) Carbon monoxide production correlates with HO-1 gene expression measured by microarray in these same 24 sickle cell disease patients. (D) Serum total bilirubin levels were measured in 27 sickle cell disease patients (14 HbSS not on hydroxyurea therapy [▴], 10 HbSS on hydroxyurea [▪], 2 HbSC and 1 HbSβ-thalassemia phenotype [○], and 13 controls [•]). Serum total bilirubin correlates with biliverdin reductase gene expression measured by microarray. Gene expression levels for biliverdin reductase also correlate with CO production (r = 0.55, P < .005, data not shown). (E) HO-1 and p21 gene expression measured by microarray and real-time PCR show increased expression in 27 sickle cell disease patients. Error bars reflect SEM. (F) Patients with sickle cell disease (n = 4) have increased cellular HO-1 protein levels in their peripheral blood mononuclear cells compared with healthy volunteers (n = 4) as measured by Western blot. Lane 1 represents an HO-1–positive control.
P21/WAF1/CIP1 (p21) is a cyclin-dependent kinase inhibitor with antiproliferative and antiapoptotic properties. The vascular protective and antiproliferative effects of HO-1 and carbon monoxide have been demonstrated to be dependent on p21.41-44 We found p21 mRNA expression to be increased in sickle cell patients relative to controls as measured by microarrays and confirmed by quantitative real-time polymerase chain reaction (Figure 5E). We also confirmed increased HO-1 mRNA expression with qRTPCR (Figure 5E). HO-1 protein measured by Western blot was elevated in mononuclear cells from sickle cell patients (Figure 5F).
Comparison of the sickle cell disease inflammatory gene expression profile to healthy volunteers receiving intravenous endotoxin
In order to confirm the uniqueness of the sickle cell inflammatory gene expression signature, we compared the gene expression signature of sickle cell patients to a general inflammatory state induced by intravenous endotoxin infusion. Gene expression data from 8 controls were evaluated before and after a single dose of endotoxin. Samples from the endotoxin study were obtained from a separate control patient population and hybridized on different dates and lots of HU95Av2 microarrays. Hierarchical clustering analysis was performed on all sickle cell patients, African-American controls, and controls before and after the endotoxin infusion using the 112 gene list specific for sickle cell disease (Figure 6).
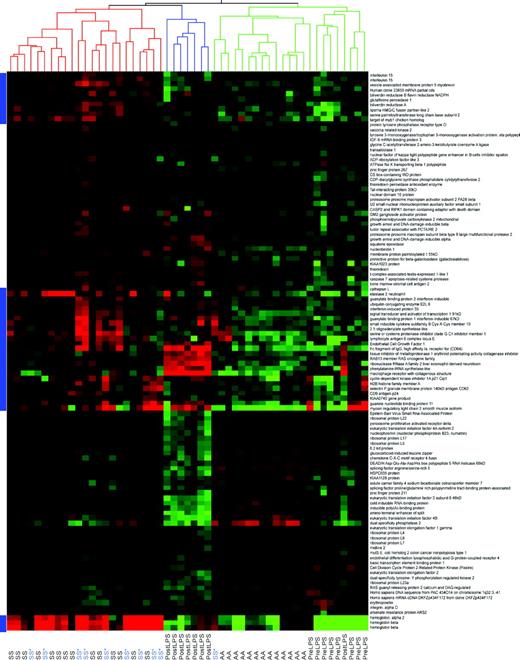
Comparison of gene expression patterns of sickle cell disease to those of a general inflammatory state. Genes were selected based on the comparison of mean gene expression levels between sickle cell disease patients and African-American healthy volunteers using a 1.2 fold-change cut off, an average difference more than 20 filter in either sickle cell disease or control group and a less than or equal to 5% false discovery rate multiple comparisons correction. Cluster analysis was applied to gene expression data from sickle cell disease patients of HbSS phenotype on (SS*) or off (SS) hydroxyurea therapy, African-American healthy volunteers (AA), a separate set of healthy volunteers (Pre LPS), and these same volunteers following intravenous endotoxin infusion (Post LPS). This figure shows that there are similarities in gene expression patterns for these 112 genes between sickle cell disease and an inflammatory state induced by endotoxin infusion. Importantly, there are clusters of genes, marked by the blue bars to the left of the figure, that show differential expression between sickle cell disease and endotoxin infusion, suggesting that the gene expression changes observed in sickle cell disease are not due solely to a generalized inflammatory state but are specific for sickle cell disease.
