Abstract
The promyelocytic leukemia (PML) tumor suppressor of acute promyelocytic leukemia (APL) is essential for a number of proapoptotic and growth-suppressive pathways as well as for the activity of differentiating agents such as retinoic acid (RA). In human APL, the dose of PML is reduced to heterozygosity given that one allele is involved in the chromosomal translocation while the status of the remaining PML allele is unknown. We have therefore used single-strand conformational polymorphism (SSCP) and sequencing analysis to screen DNA from APL patients for mutations at the PML locus. We identified DNA sequence variations resulting in a truncated PML protein in APL cases that displayed RA resistance and a very poor prognosis. Mutation analysis also led to the identification of aberrant PML sequence variations in other hematopoietic malignancies. Complete functional loss of PML is therefore selected by the APL phenotype and associates with poor prognosis and RA unresponsiveness.
Introduction
The PML tumor suppressor gene has become the object of intense research owing to its involvement in the pathogenesis of acute promyelocytic leukemia (APL) where it is found fused to the retinoic acid receptor–α (RARα) gene as a consequence of the t(15;17) chromosomal translocation.1 Promyelocytic leukemia (PML) is typically found concentrated in the nucleus in multiprotein-speckled structures termed PML nuclear bodies (PML-NBs).2 These structures are disrupted in PML-/- primary cells2 and in APL blasts.3 PML is therefore critical for the formation and stability of the NB, in turn implying that the function of multiple NB components may be impaired in the APL blasts or in cells lacking PML. A number of findings support the role for PML as a tumor suppressor.4 First, its inactivation obtained by homologous recombination lends a marked survival and proliferative advantage to cells of various histologic origins.3,5 Second, loss of PML function impairs cellular senescence in response to oncogenic stimuli.6,7 Last, PML inactivation impairs cellular differentiation upon differentiating agents such as RA and vitamin D.5,8,9 PML modulates key tumor-suppressive pathways such as the ones controlled by p53 and Rb, which are impaired in cells lacking PML's function.3,6,7,10-13 As a consequence, PML-/- mice are tumor prone.5,14 Compelling evidence that PML opposes APL leukemogenesis has been obtained in mouse models of APL. The progressive reduction of the dose of PML obtained by crossing PML-RARα transgenic mice with PML-/- mice resulted, in fact, in a dramatic increase in the incidence of leukemia and in an acceleration of leukemia onset.9 In addition, in hematopoietic cells from PML-RARα transgenic mice, PML inactivation resulted in impaired response to differentiating agents such as RA and vitamin D3 as well as in a marked survival advantage upon proapoptotic stimuli.9 In this report, we therefore investigated whether the remaining PML allele (the one not involved in the t(15;17)) would be found mutated in APL patients and whether complete PML loss would have an impact on disease onset, clinical outcome, and/or response to therapy.
Study design
Patients, samples, treatment modalities, and response criteria
The study included 131 patients with hematologic malignancies, 50 of which were APL (Table 2). All of the samples included in the present study were collected under Institutional Review Board–approved protocols and with informed consent. For most of the APL patients, summaries of the treatments administered and clinical outcome have been presented elsewhere. Briefly, out of 50 APL patients, 12 were first-relapse patients treated with all-trans retinoic acid (ATRA) and chemotherapy (daunorubicin and cytarabine)15 ; 6 were multiple-relapse patients who had experienced at least 2 relapses (range, 2-4) and had been treated with 2 or more courses of chemotherapy (range, 2-4) containing RA16 ; and 7 were patients whose pretreatment samples expressed the PML-RARα V isoform.17 Among the APL patients from the Latino population in Los Angeles, CA (n = 15), 11 received liposomal or oral RA induction followed by consolidation with 2 or more (range, 2-4) courses of chemotherapy on a variety of schedules, including idarubicin/cytarabine, etoposide/mitoxantrone, and liposomal RA. Four patients were treated with liposomal or oral RA therapy only. Two patients at relapse were treated with arsenic trioxide. One received an allogenic bone marrow transplantation (BMT). Overall, 12 of the 15 patients achieved complete remission (CR). All juvenile APL patients (n = 10; median age 6.4) received combined chemotherapy, followed by allogenic (2 cases) or autologous (1 case) BMT. Three out of 10 patients achieved CR. Of the 50 APL patients analyzed, 17 were resistant to RA. Criterion for RA resistance was the reduced in vitro sensitivity of APL blasts to RA-induced differentiation as previously reported.15 All APL patients included in this study had the t(15:17) translocation demonstrated either by karyotyping or reverse transcription–polymerase chain reaction (RT-PCR) of the PML-RARα fusion gene. APL samples used to prepare DNA were obtained from bone marrow except for a few cases, which were obtained from peripheral blood. In all cases, except for one remission case (blasts fewer than 5%), the percentage of blasts and promyelocytes in the samples analyzed was on average 80%. In addition, for most of the cases a Ficoll density gradient enrichment of APL blasts was performed, and the percentage of blasts and promyelocytes exceeded 75% in all cases and in most exceeded 90%.
