Abstract
Imatinib mesylate is a selective inhibitor of a few tyrosine kinases including KIT, and it is the first effective treatment for gastrointestinal stromal tumors (GISTs). We monitored the serum levels of KIT, KIT ligand (stem cell factor, SCF), and the vascular endothelial growth factor (VEGF) in patients with advanced GISTs treated with imatinib in a prospective randomized trial. Patients with GISTs (n = 66) had elevated pretreatment serum KIT and VEGF levels as compared with controls (median, 292 AU/mL [409 ng/mL] vs 238 AU/mL [333 ng/mL], P = .037; and median, 303 pg/mL vs 190 pg/mL, P = .013, respectively), but lower levels of SCF (median, 645 pg/mL vs 950 pg/mL; P ≤ .0001). After 1 and 6 months of imatinib treatment the average serum KIT levels decreased 31% and 52% from pretreatment levels, whereas SCF levels increased 11% and 33%, respectively. Serum VEGF levels decreased during treatment in responding patients. The median serum SCF/KIT ratio increased with treatment duration, and was 7.7-fold higher after 12 months of treatment than at baseline (range, 3.1-259-fold). A high serum SCF/KIT ratio may increase SCF-induced cell signaling with prolonged imatinib treatment, at the time when imatinib treatment is withdrawn, and in patients whose GIST has wild-type receptors. (Blood. 2004;103:2929-2935)
Introduction
Imatinib mesylate (Glivec, Gleevec, formerly known as STI571; Novartis, East Hanover, NJ) is a selective inhibitor of certain tyrosine kinases, including ABL, BCR-ABL, ARG, KIT, and the platelet-derived growth factor receptors (PDGFRs; reviewed in Capdeville et al1 ). Imatinib is highly active in the treatment of chronic myeloid leukemia, where imatinib inhibits the kinase activity of the BCR-ABL fusion protein.2 Other target diseases include chronic myelomonocytic leukemia, where imatinib may act by inhibiting the TEL-PDGFRB fusion protein produced as a result of the 5;12(q31-33, p13) translocation,3 and idiopathic hypereosinophilic syndrome by inhibiting the Fip-1-like 1 (FIP1L1)–PDGFRA fusion protein that has tyrosine kinase activity.4 Imatinib therapy may be effective also in the treatment of dermatofibrosarcoma protuberans, where it inhibits PDGFR activation related to the COL1/PDGF fusion gene resulting from translocation between chromosomes 17 and 22,5 and of some desmoid tumors, where the exact mechanism of action is not well understood.6
Imatinib is the first effective systemic therapy for gastrointestinal stromal tumors (GISTs).7 GISTs are mesenchymal tumors of the gastrointestinal tract that are likely to originate from the interstitial cells of Cajal.8,9 GISTs express the KIT receptor tyrosine kinase, which can be detected by staining for the CD117 antigen using immunohistochemistry.9,10 Up to 90% of GISTs have a gain-of-function mutation in the KIT proto-oncogene that encodes the KIT protein. Most mutations occur in the KIT exon 11, but mutations may also be found in exon 9, and rarely in exons 13 and 17.9,11 The ligand of KIT is the stem cell factor (SCF), which exists as a dimer in vivo, and upon binding to KIT induces KIT dimerization, autophosphorylation of certain tyrosine residues by KIT cross-phosphorylation, and kinase activation. This leads to a cascade of intracellular signaling events that favor cell proliferation and enhanced cell survival. Gain-of-function mutations of KIT result in ligand-independent activation of KIT signaling, leading to constitutive growth and antiapoptotic signals.
GISTs are notoriously resistant to conventional chemotherapy,7 but the majority of patients with GISTs treated with imatinib mesylate achieve a durable response.7,12 About 10% to 15% of GISTs are primarily resistant to imatinib, and a considerable proportion of the patients who first respond to imatinib later show acquired resistance.7 The mechanisms of imatinib resistance are currently not known, but the type of KIT mutation appears to be predictive for treatment outcome. Patients with exon 11 mutations have a higher likelihood of response to imatinib than those with GISTs with exon 9 mutation. Patients with wild-type KIT rarely respond to imatinib.7,42
Proteolytic cleavage at the proximal immunoglobin-like extracellular domain of KIT-expressing cells, such as the hematopoietic cells, mast cells, or melanocytes, results in the genesis of soluble KIT.13 Importantly, soluble KIT binds SCF with an affinity equal to that of membrane-bound KIT, and is able to inhibit SCF bioactivity in vitro.14 Thus, soluble KIT may be an important regulator of SCF-mediated activity. Healthy individuals have about 200 ng/mL to 400 ng/mL of soluble KIT in the serum, and the molar ratio of KIT to SCF is about 30:1 in human sera.13 No correlation has been reported between serum-soluble KIT levels and age or gender.15-17
Serum SCF is produced by proteolytic cleavage of the membrane-bound 248 and 220 amino acid residue isoforms of SCF on various cell types, including fibroblasts, endothelial cells, and hematopoietic progenitor cells. Variable median serum SCF levels have been measured in healthy individuals, the median levels ranging from 600 pg/mL to 3300 pg/mL.18-23
In the present study we longitudinally monitored serum levels of SCF, soluble KIT, and the vascular endothelial cell growth factor (VEGF) in patients with GISTs treated with imatinib in conjunction with a prospective multicenter randomized trial. Since SCF binds to and activates the wild-type KIT receptor tyrosine kinase, and soluble KIT probably functions as a regulator of the biologic activity of SCF, a change in the relative proportions of soluble SCF and soluble KIT during imatinib therapy might influence the potential for SCF-mediated cell signaling. We also monitored the serum levels of the major angiogenic growth factor, VEGF, in the same patients, because GISTs are generally highly vascular tumors and serum VEGF levels have been reported to change in parallel with treatment response in certain types of human cancer.24-26 To our knowledge, serum KIT, SCF, and VEGF levels in patients with GISTs are not known, and have not been previously evaluated during imatinib therapy.
