Abstract
The most efficient therapeutic approach for immunoglobulin light chain amyloidosis (AL) is autologous stem cell transplantation (ASCT); however, the toxicity of ASCT limits its feasibility to a minority of patients. Patients ineligible for ASCT are usually treated with standard oral melphalan and prednisone, but the response rate to this regimen is unsatisfactory, and time to response is long. High-dose dexamethasone provides a rapid response time in patients with AL. We evaluated the combination of oral melphalan and high-dose dexamethasone (M-Dex) in 46 patients with AL ineligible for ASCT. Thirty-one (67%) achieved a hematologic response and 15 (33%) a complete remission. In 22 (48%) of the responsive patients functional improvement of the organs involved was observed. Five patients (11%) experienced severe adverse events, 3 required hospitalization, and no treatment-related deaths were observed. M-Dex represents a feasible and effective therapeutic option for patients with advanced AL who are ineligible for ASCT. (Blood. 2004;103:2936-2938)
Introduction
Immunoglobulin light chain amyloidosis (AL) is caused by a usually small bone marrow plasma cell clone, synthesizing monoclonal light chains that undergo conformational modifications and aggregate into amyloid fibrils.1 In the systemic disease, the fibrils form extracellular deposits in one or more vital organs, most frequently the kidney, heart, liver, and peripheral and autonomic nervous system.2 The prognosis of patients with systemic AL is poor: the Mayo Clinic Group reported a median survival of 12 to 18 months.2,3 The main prognostic determinant is amyloid heart involvement.2 At present, the most effective approach to treating AL is high-dose chemotherapy followed by autologous stem cell transplantation (ASCT), aimed at annihilating the amyloidogenic plasma cell clone. However, ASCT-related mortality (21% even at referral centers) and toxicity limit the feasibility of transplantation to a minority of patients,4,5 although patients who cannot undergo this procedure are those in greatest need of prompt care. Patients ineligible for ASCT are usually treated with standard oral melphalan and prednisone. Although this regimen has a very low toxicity, only 28% of patients achieve a response that, in 30% of them, is obtained after more than 1 year.3 Time to response is crucial in patients with AL, particularly in patients who present with advanced disease. A previous study from our group showed that high-dose dexamethasone (HD-Dex) provides a rapid response (median, 4 months) with a 35% response rate in an unselected series of patients.6 We reasoned that combining HD-Dex with melphalan, both effective and well tolerated in patients with advanced AL, could synergize their therapeutic effect.
Study design
Forty-six patients referred between December 1999 and October 2002 to the coordinating Center of the Italian Amyloidosis Study Group in Pavia with a histologic diagnosis of amyloidosis and evidence of plasma cell dyscrasia who did not meet the eligibility criteria for ASCT were entered into the study. All patients gave oral informed consent according to the Policlinico San Matteo review board guidelines. Eligibility criteria for high-dose chemotherapy and ASCT were 2 or fewer organs involved, absence of severe cardiac involvement, creatinine 176.8 μM/L (2 mg/dL) or less, age 65 years or younger, and normal respiratory function tests.5
All patients underwent a complete physical examination, high-resolution serum and urine immunofixation electrophoresis,7 complete blood count, assessment of renal and liver function, echocardiography, and 24-hour electrocardiogram monitoring (Holter ECG). Complex ventricular arrhythmias are associated with an increased risk of sudden death in AL,8 and, in our preliminary experience with conventional high-dose dexamethasone, we observed a relevant proportion of treatment-related fatal arrhythmias.6 Therefore, patients in whom the Holter ECG detected couplets and/or ventricular tachycardia received prophylactic amiodarone (200 mg/d, 5 d/wk which was then continued indefinitely).
The patients were treated with melphalan 0.22 mg/kg and dexamethasone 40 mg given orally on days 1 to 4 every 28 days. Prophylactic omeprazole (20 mg/d), ciprofloxacin (250 mg twice daily), and itraconazole (100 mg/d) were also prescribed on days 1 to 10.