Mutational analysis
Fourteen sets of primers were generated to amplify the entire PML coding sequence and intron-exon boundaries (Table 1). PCR products were subjected to single-strand conformational polymorphism (SSCP) analysis by means of a GenePhor Electrophoresis Unit and GeneGel Excel 12.5/24 (12.5% T [acrylamide + bisacrylamide/gel volume], 2% C [methylenebisacrylamide/acrylamide + bisacrylamide]) according to the manufacturer's instructions (Amersham Pharmacia Biotech, Piscataway, NJ). Silver staining of the gels was performed with the PlusOne DNA Silver Staining Kit according to the manufacturer's instructions (Amersham Pharmacia Biotech). PCR products displaying an altered electrophoretic mobility by SSCP were reamplified and sequenced on a semiautomated sequencer (Applied Biosytems, Foster City, CA) with the use of a Taq cycle sequencing kit (ABI PRISM dye terminator cycle sequencing ready detection kit; Perkin-Elmer, Shelton, CT). Each amplicon was sequenced bidirectionally. Each sample was sequenced at least twice from independent PCR reactions.
Generation of PML mutants, primary cell transfection, Western blotting, and immunofluorescence analysis
The PML mutant 1 and PML mutant 2 were derived from a human PML cDNA cloned in the pSG5 expression vector (Stratagene, Madison, WI). PML exon 5 51272 AG deletion and PML exon 4 deletion plus the frame change (mimicking the effect of the splice site intervening sequence 3 - 1G → A [IVS3 - 1G → A] mutation) were created by means of the QuikChange site-directed mutagenesis kit (Stratagene) according to manufacturer's instructions. The constructs were verified by DNA sequencing. PML-null mouse primary embryonic fibroblasts were prepared as previously described5 and transfected with the Effectene transfection reagent (Qiagen, Valencia, CA). For Western blot analysis of PML mutants, cells were cotransfected with 500 ng PML construct (wild type [wt], mutant 1 [Mut 1], Mut 2, or empty vector) along with 100 ng green fluorescent protein (GFP) (Promega, Madison, WI) to monitor transfection efficiency. At 48 hours after transfection, cells were lysed in E1A buffer3 and analyzed by Western blotting according to standard procedures. The polyclonal antihuman PML antibody was kindly provided by Dr K. S. Chang, University of Texas (Houston, TX). For immunofluorescence analysis, cells were seeded on coverslips in 12-well plates and transfected with 100 ng PML constructs (wt, Mut 1, Mut 2, or empty vector). At 48 hours after transfection, cells were fixed and permeabilized as described5 and stained with anti-PML antibody (PGM3) (Santa Cruz Biotechnology, Santa Cruz, CA). Slides were viewed on an Olympus fluorescence microscope (Olympus America, Melville, NY) or an Axiovert 100M (Carl Zeiss, Thornwood, NY) confocal microscope.
Results and discussion
PML is mutated in RA-resistant APL
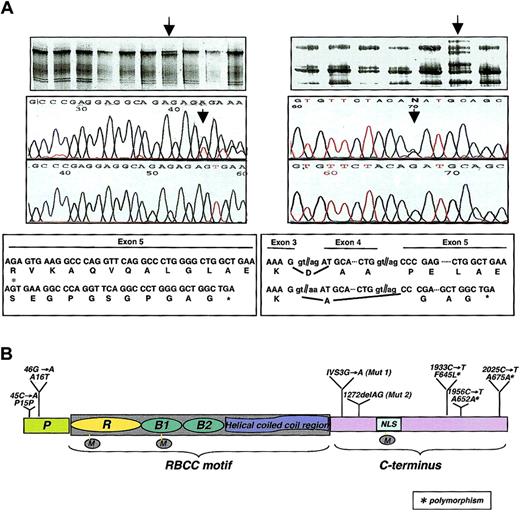
We analyzed DNA samples from RA-resistant (n = 17) and RA-sensitive (n = 33) APL, the latter including also juvenile APL (n = 10) and APL patients from the Latino population in the Los Angeles area (n = 15), where the incidence of APL among all forms of acute myeloid leukemia (AML) is much higher than in other areas of the world.18 DNA from 44 healthy individuals was also analyzed as a control. We identified DNA sequence variations predicted to result in a truncated PML protein and therefore of potential pathogenic importance in 2 of the 17 APL RA-resistant cases (Figure 1A). Both patients carrying these variants experienced a very aggressive course of the disease. The patient carrying the 1272delAG mutation in exon 5 (Figure 1A, left panel; Figure 1B, Mut 1) was a 9-year-old female (PML-RARα isoform of type L). She experienced a relapse after RA therapy alone and multiple chemotherapeutic regimens.19 No pretreatment DNA sample was available to test the presence of this variant before the beginning of the RA treatment. Sequencing analysis of the PML-RARα cDNA confirmed that the mutation occurred on the wild-type allele of PML since the mutation was not found