Patients, materials, and methods
Patients and treatment
The effect of imatinib on serum growth factor levels was investigated in a series of patients who took part in a clinical trial that evaluated the safety, tolerability, and efficacy of imatinib mesylate in patients with inoperable and/or metastatic GISTs (the CSTI571B2222 trial).7 In this open-label, randomized, multicenter trial, a total of 147 patients with histologically verified, immunohistochemically CD117 (KIT)–positive GISTs were randomly allocated to receive either 400 mg or 600 mg imatinib mesylate orally once daily until treatment failure. Treatment toxicity, blood cell counts, and blood chemistry were monitored longitudinally, and treatment response was evaluated at 1- to 3-month intervals using either computed tomography or magnetic resonance imaging (MRI). The results of the study have been published elsewhere.7 In brief, 54% of the patients had at least 50% reduction of the tumor volume (a partial response), 28% had stable disease, 14% had progressive disease during a median follow-up of about 10 months, and 5% of the patients were not evaluable for response. Patients were required to sign an informed consent for study treatment, serum sampling, and tissue biopsies before study entry, and the study protocol was approved by the institutional review board of each of the 4 participating centers.
Sera from 66 of the 147 patients enrolled in the study were available to us for measurement of soluble KIT, SCF, and VEGF levels prior to initiation of imatinib treatment, and after starting treatment. These 66 patients form the basis of the present study. Their median age was 54 years (range, 25-79 years), and 32 (48%) were male. Of the 66 patients, 9 (14%) had progressive disease despite imatinib therapy. The median age and the progressive disease rate of the 66 subjects included in the present study are identical to those of the 147 patients who were enrolled in the CSTI571B2222 trial. KIT gene mutation analysis was done with denaturing high-performance liquid chromatography (dHPLC) and DNA sequencing. Exon 11 mutation was found in 34 cases (52%), exon 9 mutation in 8 cases (12%), exon 13 and 17 mutation in 1 case (1%) each, and no mutation was detected in 4 cases (6%) (the mutational status was unknown in 18 cases [27%]). Thirty-seven patients (56%) received 400 mg and 29 (44%) received 600 mg imatinib daily. Data from 2 patients diagnosed with GIST and treated in a compassionate-use protocol in one of the study centers (Helsinki) were also included in the analysis of long-term effects of imatinib on serum KIT and growth factor levels (see Table 2).