Hematologic response to treatment was defined as a 50% or more decrease in serum and urine monoclonal component (MC). Functional improvement of the organs involved was assessed according to the Mayo Clinic Group.3 Complete hematologic remission was defined as the disappearance of serum and urine MC at high-resolution immunofixation maintained for at least 3 months. The response was evaluated every 3 months and established at the nadir of serum and urine monoclonal protein. Treatment was continued for up to 9 courses in patients who achieved the hematologic response and was discontinued if a complete hematologic remission was obtained, if the monoclonal component increased, and in the case of treatment-related toxicity.
A survival curve was plotted according to Kaplan-Meier, and the difference in survival was tested for significance with the log-rank test.
Results and discussion
Forty-six consecutive patients (33 men), were enrolled into the study. Their median age was 62 years (range, 34-79 years). A monoclonal protein was detected by high-resolution immunofixation in the serum and/or urine of all patients (32 λ, 9 κ, 5 biclonal).
High-dose chemotherapy and ASCT were contraindicated because of severe heart involvement in 32 patients (70%), involvement of more than 2 organs in 24 (52%), age older than 65 years in 17 (37%), creatinine greater than 176.8 μM/L (2 mg/dL) in 2 patients (4%), and abnormal respiratory function tests in 1 patient (2%). Twenty-four patients (52%) were not eligible for ASCT for at least 2 criteria.
The heart was involved in 32 patients (70%), the kidney in 29 (63%), the peripheral nervous system in 11 (24%), the liver in 6 (13%), the soft tissues in 4 (9%), and the lung in 1 (2%). Sixteen patients (35%) had orthostatic hypotension. Thirty-five patients (76%) had more than one organ involved. Median urinary protein loss and serum creatinine in the whole group were 4 g/24 h (range, 0-24 g/24 h) and 88.4 μM (1 mg/dL) (range, 44.2-318.24 μM [0.5-3.6 mg/dL]), respectively. Twenty-seven patients had proteinuria in the nephrotic range (≥ 3 g/24 h). The remaining 2 patients with renal amyloidosis had non-nephrotic proteinuria (1.6 and 2.7 g/24 h, respectively), and 1 patient had renal failure (serum creatinine 176.8 μM/L [2 mg/dL]). Median interventricular septum (IVS) thickness and ejection fraction were 14 mm (range, 8-23 mm) and 50% (range, 34%-75%), respectively. In 15 patients (33%) the IVS thickness was more than 15 mm.
Patients were treated with a median of 4 courses (range, 1-9 courses). Two patients died before completing the third cycle.
Thirty-one patients (67%) obtained a hematologic response, and 15 (33%) of these patients achieved complete hematologic remission. The median time to response was 4.5 months (range, 2.3-10.1 months). Twenty-two patients (48%), in whom the MC decreased by at least 50%, also achieved significant functional improvement of involved organs (Table 1).
Fourteen patients had a 50% or greater reduction of proteinuria; 6 had a 2-mm or more decrease in IVS thickness associated with resolution of heart failure; 1 patient, with lung involvement, had complete resolution of dyspnea and improvement of respiratory function tests (increase of vital capacity and forced expiratory volume); and 1 patient, with liver involvement, had normalization of alkaline phosphatase (from 451 to 178 U/L; reference, < 279 U/L). In 16 patients, organ and hematologic responses were simultaneous, whereas in 6 cases organ function improved in a period ranging from 2.6 to 18.1 months (median, 4.6 months) after the hematologic response. In 2 patients in complete remission, reported in Table 1, the organs involved (heart and kidney) did not improve, and the patients are alive. In 14 of the 15 patients who achieved complete hematologic remission, the response is maintained off therapy after a median follow-up of 16.4 months (range, 3-34 months). In one patient the monoclonal component reappeared after 29 months.
Five patients (11%) experienced severe (Common Toxicity Criteria [CTC] grade ≥ 3) adverse events. Three patients had respiratory infections, requiring admission to hospital. Two of them were the only patients who had not followed the antibiotic prophylaxis as prescribed. One patient, after the eighth cycle of M-Dex, developed reversible severe cytopenia which needed granulocyte colony-stimulating factor and platelet transfusional support. One patient, who had received 2 courses of M-Dex, developed a myelodysplastic syndrome.