in the fusion cDNA. A missense mutation (G289R) in the ligand-binding domain of the RARα portion of PML-RARα of this patient was also previously reported.16 The patient carrying the IVS3 - 1G → A variant (Figure 1A, right panel; Figure 1B, Mut 2) was a 19-year-old male affected by APL expressing the V form of PML-RARα with a relatively large deletion of PML exon 6 and insertion of a long overcompensatory sequence from RARα intron 2 in the fusion gene.20 Notably, this patient had evidence of decreased sensitivity to ATRA even before beginning therapy by nitroblue tetrazolium (NBT) test21 (NBT positivity lower than 20% at 100 nM RA), but not by the CD11b marker. He received only 3 weeks of ATRA owing to development of a fungal infection. After a relapse, this patient displayed a markedly reduced in vitro RA sensitivity compared with the pretreatment sensitivity. He survived 35 months after the diagnosis. To determine whether this mutation was selected by the RA treatment, we sequenced genomic DNA from the leukemic blasts during the pretreatment phase and found this variant to be present. Sequencing analysis of genomic DNA obtained during the remission phase demonstrated the absence of the mutation, thus excluding the presence of a germ line mutation in the PML gene predisposing to the onset of APL. No APL blasts were available to check the status of the PML protein.
PML mutational analysis in APL. (A) SSCP (top panels) and sequencing analysis (2 middle panels, with the mutated sequences on the upper middle panel) identified 2 DNA sequence variations (arrows) predicted to result in a truncated PML protein (bottom panels) among the 17 APL RA-resistant samples. The first is a small deletion (1272delAG) and the second a splice site mutation (IVS3 - 1G → A). (B) PML missense, truncating, and silent mutations in hematopoietic malignancies. The schematic structure of the PML protein is shown: P indicates proline-rich region; R, RING finger domain; B1 and B2, B-boxes 1 and 2; NLS, nuclear localization signal; M, sumoylation sites. The 1272delAG is indicated as Mut 1 and IVS3 - 1G → A as Mut 2. The polymorphic mutation is also indicated.
PML mutational analysis in APL. (A) SSCP (top panels) and sequencing analysis (2 middle panels, with the mutated sequences on the upper middle panel) identified 2 DNA sequence variations (arrows) predicted to result in a truncated PML protein (bottom panels) among the 17 APL RA-resistant samples. The first is a small deletion (1272delAG) and the second a splice site mutation (IVS3 - 1G → A). (B) PML missense, truncating, and silent mutations in hematopoietic malignancies. The schematic structure of the PML protein is shown: P indicates proline-rich region; R, RING finger domain; B1 and B2, B-boxes 1 and 2; NLS, nuclear localization signal; M, sumoylation sites. The 1272delAG is indicated as Mut 1 and IVS3 - 1G → A as Mut 2. The polymorphic mutation is also indicated.
Detection of PML gene variants in non-APL hematopoietic malignancies
We next analyzed samples from hematopoietic malignancies other than APL (Table 2). PML variants not found in the healthy population were identified in these samples (Table 2; Figure 1B). Among the AML non-APL cases, we detected a missense mutation in exon 1 46G → A (A16T) localized in the PML amino terminus proline-rich domain (Figure 1B). In addition, a number of variants were observed in the untranslated UTR 5′ (–14C → G) in MDS, in exon 1 (45C → A) in AML non-APL, in intron 4 (IVS4 + 24G 3 C) in multiple myeloma and lymphomas, in intron 7 (IVS7 - 22C → T) in AML non-APL, and in exon 9 (1933C → T, 1956C → T, 2025C → T). The variants identified in exon 9 very likely represent polymorphisms since they were also frequently found in healthy individuals, while all the other PML variants were found only in the samples from affected individuals. PML mutations were not accompanied by loss of protein expression in multiple myeloma and lymphoma cases. For the mutations identified in AML non-APL and MDS cases, cells were not available for the protein expression analysis.