Average change in soluble KIT, SCF, and VEGF in patients with GISTs treated with imatinib mesylate
Factor . | Pretreatment . | 1 month on therapy* . | Average change, % . | 6 months on therapy† . | Average change, % . | P (change from pretreatment level)‡ . | P (responders vs nonresponders)‡ . |
|---|---|---|---|---|---|---|---|
| KIT | 292 AU/mL | 204 AU/mL | −31 | 132 AU/mL | −52 | .0012 | — |
| Responders | 298 AU/mL | 202 AU/mL | −35 | 140 AU/mL | −52 | — | .28 |
| Nonresponders | 258 AU/mL | 204 AU/mL | −17 | 113 AU/mL | −62 | — | — |
| SCF | 645 pg/mL | 770 pg/mL | 11 | 875 pg/mL | 33 | .0001 | — |
| Responders | 660 pg/mL | 740 pg/mL | 9 | 880 pg/mL | 33 | — | .18 |
| Nonresponders | 620 pg/mL | 780 pg/mL | 20 | 880 pg/mL | 23 | — | — |
| VEGF | 295 pg/mL | 180 pg/mL | −42 | 205 pg/mL | −31 | .14 | — |
| Responders | 320 pg/mL | 180 pg/mL | −45 | 220 pg/mL | −34 | — | .22 |
| Nonresponders | 160 pg/mL | 145 pg/mL | 0 | 185 pg/mL | −.9 | — | — |
Factor . | Pretreatment . | 1 month on therapy* . | Average change, % . | 6 months on therapy† . | Average change, % . | P (change from pretreatment level)‡ . | P (responders vs nonresponders)‡ . |
|---|---|---|---|---|---|---|---|
| KIT | 292 AU/mL | 204 AU/mL | −31 | 132 AU/mL | −52 | .0012 | — |
| Responders | 298 AU/mL | 202 AU/mL | −35 | 140 AU/mL | −52 | — | .28 |
| Nonresponders | 258 AU/mL | 204 AU/mL | −17 | 113 AU/mL | −62 | — | — |
| SCF | 645 pg/mL | 770 pg/mL | 11 | 875 pg/mL | 33 | .0001 | — |
| Responders | 660 pg/mL | 740 pg/mL | 9 | 880 pg/mL | 33 | — | .18 |
| Nonresponders | 620 pg/mL | 780 pg/mL | 20 | 880 pg/mL | 23 | — | — |
| VEGF | 295 pg/mL | 180 pg/mL | −42 | 205 pg/mL | −31 | .14 | — |
| Responders | 320 pg/mL | 180 pg/mL | −45 | 220 pg/mL | −34 | — | .22 |
| Nonresponders | 160 pg/mL | 145 pg/mL | 0 | 185 pg/mL | −.9 | — | — |
For each group (KIT, SCF, VEGF), study populations are as follows: responders, n = 57; and nonresponders, n = 9. The “baseline” and “on therapy” data are given as the medians observed; the average change is the median percentage of the change from the baseline value. —indicates not applicable.
N = 66.
N = 45.
Repeated measures ANOVA test.
Serum and blood samples
A pretreatment (baseline) serum and blood sample for growth factor analysis was taken 0 to 7 days before starting imatinib treatment. The first follow-up samples were collected about 1 month (range, 2-7 weeks) after taking the first dose of imatinib, and the subsequent samples were collected a few months after the first imatinib dose. Serum (> 1 mL) was collected, centrifuged at 2000g for 10 minutes at +4°C, and then stored as aliquots at -20°C or colder. Whole blood samples (> 2 mL) were collected in test tubes containing sodium citrate as an anticoagulant. The whole-blood samples were lysed by adding 2 volumes of sterile aqua, and subsequently freeze-thawing the samples twice.27
Control subjects
Serum KIT, SCF, and VEGF were measured in 40 control subjects. We obtained sera from 24 generally healthy hospital workers who consented to give a blood sample for the study, and 16 male individuals who took part in a prostate-specific antigen (PSA)–based screening trial for prostate cancer. The PSA-screening trial participants were identified from the Population Registry of Finland,28 and the analyses were done from the leftover sera after serum PSA had been measured. The samples were frozen and stored for 4 to 6 months at -20°Corat -80°C before analysis. The median age of the control subjects was 52 years (range, 23-71 years), and 55% were male.
Serum concentrations of KIT, SCF, and VEGF
The serum levels of KIT, SCF, and VEGF were determined by enzyme-linked immunosorbent assay (ELISA). The analyses were carried out according to the manufacturer's recommendations, and as described in detail for VEGF elsewhere.27 Serum and whole-blood VEGF was measured on anti–human VEGF–coated microwells, and horseradish peroxidase (HRP)–conjugated anti-VEGF antibody (Ab) was used as the secondary Ab (R&D Systems, Minneapolis, MN). The assay detects the VEGF isoforms VEGF121 and VEGF165. The ELISA protocol used to measure serum KIT (Nichirei Corporation, Tokyo, Japan) detects both normal and mutated serum KIT.29 The assay uses mouse anti-human KIT Ab–coated microwells, and alkaline phosphatase–conjugated anti-KIT Ab as the secondary Ab. The results were read as AU/mL, where one unit equals to 1.4 ng/mL circulating KIT. SCF levels were measured with an ELISA that uses mouse anti-human SCF Ab–coated microwells as the capturing antibody, and an HRP-conjugated Ab against recombinant human SCF as the secondary Ab (R&D Systems). According to the manufacturer, addition of KIT protein to the sample does not cause any significant crossreactivity or interference with the SCF analysis.
Serum analyses were done blinded to the clinical data. Intraassay variability was assessed by analyzing 2 replicates of 15 serum samples, and the interassay variation by assaying 15 samples twice in 2 separate assays. Both interassay and intraassay variability were less than 10%. The effect of sample freezing on serum KIT and SCF levels was investigated by measuring serum KIT and SCF concentration before and after a freeze-thaw cycle in samples taken from 6 cancer patients and 6 controls, but no significant difference was found in the serum levels when measured before and after freezing the samples (data not shown). Freezing and thawing of serum samples does not influence serum VEGF concentrations.30
Statistical analysis
The statistical analyses were performed using StatView version 5.0 (SAS Institute, Cary, NC). Serum concentrations of SCF, KIT, and VEGF between 2 groups were compared using the Mann-Whitney test. The Spearman rank correlation coefficient was computed to study the association between serum and lysed whole-blood VEGF concentrations. Changes in SCF, KIT, and VEGF levels over time were compared using repeated measures analysis of variance (ANOVA). All P values are 2-sided.