Comparison of gene expression patterns of sickle cell disease to those of a general inflammatory state. Genes were selected based on the comparison of mean gene expression levels between sickle cell disease patients and African-American healthy volunteers using a 1.2 fold-change cut off, an average difference more than 20 filter in either sickle cell disease or control group and a less than or equal to 5% false discovery rate multiple comparisons correction. Cluster analysis was applied to gene expression data from sickle cell disease patients of HbSS phenotype on (SS*) or off (SS) hydroxyurea therapy, African-American healthy volunteers (AA), a separate set of healthy volunteers (Pre LPS), and these same volunteers following intravenous endotoxin infusion (Post LPS). This figure shows that there are similarities in gene expression patterns for these 112 genes between sickle cell disease and an inflammatory state induced by endotoxin infusion. Importantly, there are clusters of genes, marked by the blue bars to the left of the figure, that show differential expression between sickle cell disease and endotoxin infusion, suggesting that the gene expression changes observed in sickle cell disease are not due solely to a generalized inflammatory state but are specific for sickle cell disease.
As further validation of the fidelity of gene expression data, the previously described healthy African-American volunteers and the healthy pre-endotoxin volunteers (from the endotoxin study) clustered together. One postendotoxin patient did cluster with the pre-endotoxin and African-American controls and may represent a weak response to endotoxin infusion. Moreover, there was a remarkable similarity in gene expression between the sickle cell disease and postendotoxin-treated controls, highlighting the intense level of inflammation and stress in steady-state sickle cell disease. However, there were several distinct islands of genes that had a unique expression pattern specific for sickle cell disease (Figure 6). Therefore, much of the gene expression pattern observed in sickle cell patients are effects specific to sickle cell disease and are not due to a nonspecific systemic inflammatory state.
Limited contribution of neutrophil- and platelet-derived genes to the peripheral blood mononuclear cell gene expression profile
To determine whether contaminating RNA from platelets in our PBMC preparation could contribute to our gene expression profile, we isolated RNA from platelet-rich plasma of 2 additional sickle cell patients and 2 control subjects and found that even this platelet-rich fraction contained insufficient RNA (571 ng and 189 ng in sickle cell and control patients respectively) for use in microarray experiments. Additionally, based on the amount of platelet contamination measured in our PBMC preparations by flow cytometry (see Supplemental Materials), we found that contaminating platelets would account for less than 0.5% of total RNA of the PBMC preparation, an amount of RNA insufficient for microarray experiments.
To determine whether neutrophil-specific genes contributed to our gene list we isolated neutrophils from blood of 6 control subjects before and after endotoxin (100 ng/mL) exposure (to evoke the expression of a broad range of neutrophil-derived genes). Neutrophil-derived gene expression was compared with peripheral blood mononuclear cell gene expression of 3 additional sickle cell patients, and 88 neutrophil-specific genes were identified. Only 4 neutrophil-specific genes overlapped with our sickle cell gene expression list (see Supplemental Table S2 and Supplemental text). Additionally, the expected average yield of total RNA from contaminating neutrophils in our PBMC preparation would account for less than 0.25% of the total RNA (see Supplemental Materials). Therefore, the inflammatory genes on our list of 385 that appear to be of neutrophil or platelet origin are unlikely to be derived from these cells—as our PBMC preparation had insufficient platelet and neutrophil RNA for microarray experiments.45 However, it remains possible that a few highly expressed genes from platelets or neutrophils have contributed to our gene list.
Discussion
Sickle cell disease is caused by the downstream effects of hemoglobin S polymerization, leading to chronic cycles of ischemia-reperfusion vascular and tissue injury. This direct tissue ischemia and factors such as plasma hemoglobin-induced endothelial dysfunction, free radical generation, and cytokine activation produce a characteristic and unique chronic inflammatory state that further promotes and propagates vascular insufficiency and ultimately results in tissue infarction.7,27 This thesis is supported by the observation that leukocytosis and dactilitis in infants predict subsequent morbidity and mortality in children and adults. Furthermore, systemic markers of inflammation such as C-reactive protein, soluble adhesion molecules, endothelin-1, and cytokines are increased in plasma during steady-state and vaso-occlusive pain crisis.6,13,15,23,24,46-48 To better understand the contribution of oxidant stress and inflammation to the pathogenesis of sickle cell disease we have interrogated the abundant and readily accessible circulating leukocyte pool using global transcriptional analysis and find that sickle cell disease evokes a highly specific transcriptional response.