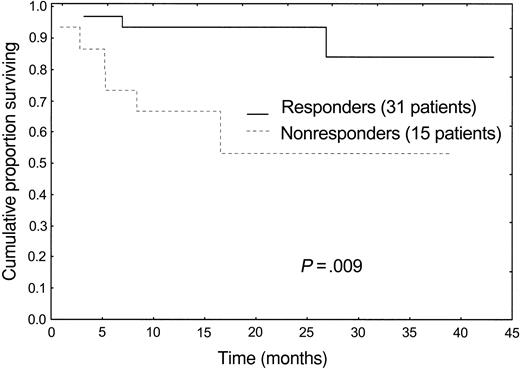
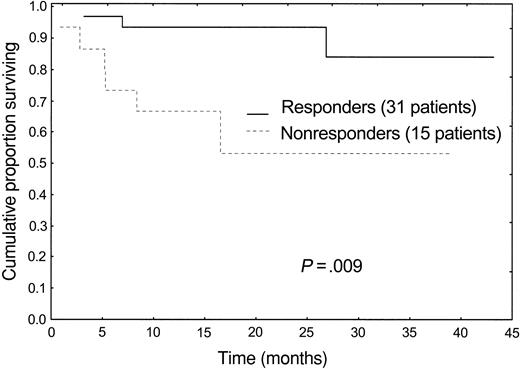
The median follow-up of living patients is 20 months (range, 6-43 months) from starting therapy. Overall, 9 patients (20%) died after a median follow-up of 5 months (range, 1-27 months). Eight patients had severe heart involvement and died of heart failure (6 patients) and sudden death (2 patients), despite 2 of them having achieved a hematologic response, although not complete remission. One patient died as a result of a preexisting not amyloid-related obstructive lung disease, still in complete hematologic remission, 27 months after therapy. Hematologic response to therapy translated into a significant survival advantage (Figure 1).
These data show that treatment with M-Dex (1) is feasible and well tolerated in patients who are ineligible for ASCT because of advanced organ damage, (2) provides a high rate (67%) of durable responses in a short time (median, 4.5 months) with a positive affect on survival, and (3) produces significant organ function improvement in nearly half the patients.
Despite the fact that we selected patients with advanced disease, ineligible for ASCT, there was no treatment-related mortality, and toxicity was low. M-Dex appeared to be a feasible option in this subset of patients and showed a good cost-effectiveness profile: During therapy, only 3 patients (6%), who developed respiratory infections, required admission to hospital. The response rate observed in this series (67%, 33% complete remission) compares favorably with that achievable in unselected patients with standard melphalan and prednisone (28%, complete remission not specified)3 and also with the results obtained with HD-Dex (35% response rate, 9% complete remission) of our previous study.6 The short time to response (median, 4.5 months) is crucial in patients with advanced disease. One third of the patients achieved a durable complete hematologic remission, a rare event in patients with AL who cannot undergo transplantation. Despite advanced functional impairment, the reduction of the MC translated into improved function of the organs involved by the disease in 48% of patients and resulted into a significant survival benefit (Figure 1). Most important, we observed resolution of heart failure in 6 of 32 cases. The observation of fatal heart failure despite reduction of the amyloidogenic protein more than 50% is not surprising, considering the possible irreversible organ damage caused by the disease, and highlights the paramount importance of early diagnosis.
Patients who do not reach complete remission with M-Dex, but who obtain significant organ improvement, might be re-evaluated for transplantation, although the exposure to the alkylating agent may jeopardize stem cell harvesting. In this respect, in patients who present with a potentially reversible contraindication for ASCT (ie, isolated severe heart involvement), treatment with the modified HD-Dex regimen6 may be considered, carefully weighing the lower response rate (67% versus 35%) versus the preserved possibility of harvesting stem cells. Thalidomide is also an effective alternative, but, unfortunately, it is poorly tolerated by these fragile patients.9
Supported by grants from the Italian Ministry of Health, the IRCCS–Policlinico San Matteo, the CARIPLO Foundation, Milan, and the Carlo Bernasconi Family Research Fund.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
Prepublished online as Blood First Edition Paper, December 18, 2003; DOI 10.1182/blood-2003-08-2788.