Results
Association between serum and lysed whole-blood VEGF levels
Because we and others have found that most VEGF in the blood circulation is carried within platelets and the white blood cells, handling of the blood samples might influence the serum VEGF levels.27,31 To investigate this, we first compared serum and lysed whole-blood sample VEGF levels taken from the same individuals at the same time (n = 32). The median pretreatment VEGF concentration in these sera was 310 pg/mL (range, 24-2400 pg/mL), and in the corresponding whole-blood samples it was 810 pg/mL (range, 234-3150 pg/mL, P < .0001). High pretreatment serum VEGF levels were associated with high whole-blood VEGF concentrations, suggesting that serum VEGF can be used as a reasonable surrogate marker for the whole-blood VEGF content (Spearman rank correlation coefficient 0.66, P = <.0001). Similarly, high serum VEGF levels were associated with high lysed whole-blood VEGF levels when measured from samples taken during imatinib therapy (correlation coefficient 0.56; P = .0027).
Serum KIT, SCF, and VEGF concentrations in patients with GISTs and controls
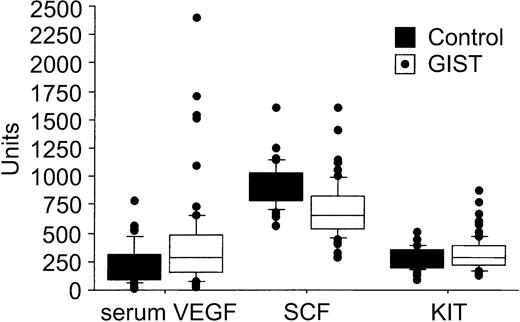
The pretreatment serum KIT concentrations were higher in patients with GISTs than in the healthy controls (median, 292 AU/mL [409 ng/mL] vs 238 AU/mL [333 ng/mL]; P = .037; Table 1). This median value of the controls is close to the one given by the manufacturer of the ELISA kit for healthy individuals (188 AU/mL ± 57 AU/mL), and similar to the values found by Kawakita et al.29 No significant associations were found between patient age at the time of sampling, gender, and the pretreatment levels of serum KIT, SCF, VEGF, or whole-blood VEGF (P > .10 for all analyses). Serum VEGF levels were generally higher in patients with GISTs than in the control subjects (median, 303 pg/mL vs 190 pg/mL; P = .013), whereas the pretreatment serum SCF concentrations were lower in patients with GISTs than in the controls (645 pg/mL vs 950 pg/mL, respectively; P < .0001; Table 1; Figure 1).
Pretreatment serum KIT, SCF, and VEGF concentrations of GIST patients and controls
. | Patients with GISTs, median (range)* . | Controls, median (range)† . | P‡ . |
|---|---|---|---|
| KIT, AU/mL | 292 (130-875) | 238 (90-514) | .037 |
| SCF, pg/mL | 645 (290-1600) | 950 (565-1600) | < .0001 |
| VEGF, pg/mL | 303 (24-2400) | 190 (10-775) | .013 |
. | Patients with GISTs, median (range)* . | Controls, median (range)† . | P‡ . |
|---|---|---|---|
| KIT, AU/mL | 292 (130-875) | 238 (90-514) | .037 |
| SCF, pg/mL | 645 (290-1600) | 950 (565-1600) | < .0001 |
| VEGF, pg/mL | 303 (24-2400) | 190 (10-775) | .013 |
N = 66.
N = 40.
P value determined by Mann-Whitney test.
A box plot of serum VEGF (pg/mL), SCF (pg/mL), and soluble KIT levels (AU/mL) in 66 patients with GISTs and 40 controls. The horizontal lines indicate the 10th, 25th, 50th (median), 75th, and 90th percentiles of the variables, and values above the 90th and below the 10th percentiles are plotted as closed circles.
A box plot of serum VEGF (pg/mL), SCF (pg/mL), and soluble KIT levels (AU/mL) in 66 patients with GISTs and 40 controls. The horizontal lines indicate the 10th, 25th, 50th (median), 75th, and 90th percentiles of the variables, and values above the 90th and below the 10th percentiles are plotted as closed circles.