Hierarchical clustering of all subjects based on the expression pattern of the 112 significant genes separated subjects into distinct groups: patients with sickle cell disease, African-American and endotoxin controls, and controls treated with a single dose of endotoxin (an inflammatory control group). Interestingly, hydroxyurea treatment did not significantly affect gene expression profiles, even using less stringent multiple comparisons corrections (≤ 20% false discovery rate). These data suggest that hydroxyurea does not have a direct effect on leukocyte gene expression in sickle cell patients and weighs on an ongoing controversy, whether the mechanism of action of hydroxyurea is secondary to a direct anti-inflammatory effect or the antipolymerization effect of fetal hemoglobin induction.49 The beneficial effects of hydroxyurea-mediated leukoreduction on clinical outcomes have been reassessed in the multicenter hydroxyurea study. With additional follow-up, the association between leukoreduction and clinical improvement has weakened, while the association of fetal hemoglobin induction and clinical improvement remains robust.49,50 The persistence of inflammatory and oxido-reductase gene expression profiles in patients on hydroxyurea therapy may be explained by the relatively limited hemoglobin F induction, lack of full F-cell penetrance, and persistent hemolysis and inflammation in most adult patients on treatment. Analysis of polymerization tendencies suggests that levels of hemoglobin F more than 25% in a pancellular distribution are required to completely eliminate intracellular polymerization.51,52 An alternate explanation is that the effect of hydroxyurea on gene expression is smaller than the patient-to-patient variability in gene expression found in sickle cell disease.
A single dose of endotoxin in controls produces an inflammatory transcriptional response that has some similarities to that seen in steady-state sickle cell disease. At less than or equal to 10% false discovery rate a large majority of 385 genes were similarly differentially regulated in patients with sickle cell disease, including cell cycle regulation, apoptosis, adhesion molecules, interferon-induced genes, kinases, and signaling molecules. However, the gene expression profile for sickle cell disease is unique for a large number of related gene families including heme-processing enzymes, growth factors (IL-15, endothelial cell growth factor-1 [ECGF-1]), antioxidant systems, adhesion molecules such as integrins and P-selectin, and globin genes (Table 3). Some of the genes on our gene list may be coexpressed on multiple cell types; other genes such as P-selectin and platelet glycoprotein genes may also be coexpressed on other cells or may be derived from small amounts of platelet contamination secondary to platelet-monocyte aggregation. IL-15 is important in the maintenance of immune cell function but also stimulates inflammatory cytokine production such as IFNγ, TNFα, and IL-1β, cytokines that have been shown to be increased in patients with sickle cell disease.48 IL-15 is also angiogenic and increased expression has been demonstrated in other inflammatory diseases such as rheumatoid arthritis, inflammatory bowel disease, multiple sclerosis, and sarcoidosis.53 The exact role of this cytokine in the pathogenesis of sickle cell disease remains to be explored. ECGF-1, usually produced from platelets but perhaps from peripheral blood mononuclear cells as well, is chemotactic for endothelial cells and monocytes and promotes angiogenesis. The gene expression profile of sickle cell disease shows a global up-regulation of pro-inflammatory markers that may contribute to disease pathogenesis but it also shows up-regulation of several compensatory mechanisms. The HO-1 pathway and downstream effectors may play a significant role in the anti-inflammatory and vascular protective responses to ischemia-reperfusion injury in sickle cell disease.
Chronic hemolysis in sickle cell disease requires the up-regulation of enzyme systems to catabolize over 30 g of potentially toxic and pro-oxidant hemoglobin released per day from hemolyzed erythrocytes.27 Heme oxygenase-1, the inducible isoform of heme oxygenase, is the rate-limiting enzyme in heme catabolism.24,54-56 HO-1 is found predominantly in Kuppfer cells of the liver and circulating and tissue monocytes; however, it has been demonstrated in other cell types throughout the body in response to heme exposure, inflammation, oxidant injury, and other stressors. Heme is broken down to biliverdin by HO-1, releasing carbon monoxide and iron. Biliverdin is converted to bilirubin by the enzyme biliverdin reductase. Biliverdin reductase has recently been proposed as a major catalytic antioxidant system.57 The functional role of HO-1 extends beyond heme catabolism; its induction is a compensatory response to tissue stress or injury and protects from the deleterious effects of inflammation. Beneficial effects of HO-1 include inhibition of inflammation in models of ischemia-reperfusion and xenograft rejection, protection from oxidant-induced injury, enhanced induction and mediation of the anti-inflammatory effects of IL-10, inhibition of vascular smooth muscle proliferation in response to vascular injury, antiatherogenesis, and vascular relaxation.24,42-44,57-62
These data are consistent with the recent observation of increased HO-1 expression in renal tissue and circulating endothelial cells of patients with sickle cell disease.63 We also show the concurrent up-regulation of biliverdin reductase (α and β) and p21. In aggregate, these data suggest that circulating mononuclear cells participate in a compensatory response to the repetitive vascular injury characteristic of sickle cell disease.43,44,63,64 For example, despite having reduced nitric oxide bioavailability and endothelial dysfunction,27,28 patients with sickle cell disease do not develop atherosclerotic coronary disease,65-67 which may be due to the vascular protective functions of the HO-1 and p21 systems. Other antioxidant genes such as glutathione peroxidase, thioredoxin, and thioredoxin peroxidase are up-regulated in sickle cell patients, demonstrating a compensatory response to chronic ischemia-reperfusion–induced oxidant injury.