PML mutants display an aberrant cellular localization pattern
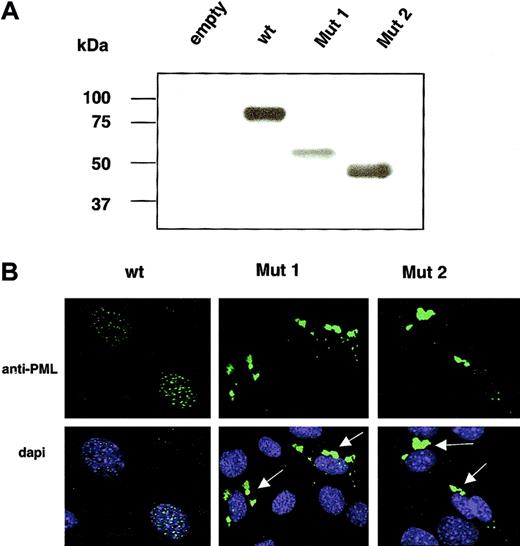
To assess the molecular consequences of the PML mutations detected in RA-resistant APL, expression vectors encoding the 2 mutated forms of PML (Mut 1 and Mut 2) were generated and transfected in PML-/- cells (mouse fibroblasts) (Figure 2). As expected, they were found to encode truncated PML proteins (Figure 2A). Strikingly, both PML Mut 1 and PML Mut 2 proteins displayed an aberrant patchy cytoplasmic localization pattern and failed to accumulate in the NBs or the nucleus (Figure 2B). These results are consistent with the fact that the PML NLS is not retained in the truncated variants, while these mutants retain the RING B box-coiled coil motif (RBCC) motif, which is responsible for PML homo-oligomerization5 (Figure 1B).
Molecular characterization of PML Mut 1 and Mut 2 variants. (A) Western blot analysis of wt PML, Mut 1, and Mut 2 overexpressed in PML-/- mouse fibroblasts. (B) Immunofluorescence analysis reveals that PML Mut 1 and Mut 2 localize aberrantly in the cytoplasm as indicated by the arrows. Original magnification × 60.
Molecular characterization of PML Mut 1 and Mut 2 variants. (A) Western blot analysis of wt PML, Mut 1, and Mut 2 overexpressed in PML-/- mouse fibroblasts. (B) Immunofluorescence analysis reveals that PML Mut 1 and Mut 2 localize aberrantly in the cytoplasm as indicated by the arrows. Original magnification × 60.
In summary, we identified for the first time PML gene sequence variations in hematopoietic malignancies. Truncating mutations in the PML gene were identified in APL cases characterized by resistance to RA treatment and associated with a poor clinical outcome. We demonstrated that these mutations result in a truncated protein that no longer concentrates in the nucleus and in the PML-NBs, but instead displays an aberrant cytoplasmic localization pattern. These variants are therefore unable to exert the various nuclear and NB-dependent functions that have been attributed to PML.2 In APL, as a consequence of the t(15;17) translocation, PML is reduced to heterozygosity. Thus, the truncating mutation in the other PML allele results in its complete inactivation. By contrast, the consequence of PML mutations in non-APL malignancies remains to be determined, as it is presently unclear whether these PML mutants can act aberrantly.
In APL, PML-RARα causes the disruption of the PML-NB and the relocalization of PML to aberrant nuclear sites, interfering with its normal cellular function.5 This dominant negative activity of the fusion protein on PML is crucial for APL leukemogenesis, as demonstrated by the fact that PML-RARα caused leukemia with APL features while dominant negative RARα mutants that cannot interfere with PML function failed to do so.22,23 Since the effects of PML-RARα on PML are dose dependent, the product of the remaining PML wild-type allele may in turn oppose PML-RARα's oncogenic role. Indeed, we have previously demonstrated that the progressive reduction of the dose of PML obtained by crossing PML-RARα transgenic mice with PML-/- mutants accelerated leukemogenesis and exacerbated its unresponsiveness to differentiating, growth-inhibitory, and proapoptotic stimuli.9 Therefore, as in murine models, complete PML loss in human APL could favor PML-RARα leukemogenesis by rendering leukemic blasts less responsive to differentiating stimuli, such as RA and vitamin D, and conferring a proliferative, survival advantage and unresponsiveness to treatment.
Prepublished online as Blood First Edition Paper, November 20, 2003; DOI 10.1182/blood-2003-07-2200.
Supported by the National Cancer Institute (NCI) (CA71692 and CA74031) (P.P.P.), and a Leukemia and Lymphoma Society Specialized Center of Research (SCOR) grant (S.N. and P.P.P.)
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Ilhem Guernah, Francesco Piazza, Davide Ruggero, Paolo Salomoni, Vibha Jain, Riccardo Dalla-Favera, Alan Houghton, Robert Hromas, and Kun-Sang Chang for technical help, material, and discussions.