Effect of imatinib treatment on serum KIT, SCF, and VEGF concentrations
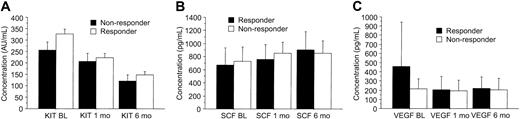
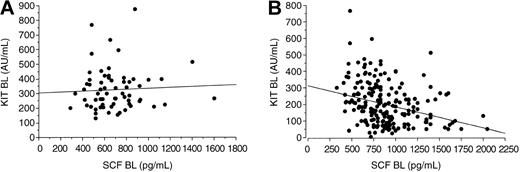
Treatment with imatinib mesylate resulted in a marked reduction in the levels of circulating KIT, whereas the SCF concentrations increased during therapy as compared with the pretreatment levels. The serum KIT levels decreased and the SCF levels increased regardless of the response to treatment (P = .0012 and .0001, respectively; Table 2; Figure 2). Patients treated with either 400 mg or 600 mg imatinib mesylate had similar serum SCF and VEGF levels during treatment (P = .31 and .65, respectively), but patients treated with 600 mg imatinib tended to have a larger decrease in serum KIT concentrations with time than those treated with 400 mg (P = .078). Before treatment, we found no significant association between the KIT and SCF levels in the sera of patients with GISTs (r = .069; P = .84) or in the sera of the controls (P = .34), whereas in samples taken during imatinib therapy high serum SCF levels were associated with low soluble KIT levels (r = -.34; P < .0001; Figure 3).
Serum KIT, SCF, and VEGF levels shown according to response to imatinib. Patients who had stable disease are included in the category of responding patients. The best response achieved was recorded. (A) KIT. (B) SCF. (C) VEGF. Mean values ± SE are shown.
Serum KIT, SCF, and VEGF levels shown according to response to imatinib. Patients who had stable disease are included in the category of responding patients. The best response achieved was recorded. (A) KIT. (B) SCF. (C) VEGF. Mean values ± SE are shown.
Association between serum KIT and SCF levels. (A) Samples taken within 1 week of starting imatinib therapy (BL indicates baseline; Spearman rank correlation coefficient .069; P = .84). (B) Samples taken during imatinib therapy (correlation coefficient -.34; P = <.0001).
Association between serum KIT and SCF levels. (A) Samples taken within 1 week of starting imatinib therapy (BL indicates baseline; Spearman rank correlation coefficient .069; P = .84). (B) Samples taken during imatinib therapy (correlation coefficient -.34; P = <.0001).
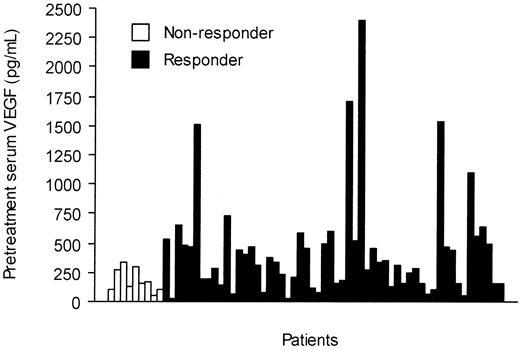
The pretreatment serum KIT levels were slightly higher in responding patients than in those who did not respond to imatinib treatment (298 AU/mL vs 258 AU/mL, respectively; P = .087). Patients who responded to imatinib had higher pretreatment serum VEGF levels than those who did not respond (median, 320 pg/mL vs 160 pg/mL; P = .035; Figure 4), and the median serum VEGF levels tended to decrease during imatinib therapy in patients who responded to imatinib therapy, who had declining values during the first month of therapy (Figure 2). However, no significant difference in serum VEGF levels was found between the responding and nonresponding patients in a repeated measures analysis of variance (P = .14, Table 2).
Pretreatment serum VEGF levels in 66 patients with GISTs according to response to imatinib therapy.
Pretreatment serum VEGF levels in 66 patients with GISTs according to response to imatinib therapy.