Circulating leukocytes represent a readily accessible cell population centrally involved in sickle cell disease vasculopathy. Our results highlight the intense pro-oxidant, hemolytic, and inflammatory nature of steady-state sickle cell disease and support the precis that sickle cell patients suffer from chronic ischemia-reperfusion vascular injury.3,4 These data also provide novel insight into the broad compensatory responses to sickle cell vascular injury with dramatic up-regulation of catalytic antioxidant and stress response systems and angiogenic factors. Such pathways are ideally suited for polymorphism studies to explain phenotypic heterogeneity. Given the limited predictive value of current biomarkers of sickle cell disease severity such as white blood count and transcranial Doppler, inflammatory fingerprints of peripheral blood may provide a better prognostic tool for identifying patients at high risk of debilitating clinical events who would be candidates for more aggressive therapies such as bone marrow transplantation or gene therapy.
Prepublished online as Blood First Edition Paper, March 18, 2004; DOI 10.1182/blood-2003-08-2760.
The online version of the article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We would like to thank Alan Schechter and Greg Kato for their careful review of our manuscript; Jennifer Kawwass, Pat Madara, Xunde Wang, and Christopher Reiter for their contributions and technical expertise; and Patricia Smatlak and Wynona Coles for recruitment and care of research subjects.

![Figure 5. Validation of gene expression data for the HO-1 pathway and p21. Gene expression levels are reflected as arbitrary units of fold-change of expression relative to mean control levels. Black lines in B, C, and D are regression lines. (A) Sickle cell disease patients (red bars) have increased mean gene expression (left y-axis) of all enzymes in the heme catabolism pathway and have higher mean serum total bilirubin (right y-axis), the end product of heme breakdown, compared with healthy volunteers (gray bars). Error bars reflect SEM. (B) Carbon monoxide production (determined by carboxy hemoglobin levels measured by co-oximetry) was measured in 24 patients with sickle cell disease (13 HbSS not on hydroxyurea therapy [▴], 8 HbSS on hydroxyurea [▪], 2 HbSC and 1 HbSβ-thalassemia phenotype [○]). Carbon monoxide production correlates with plasma heme levels measured by benzidine assay. (C) Carbon monoxide production correlates with HO-1 gene expression measured by microarray in these same 24 sickle cell disease patients. (D) Serum total bilirubin levels were measured in 27 sickle cell disease patients (14 HbSS not on hydroxyurea therapy [▴], 10 HbSS on hydroxyurea [▪], 2 HbSC and 1 HbSβ-thalassemia phenotype [○], and 13 controls [•]). Serum total bilirubin correlates with biliverdin reductase gene expression measured by microarray. Gene expression levels for biliverdin reductase also correlate with CO production (r = 0.55, P < .005, data not shown). (E) HO-1 and p21 gene expression measured by microarray and real-time PCR show increased expression in 27 sickle cell disease patients. Error bars reflect SEM. (F) Patients with sickle cell disease (n = 4) have increased cellular HO-1 protein levels in their peripheral blood mononuclear cells compared with healthy volunteers (n = 4) as measured by Western blot. Lane 1 represents an HO-1–positive control.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/104/1/10.1182_blood-2003-08-2760/6/m_zh80130463560005.jpeg?Expires=1763810993&Signature=15YjD9Lqf50xMftXPj-SvQOiphI1A2dj0g4s7kL0esCZLbYzg8YTk9gsOPauPMqPYTCvzL0IOScSK7rWtvi8j8jzRPyM-owaFyYM7n1zxCPA4iB9HIsYohMXNNPSvatyYK43pidtGwuVlK4v7s8BJc-MeEW5hHs~tbAuq4SYVluM7u65mSHj1Lt~ktyiRzD~MUmwTqsD7QTq9~0gKVpGFwlzIt1ap-z47qohmUYePhUaN571nBARxmrN-8sBw8d3-Us0T0ACbo281f4UyKNz5l3wH38KEWgIRDx5tZ9BG7yVZzw5xQ9dbpxyjtI2~7Y7R9E6GXZrOP0-A-miNRYLKQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)