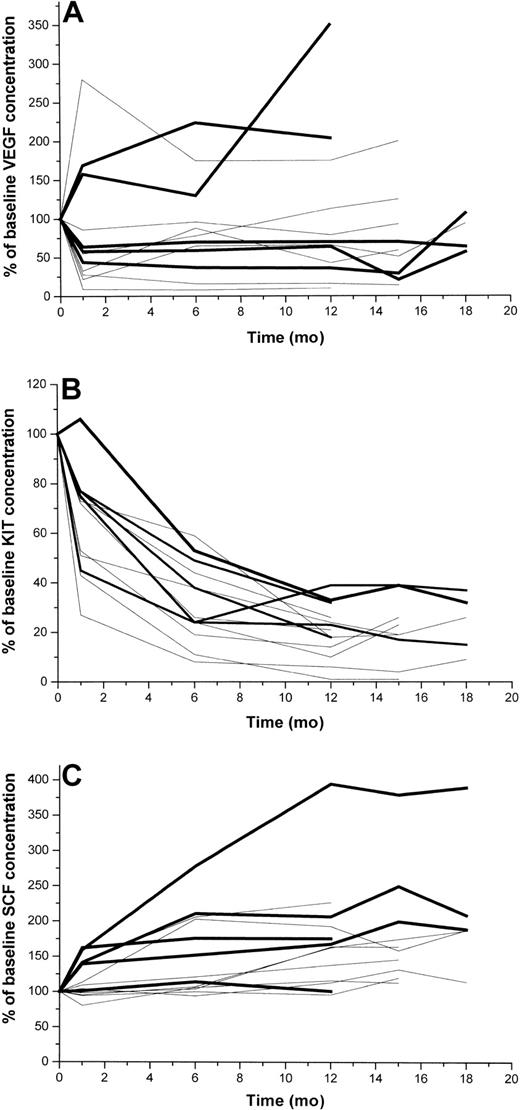
Effects of long-term imatinib administration on serum KIT, SCF, and VEGF levels
Longitudinally taken serum samples beyond the first 6 months of treatment were available from one study center (Helsinki; n = 13). Serum KIT concentrations decreased more than 60% from the baseline level in all 13 patients during the first year of treatment and remained low during the subsequent 6 months of therapy (Figure 5). In contrast, serum SCF levels increased with time, most patients showing a 25% to 250% increase after 15 months of treatment. Serum VEGF levels behaved in a more heterogeneous manner, 3 of the 13 patients had increasing and the rest decreasing levels during the first months of therapy. Interestingly, 2 of the 3 patients who progressed during the first 12 months of treatment had markedly rising VEGF levels during the first 12 months, and 1 of them had markedly increasing serum SCF levels (Figure 5). The serum KIT levels of the 3 patients who had documented disease progression during the first 12 months of imatinib therapy decreased about 70% during this time period, suggesting that serum KIT levels alone cannot be used for monitoring of response to imatinib therapy in patients with GIST.
Serum levels of VEGF, KIT, and SCF in 13 patients with GISTs with longitudinally taken serum samples available at least for 12 months. Patients with early progressive disease are shown with a bold black line (n = 5), and the rest of the patients are shown with thin lines. (A) VEGF, (B) KIT, (C) SCF.
Serum levels of VEGF, KIT, and SCF in 13 patients with GISTs with longitudinally taken serum samples available at least for 12 months. Patients with early progressive disease are shown with a bold black line (n = 5), and the rest of the patients are shown with thin lines. (A) VEGF, (B) KIT, (C) SCF.
Effect of imatinib treatment on the ratio of SCF to soluble KIT
Since one of the functions of the soluble KIT may be to regulate exposure of cells to unbound SCF, we calculated the ratios of SCF to soluble KIT in patients with GISTs treated with imatinib. The mean ratio increased 7.7-fold during the first 12 months of treatment, and was 13.6-fold higher than the baseline ratio in the few patients followed for 18 months or longer (Table 3). When the ratio of SCF to soluble KIT was analyzed from all 66 study patients using the 1- and 6-month follow-up data available, 59 (89%) of the patients had an increasing level (ratio > 1.0). No association was found between response to treatment and the size of the ratio of SCF to soluble KIT (P = .60).
Ratios of serum stem cell factor to serum KIT in GIST patients treated with imatinib
. | Ratio of serum stem cell factor (SCF) to KIT during treatment . | . | . | . | . | . | . | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
. | Pretreatment . | 1 mo . | 5 mo . | 12 mo . | 15 mo . | 18 mo . | Follow-up . | |||||
| Patients with 12-month ratio available | ||||||||||||
| 1 | 1.0 | 2.2 | 9.4 | 259.0 | 318.0 | 341.0 | PR 31 mo | |||||
| 2 | 1.0 | 4.1 | 25.8 | 33.4 | 39.2 | 20.5 | PR 30 mo | |||||
| 3 | 1.0 | 2.1 | 2.5 | 4.8 | 7.0 | 4.4 | PR 30 mo | |||||
| 4 | 1.0 | 1.8 | 8.0 | 10.9 | NA1 | NA | PR 24 mo | |||||
| 5 | 1.0 | 1.8 | 4.5 | 11.2 | 5.0 | NA | PR 23 mo | |||||
| 6 | 1.0 | 1.3 | 1.7 | 5.3 | 6.2 | NA | PR 23 mo | |||||
| 7 | 1.0 | 1.4 | 3.2 | 7.7 | 8.3 | NA | PR 22 mo | |||||
| 8 | 1.0 | 1.8 | 5.5 | 12.0 | 6.6 | NA | PR → PD 25 mo | |||||
| 9 | 1.0 | 1.3 | 4.0 | 6.2 | 6.5 | 6.6 | PR → PD 17 mo | |||||
| 10 | 1.0 | 3.1 | 6.4 | 4.3 | 5.2 | 5.1 | SD → PD 15 mo | |||||
| 11 | 1.0 | 1.2 | 11.5 | 16.9 | 22.1 | 26.3 | PR → PD 11 mo | |||||
| 12 | 1.0 | 1.3 | 2.3 | 3.1 | NA | NA | PR → PD 6 mo | |||||
| 13 | 1.0 | 2.2 | 4.1 | 6.9 | NA | NA | PD 12 mo | |||||
| Median | 1.0 | 1.8 | 4.5 | 7.7 | 6.8 | 13.6 | — | |||||
| Median for all other patients; N = 53 | 1.0 | 1.8 | 2.7 | NA | NA | NA | — | |||||
. | Ratio of serum stem cell factor (SCF) to KIT during treatment . | . | . | . | . | . | . | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
. | Pretreatment . | 1 mo . | 5 mo . | 12 mo . | 15 mo . | 18 mo . | Follow-up . | |||||
| Patients with 12-month ratio available | ||||||||||||
| 1 | 1.0 | 2.2 | 9.4 | 259.0 | 318.0 | 341.0 | PR 31 mo | |||||
| 2 | 1.0 | 4.1 | 25.8 | 33.4 | 39.2 | 20.5 | PR 30 mo | |||||
| 3 | 1.0 | 2.1 | 2.5 | 4.8 | 7.0 | 4.4 | PR 30 mo | |||||
| 4 | 1.0 | 1.8 | 8.0 | 10.9 | NA1 | NA | PR 24 mo | |||||
| 5 | 1.0 | 1.8 | 4.5 | 11.2 | 5.0 | NA | PR 23 mo | |||||
| 6 | 1.0 | 1.3 | 1.7 | 5.3 | 6.2 | NA | PR 23 mo | |||||
| 7 | 1.0 | 1.4 | 3.2 | 7.7 | 8.3 | NA | PR 22 mo | |||||
| 8 | 1.0 | 1.8 | 5.5 | 12.0 | 6.6 | NA | PR → PD 25 mo | |||||
| 9 | 1.0 | 1.3 | 4.0 | 6.2 | 6.5 | 6.6 | PR → PD 17 mo | |||||
| 10 | 1.0 | 3.1 | 6.4 | 4.3 | 5.2 | 5.1 | SD → PD 15 mo | |||||
| 11 | 1.0 | 1.2 | 11.5 | 16.9 | 22.1 | 26.3 | PR → PD 11 mo | |||||
| 12 | 1.0 | 1.3 | 2.3 | 3.1 | NA | NA | PR → PD 6 mo | |||||
| 13 | 1.0 | 2.2 | 4.1 | 6.9 | NA | NA | PD 12 mo | |||||
| Median | 1.0 | 1.8 | 4.5 | 7.7 | 6.8 | 13.6 | — | |||||
| Median for all other patients; N = 53 | 1.0 | 1.8 | 2.7 | NA | NA | NA | — | |||||
NA indicates not available; —, not applicable; PR, partial response; PD, progressive disease; and SD, stable disease.
Discussion
We found serum-soluble KIT levels to decrease markedly during imatinib therapy regardless of whether patients with GISTs with advanced disease responded to treatment or not, whereas serum SCF levels increased in the great majority of patients. In general, the proportional increase in the serum SCF levels was greater the longer the patients had been treated with imatinib. This resulted in highly elevated ratios of SCF to soluble KIT in patients who were treated with imatinib for several months for GISTs. High serum SCF/KIT ratios may be important, since soluble KIT binds SCF and may regulate the concentrations of unbound SCF in the blood circulation.14 Interestingly, increasing soluble VEGF levels were recently measured in patients with colon cancer treated with an antiangiogenic tyrosine kinase inhibitor SU11248, also suggesting that tyrosine receptor–targeted anticancer therapy may lead to gradual increase of the soluble ligand level.32
Little is known about the clinical significance of imatinib therapy–associated changes in serum KIT and SCF levels. Serum KIT has been suggested to originate from proteolytic cleavage of the extracellular domain of KIT on KIT-expressing cells.13 Cells that normally express KIT include mast cells, melanocytes, gastrointestinal tract pacemaker cells (Cajal cells), Leydig cells, spermatogonia, spermatids, hematopoietic stem cells, cutaneous basal cells, and breast epithelial cells.7,13,33,34 Patients with chronic myeloid leukemia (CML) often have elevated concentrations of circulating KIT in the serum,29 and a proportion of soluble KIT in patients with GISTs might originate from tumor cells. This hypothesis is supported by the presence of slightly higher serum KIT levels in patients with GISTs than in healthy controls (Table 1), and the declining serum KIT levels during imatinib treatment might thus in part reflect a decrease in the GIST tumor volume. However, we found no significant difference in the decline of serum KIT levels between responding and nonresponding patients, and no increase in the serum KIT levels was found in the few patients who progressed during therapy. We have also monitored serum KIT levels in 3 patients treated with imatinib for metastatic KIT-immunopositive melanoma. All melanoma patients had declining serum KIT levels, although all of them progressed clinically during treatment with imatinib (P.B. and H.J., unpublished data, 2002). These findings suggest that declining serum KIT levels during imatinib therapy may reflect inhibition of the overall KIT receptor tyrosine kinase turnover in the normal tissues rather than tumor response, and that soluble KIT levels cannot be used as a reliable predictor for imatinib response in patients with GISTs.
Both KIT and SCF function are essential in the early phases of hematopoiesis and erythropoiesis. Mutations in either the Sl (steel factor) locus, which encodes for SCF, or in the W locus, which encodes for KIT, provide information about the functions of this ligand-receptor pair in vivo.14 Mice that lack either SCF or KIT are nonviable and die in utero or during the perinatal period with severe macrocytic anemia.35 The principal effect of KIT on erythropoiesis appears to be quantitative, since red blood cells are formed in the absence of KIT, but are fewer in number.36 The present finding that serum SCF levels increase during imatinib treatment in virtually all patients with GISTs regardless of treatment response might be a consequence of a compensatory body response to preserve KIT signaling in the bone marrow and other tissues in order to maintain the normal body functions including blood cell production.
The persistent rise in the unbound serum SCF levels with prolonged treatment is of particular interest, since upon binding to the extracellular domain of the KIT receptor SCF causes dimerization and activation of the receptor, and may enhance signaling of both wild-type and mutated receptors. KIT (CD117)–expressing cells may still be found in core needle biopsies taken from GIST lesions of patients who respond to imatinib, and such tumor cells may represent imatinib-resistant and/or dormant tumor cells that have failed to undergo apoptosis in spite of otherwise successful imatinib treatment.37 Hypothetically, the gradually increasing levels of serum SCF during prolonged imatinib therapy might enhance survival of such dormant tumor cells. Rising serum SCF levels, coupled with genetic instability of the GIST cells, may favor selection and give a growth advantage to imatinib-resistant cells in vivo, and ultimately lead to treatment failure. Hence, rising unbound serum SCF levels during imatinib treatment might contribute to development of acquired resistance to imatinib in the treatment of patients with GISTs.
The mechanisms that are responsible for imatinib resistance in GISTs are likely to be heterogeneous. New mutations, sometimes affecting the binding site of imatinib, have now been described both in patients with CML and those with GISTs who have developed acquired resistance to imatinib, and the target gene amplifications may also occur in cancers resistant to imatinib.38 In a few imatinib-resistant GISTs, a receptor tyrosine kinase other than KIT has been found to be activated accompanied by a loss of the KIT oncoprotein expression. However, in many GISTs with acquired resistance to imatinib no specific molecular genetic mechanism for imatinib resistance could be identified, and either KIT or PDGFRA tyrosine kinase receptor remain constitutively activated.39
SCF levels were lower and serum VEGF levels higher in patients with GISTs prior to initiating imatinib therapy than in the controls. Low SCF levels might be explained by sequestration of soluble SCF from the blood to the tumor tissue in patients with GISTs. The relatively high VEGF levels measured in untreated patients with GISTs might be explained by production of VEGF in tumors as has been described for a few other types of human cancer.40 Serum VEGF levels change in parallel with treatment outcome in certain types of human cancer,24-26 and have recently been found to provide an indirect estimate of tumor VEGF expression despite that much of the blood VEGF is carried in platelets.41 We measured decreasing serum levels in patients who responded to imatinib, but this was not a significant finding in a multivariate repeated measures ANOVA model.
We conclude that the serum concentrations of the SCF increase and the KIT levels decrease during imatinib treatment of patients with GISTs, resulting in high SCF/KIT ratios with prolonged treatment. Much of the soluble KIT is likely to originate from the normal tissues, and the decrease in serum KIT may rather reflect decreased KIT turnover than tumor volume shrinkage. The reasons for SCF elevation remain hypothetical, but SCF elevation might result from a compensatory mechanism to maintain hematopoiesis and other KIT-related body functions. Prolonged imatinib treatment results in high ratios of SCF to soluble KIT. The clinical significance of these are not known, but high SCF/KIT ratios may increase the potential for SCF-induced cell signaling particularly at the time when imatinib treatment is withdrawn, or when stimulation of GISTs with wild-type KIT receptors occurs.
Prepublished online as Blood First Edition Paper, January 8, 2004; DOI 10.1182/blood-2003-10-3443.
Supported by research grants from the Cancer Society of Finland (H.J.), Helsinki University Central Hospital Research Funds (P.B., H.J.), and Novartis Pharma AG, Basel, Switzerland.
Several of the authors (M. von M., M.C.H., C.D.B., G.D.D., and H.J.) have declared a financial interest in Novartis, whose product was studied in the present work. S.D. and A.K. are employees of Novartis.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
The authors thank Onerva Levälampi for technical assistance and Henrik Alfthan (Department of Clinical Chemistry, Helsinki University Central Hospital, Finland) for the control sera.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal